Abstract
In this study, we examined whether topical treatment of glutamate receptor antagonists attenuate hyperexcitability of lumbar spinal dorsal horn neurons following low thoracic hemisection spinal cord injury in rats. Four weeks after spinal hemisection, neuronal activity in response to mechanical stimuli applied on the peripheral receptive field was significantly increased in three different phenotypes of lumbar spinal dorsal horn neurons: wide dynamic range (WDR), low threshold (LT) and high threshold (HT). Topical application of MK-801 (NMDA receptor antagonist, 50 μg) significantly attenuated the activity of WDR, but not LT and HT neurons; whereas, NBQX (AMPA receptor antagonist, 0.5 and 1 μg) significantly attenuated neuronal activity in all three phenotypes of neurons (*p<0.05). However, MCPG (group I/II metabotropic glutamate receptor antagonist, 100 μg) had no effect. The present study, in the context of previous work, suggests that ionotropic glutamate receptor activation play critical roles in the maintenance of neuronal hyperexcitability and neuropathic “below level” pain behavior following spinal hemisection injury.
Keywords: Central Neuropathic Pain, Hyperexcitability, NBQX, MCPG, MK-801, Spinal Cord Injury
Spinal cord injuries (SCI) result in maladaptive synaptic plasticity via extra- and intracellular biochemical events in the spinal dorsal horn. The enhanced synaptic plasticity causes abnormal dorsal horn neuronal hyperexcitability in response to mechanical, chemical and thermal stimuli, respectively, and is called central sensitization. Persistent neuronal hyperexcitability produces enhanced long-lasting transmission of somatosensory input- that is interpreted to be nociceptive in response to non-noxious (allodynia) or noxious (hyperalgesia) stimuli applied to the peripheral receptive fields.
Spinal cord injury increases the concentration of extracellular glutamate and results in increased glutamate receptor expression over time in the lumbar spinal cord (Liu et al., 1991). There is accumulating evidence that supports the involvement of glutamate receptors as a critical component in maintained neuropathic behavior and persistent neuronal hyperexcitability after spinal cord injury. Glutamate, an excitatory amino acid in the nervous system, acts via ligand-gated ionotropic receptors, such as the NMDA and AMPA receptors, as well as G-protein coupled metabotropic glutamate receptors (mGluRs) (Bennett et al., 2000; Gwak et al., 2005; Bleakman et al., 2006). We previously demonstrated that low thoracic spinal hemisection results in overexpression of group I/II mGluR in the lumbar spinal dorsal horn (Gwak et al., 2005) and treatment of ionotropic glutamate receptor antagonist attenuated mechanical allodynic behaviors (Bennett et al., 2000; Gwak et al., 2007). However, it is unknown whether group I/II mGluR or other glutamate receptors modulates the electrophysiological properties of lumbar dorsal horn neurons following spinal hemisection.
In the present study, we examined the electrophysiological outcomes in the maintenance of neuronal hyperexcitability by topical administration of NMDA, AMPA and mGluR I/II receptor antagonists to evaluate the role of these receptors in segmental regions below the level of spinal hemisection injury. Although the evidence suggests that activation of GluRs critically modulates neuronal excitability following SCI, little is known regarding their roles in the maintenance of neuronal hyperexcitability following SCI.
A total of 120 male Sprague-Dawley rats (200–250 mg) were used in this study. SCI was produced by transverse hemisection by using a micro dissecting knife and a 28-gauge needle, from dorsal to ventral, of spinal segment T13 (Christensen et al., 1996; Gwak et al., 2003) under masked-inhalation anesthesia (enflurane: induction 3% and maintenance 2%) using a surgical microscope. All surgeries and experiments were done in compliance with the approval by the Institutional Animal Care and Use Committee. As a control group, sham surgery was done as the hemisection surgery, with the exception that the spinal cord was not cut. The animals were allowed to survive for 28 days after spinal hemisection at which time electrophysiology experiments were performed. By 28 days, mechanical allodynia has stabilized based on previous behavioral studies (Gwak et al., 2003, 2007, 2008; Christensen, et al., 1996).
For electrophysiology, a tracheal cannula was used for artificial ventilation (ventilator, CWE Inc, U.S.A.) and a jugular vein cannula for supplemental paralysis with pancronium bromide (2–4 mg/kg/h) was inserted, respectively, under masked-inhalation of enflurane (induction at 3% and maintenance at 2%). A laminectomy (T12-L3) exposed the lumbar enlargement and the rat was held in place by a stereotaxic apparatus and end-tidal CO2 concentration (3.5%–4.5%, a carbon dioxide analyzer) and body temperature (37 °C, homeothermic blanket control unit) were monitored, respectively. The phenotypes of dorsal horn neurons were classified into one of three categories: wide dynamic range (WDR), low threshold (LT) and high threshold (HT) neurons according to the stimulus-response properties. Briefly, neurons that showed the best response activity to brush stimuli were classified as LT neurons; neurons that showed the best response activity to both pressure and pinch stimuli were classified as HT neurons; and neurons that showed graded response patterns to increased mechanical intensity were classified as WDR neurons (Chung et al., 1986, Gwak et al., 2003).
The single-unit activity was recorded with single carbon filament-filled glass microelectrodes. The mechanical stimuli was applied for 10 seconds and included brushing the skin (brush stimuli), followed by application of large clamp (pressure stimuli, Bulldog Clamp, Tiemann) and a small clamp (pinch stimuli, Serrefines, Tiemann). After isolating a single unit, the background activity was recorded for 20 seconds prior to stimulus application to the peripheral receptive field. Topical spinal application near the recording electrode was performed in separate recording sessions of two doses of NBQX [Tocris, 0.5 and 1 μg, disodium salt, competitive AMPA/Kainate receptor antagonist, 2,3-Dioxo-6-nitro-1,2,3,4,-tetrahydrobenzo[f]-quinoxaline-7-sulfonamide, Tocris, MO, U.S.A]; two doses of MK-801 [Tocris, 25 and 50 μg, non-competitive NMDA receptor antagonist, (5R, 10S)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate] or two doses of MCPG [Tocris, 50 and 100 μg, competitive Group I and II mGluR receptor antagonist, ±-α-Methyl-(4-carboxylphenyl)glycine] given in 50 μl volumes. The concentrations of antagonists were based on previous reports (Bennett et al., 2000, Gwak et al., 2007, Zahn and Brennan, 1998). We were able to record from 7–8 identified neurons (combined from injured and uninjured sides of spinal dorsal horn) from each animal for the electrophysiological comparisons (hemisection versus sham control). In the pharmacology studies, only one identified neuron per animal was used for comparison of vehicle (PBS) or drug effect to avoid multiple drug influences (pre drug level versus post drug level). A t-test was used to assess differences comparing pre- to post drug in the spinal dorsal horn neuron’s responses. The data from the left and the right lumbar dorsal horn recordings are combined because there was no significant difference in side-by-side comparisons (injured side vs. uninjured side). Alpha level of significance was set at 0.05 for all statistical tests using SigmaStat (ver 3.0). All data values are displayed as means (X) ± standard errors (SE.).
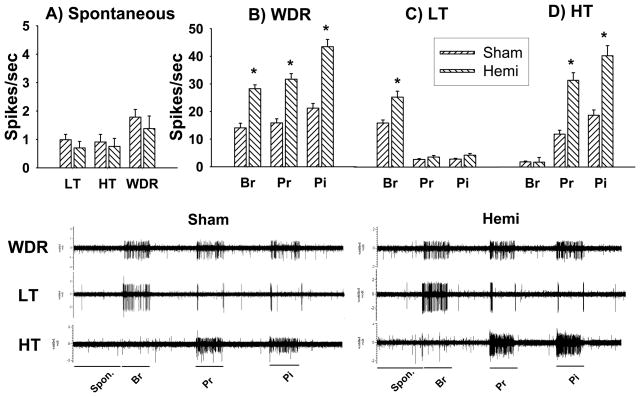
In the first set of electrophysiological studies after hemisection, the mean spontaneous activity of LT, HT and WDR neurons did not show significant changes compared to sham controls (Figure 1A). However, LT, HT and WDR neurons showed significantly increased neuronal activity in response to mechanical stimuli compared to sham controls. The mean activity of WDR neurons (43 cells) was significantly increased with values of 28.3 ± 1.4, 31.7 ± 2, and 43.5 ± 2.7 spikes/sec to brush, pressure and pinch stimuli, respectively compared to sham control values of 14.1 ± 1.7, 15.9 ± 1.5, 21.3 ± 1.7 spikes/sec, (25 cells, *p<0.05, Figure 1B). The mean activity of LT neurons (25 cells) in the hemisection group to brush stimuli was 26.1 ± 2.2 spikes/sec, which was significantly increased compared to sham control values of 16.4 ± 1.2 spikes/sec (37 cells, *p<0.05, Figure 1C). The evoked activity of HT neurons (15 cells) was 32 ± 2.8 (pressure) and 41 ± 3.7 spikes/sec (pinch), respectively and showed significantly increased activity compared to sham controls which had values of 12.1 ± 1.5 and 19.1 ± 2 spikes/sec (24 cells, *p<0.05, Figure 1D).
Figure 1.
Hyperexcitability of lumbar spinal dorsal horn neurons following spinal hemisection. (Top) four weeks after hemisection, the spontaneous activity (A) did not show significant differences among three different types of neurons compared to sham controls. (B) WDR neurons showed significantly increased evoked activity to brush, pressure and pinch stimuli compared to sham controls. (C) LT neurons showed significantly increased brush-evoked activity whereas HT neurons (D) showed significantly increased pressure- and pinch-evoked activity, respectively. Values shown as means ± S.E.s. (Bottom) shows the typical waveforms of each type of neuron in the absence of stimuli (20 seconds) and in response to stimuli applied for 10 seconds to the peripheral receptive field. Spon : spontaneous, Br: brush, Pr: pressure, Pi: pinch stimuli. *p<0.05.
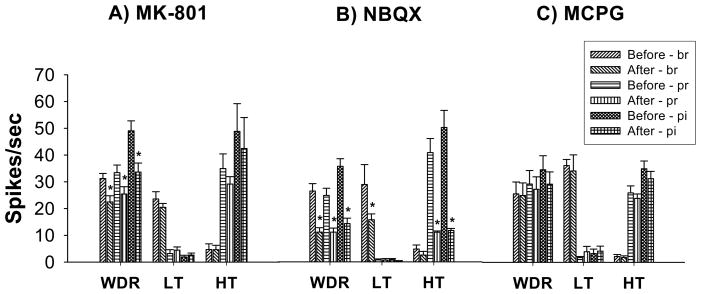
In the second set of electrophysiological studies, application of MK801 and NBQX significantly attenuated neuronal hyperexcitability after spinal hemisection. After application of MK-801 (50 μg), the mean activity of WDR neurons (10 cells) was 22.5 ± 2.3, 25.5 ± 2.7 and 33.7 ± 3.4 spikes/sec to brush, pressure and pinch stimuli, respectively, which was significantly attenuated compared to pre-drug levels of activity (31 ± 1.9, 33 ± 2.8 and 49 ± 3.7 spikes/sec, respectively) whereas the mean activity of LT (9 cells) and HT (3 cells) neurons did not show significant changes (Figure 2A, *p<0.05). However, NBQX application (19 cells for 1 μg and 15 cells for 0.5 μg) significantly attenuated the electrophysiological activity in all phenotypes of dorsal horn neurons compared to pre-drug levels of activity. Prior to application of NBQX, the mean activity of WDR neurons were 26 ± 2.7, 24.1 ± 2.6 and 35.1 ± 2.8 spikes/sec, respectively. After application of NBQX (1 μg, 19 cells), the mean activity for WDR neurons (10 cells) were 10.9 ± 1.6, 10.9 ± 1.4 and 14.1 ± 2 spikes/sec, respectively which demonstrated significant differences. In addition, NBQX application significantly attenuated the mean activity of LT neurons (5 cells, Figure 2B) and HT neurons (4 cells, Figure 2C) (*p<0.05). However, values after MK-801 (25 μg, 14 cells) and MCPG application (15 cells for 50 μg and 18 cells for 100 μg, Figure 2C) did not show significant changes compared to pre-drug levels of activity.
Figure 2.
Inhibition of lumbar dorsal horn neuronal hyperexcitability by topical spinal application of glutamate receptor antagonists four weeks after thoracic spinal hemisection. (A) Topical application of MK-801 (50 μg) significantly inhibited hyperexcitability of spinal lumbar WDR neurons (10 cells) whereas LT (9 cells) and HT (3 cells) neurons did not show significant differences compared to pre-drug levels of activity. (B) Topical application of NBQX (1 μg) significantly inhibited hyperexcitability of WDR (10 cells), LT (5 cells) and HT (4 cells) spinal lumbar dorsal horn neurons. However, topical application of MCPG (C, 100 μg, WDR =7 cells, LT =6 cells, HT=5 cells) did not show any significant changes. *p<0.05.
The present study demonstrates that blockade of NMDA and AMPA ionotropic glutamate receptors, but not group I and II metabotropic (mGluRI/II) glutamate receptors, attenuates hyperexcitability of spinal lumbar dorsal horn neurons following low thoracic spinal hemisection injury. Thus, the present study, taken together with previous studies, suggests that ionotropic, not metabotropic, glutamate receptors contribute to the maintenance of neuronal hyperexcitability and play critical roles in persistent “below-level” neuropathic pain behavior (Bennett et al., 2000; Gwak et al, 2007; however, see Mills, et al., 2000) in remote regions below the level of spinal hemisection injury.
Immediately after spinal cord injury, activation of glutamate receptors results in an excessive influx of Ca2+ ions into the intracellular compartment followed by activation of calcium-dependent and -independent kinases, such as PKC, PKA, CAMKII, PLA2, and MAPK family (Choi, 1992). Activation of intracellular downstream pathways initiates development of abnormal response properties in the spinal dorsal horn neurons, such as increased spontaneous activity, decreased threshold for activation and enhanced neuronal activity in response to both non-noxious and noxious stimuli, a condition known as neuronal hyperexcitability or central sensitization (Gwak et al., 2003; Hains et al., 2003).
Spinal hyperexcitability is a predominant spinal mechanism for behavior consistent with mechanical allodynia, a neuropathic pain-like behavior following neural injury that can be tested in rodent neuropathic pain models (Christensen et al., 1996). Several previous reports showed that mechanical allodynia-like behavior was attenuated by administration of an NMDA and AMPA receptor antagonist in rodent spinal hemisection injury models (Bennett et al., 2000; Gwak et al., 2007); however the mechanisms are not know. In the present study, we demonstrate that blockade of the NMDA receptor attenuated hyperexcitability of lumbar WDR neurons whereas LT and HT neurons did not show any significant changes following low thoracic spinal hemisection. In addition, blockade of the AMPA receptor attenuated neuronal hyperexcitability in all three types of neurons, WDR, HT and LT, respectively. By contrast, blockade of group I and II mGluR receptor did not show attenuation of neuronal hyperexcitability in any phenotype of lumbar dorsal horn neurons. It is interesting to note that we previously reported that spinal hemisection resulted in increased expression of mGluR I and II receptors in neurons and astrocytes in the lumber spinal dorsal horn (Gwak and Hulsebosch, 2005). In addition, the literature suggests that administration of intrathecal group I mGluRs antagonists attenuates mechanical allodynia-like behaviors following spinal contusion injury (Mills et al., 2000). This discrepancy suggests that the change of mGluRs receptor expression in the spinal dorsal horn may not be an indicator of central neuropathic pain since spinal contusion, but not hemisection injury, produces mGluRs-mediated maintenance of central neuropathic pain. Although we have not confirmed expression of activated glutamate receptors, such as the phosphorylated form in the hemisected model, we report that activation of ionotropic glutamate receptors are major contributors to “below-level” neuropathic pain following low thoracic spinal hemisection injury(Bennett, et al., 2000; Gwak et al., 2007).
We have consistently reported that unilateral SCI results in reproducible, bilateral hyperexcitability in lumbar spinal dorsal horn neurons in regions remote and below the injury (Gwak et al., 2003, 2007, 2008). The bilateral mechanism is not fully understood. However, the neuroanatomical architecture of the spinal cord may provide the substrate for the bilateral mechanisms. Firstly, multisegmental propriospinal pathways relay the somatosensory input over a few segments of spinal cord both ipsilaterally and contralaterally; and commissural pathways are able to relay these inputs from ipsilateral to contralateral sides of the spinal dorsal horn (Basbaum, 1973; Chung and Coggeshall, 1983). Secondly, a few descending pathways decussate at the segment of termination (Skagerberg and Bjorklund, 1985) which would cause bilateral loss of endogenous descending inhibitory input to somatosensory circuits below the lesion.
In conclusion, the present study demonstrates that ionotropic glutamate receptor antagonists may be a useful treatment for below-level chronic central neuropathic pain following SCI.
Acknowledgments
This work was supported by a grant (1999-2-21300-004-3) from the Basic Research Program of the Korea Science & Engineering Foundation and NIH grants NS11255 and NS39161.
References
- Basbaum AI. Conduction of the effects of noxious stimulation by short-fiber multisynaptic systems of the spinal cord in rat. Exp Neurol. 1973;40:699–716. doi: 10.1016/0014-4886(73)90105-2. [DOI] [PubMed] [Google Scholar]
- Bennett AD, Everhart AW, Hulsebosch CE. Intrathecal administration of an NMDA or a non-NMDA receptor antagonist reduces mechanical but not thermal allodynia in a rodent model of chronic central pain after spinal cord injury. Brain Res. 2000;859:72–82. doi: 10.1016/s0006-8993(99)02483-x. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1999;23(9):261–276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- Chung K, Coggeshall RE. Propriospinal fibers in the rat. J Comp Neurol. 1983;217:47–53. doi: 10.1002/cne.902170105. [DOI] [PubMed] [Google Scholar]
- Chung JM, Surmeier DJ, Lee DJ, Sorkin LS, Honda CN, Tsong Y, Willis WD. Classification of primate spinothalamic and somatosensory thalamic neurons based on cluster analysis. J Neurophysiol. 1986;56:308–327. doi: 10.1152/jn.1986.56.2.308. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Nam TS, Paik KS, Hulsebosch CE, Leem JW. Attenuation of mechanical hyperalgesia following spinal cord injury by administration of antibodies to nerve growth factor in the rat. Neurosci Lett. 2003;336:117–120. doi: 10.1016/s0304-3940(02)01251-x. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Upregulation of Group I metabotropic glutamate receptors in neurons and astrocytes in the dorsal horn following spinal cord injury. Exp Neurol. 2005;195:236–243. doi: 10.1016/j.expneurol.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Kang J, Leem JW, Hulsebosch CE. Spinal AMPA receptor inhibition attenuates mechanical allodynia and neuronal hyperexcitability following spinal cord injury in rats. J Neurosci Res. 2007;85:2352–2359. doi: 10.1002/jnr.21379. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Profentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 2008;138:410–422. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Johnson KM, Eaton MJ, Willis WD, Hulsebosch CE. Serotonergic neural precursor cell grafts attenuate bilateral hyperexcitability of dorsal horn neurons after spinal hemisection in rat. Neuroscience. 2003;116:1097–1110. doi: 10.1016/s0306-4522(02)00729-7. [DOI] [PubMed] [Google Scholar]
- Liu D, Thangnipon W, McAdoo DJ. Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res. 1991;547:344–348. doi: 10.1016/0006-8993(91)90984-4. [DOI] [PubMed] [Google Scholar]
- Mills CD, Xu GY, Johnson KM, Hulsebosch CE. AIDA reduces glutamate release and attenuates mechanical allodynia after spinal cord injury. Neuroreport. 2000;11:3067–3070. doi: 10.1097/00001756-200009280-00007. [DOI] [PubMed] [Google Scholar]
- Skagerberg G, Bjoprklund A. Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. Neuroscience. 1985;15:445–480. doi: 10.1016/0306-4522(85)90225-8. [DOI] [PubMed] [Google Scholar]
- Zahn PK, Brennan TJ. Intrathecal metabotropic glutamate antagonists do not decrease mechanical hyperalgesia in a rat model of postoperative pain. Anesth Analg. 1998;87:1354–1359. [PubMed] [Google Scholar]