Abstract
Phase-change materials (PCMs), including 1-tetradecanol with a melting point at 38–39 °C and dodecanoic acid with a melting point at 43–46 °C were exploited as thermosensitive materials to demonstrate a new temperature-regulated drug release system. In this approach, colloidal particles containing FITC-dextran were embedded in the PCM matrix and processed as spheres or rods. When temperature was below the melting point of the PCM, there was no release of FITC-dextran due to the hydrophobic nature of the PCM. As the temperature was increased beyond the melting point, the PCM began to melt, the encapsulated particles leached out, and eventually FITC-dextran was released from the colloidal particles. By using colloidal particles made of gelatin, chitosan, and poly(lactic-co-glycolic acid) that have different solubility in water, we could manipulate the release pattern of FITC-dextran. We also demonstrated a dual temperature-regulated drug release system by incorporating two different PCMs into the same device. As an attractive feature, we could easily alter the initiation temperature and release pattern of drugs by judiciously selecting different combinations of PCMs and materials for the colloidal particles.
Stimuli-sensitive drug delivery is a promising strategy for achieving on-demand release of drugs. External and internal stimuli, including temperature, pH, ultrasound, and specific molecules (e.g., glucose), can all be utilized to regulate the release of drugs in various delivery systems.[1] Among them, temperature has been commonly used as a stimulus to trigger drug release due to the fact that local body temperature can vary in response to ambient conditions and diseases in some cases. So far, only a handful of materials with thermosensitive properties have been demonstrated for temperature-sensitive drug release, and typical examples include poly(N-isopropylacrylamide) (poly-NIPAAm) and its derivatives,[2] biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA) and poly(ethylene glycol) (PEG),[3] poly(organophosphazenes),[4] as well as polysaccharide and its derivatives.[5] However, the drug release systems fabricated from these materials have a number of shortcomings that will limit their use in clinical applications: non-biodegradability and significant toxicity (for poly-NIPAAm and poloxamer); need of a high temperature process to load the drugs (for block copolymers based on PLGA and PEG); need of an additional heating system for initiation; inconsistent drug release behaviors; low encapsulation efficiency, and insufficient amount of drug loading.[6] In addition, most of the delivery systems based on hydrogels show a noticeable amount of released drugs at the initial point due to the free diffusion of drugs through the porous structure of a hydrogel. In this regard, the hydrogels and colloidal particles based on poly-NIPAAm would be in the “on” state of drug release immediately after their fabrication, making it difficult to achieve precise control over the release profile.[7]
There are a number of generic requirements for an ideal stimuli-sensitive drug delivery system: i) the material used for fabricating the matrix must be biocompatible or at least bio-inert; ii) the drug can be encapsulated without losing the activity; iii) drug release can be initiated in a non-invasive manner and favorably without the involvement of any external apparatus; and iv) no drug should be released until the system is turned on.[6b]
Here we introduce two new classes of thermosensitive materials: fatty alcohols and fatty acids. These materials, capable of reversible solid-liquid transition in response to temperature variation, are known as phase-change materials (PCMs).[8] Specifically, 1-tetradecanol with a melting point (m.p.) of 38–39 °C and dodecanoic acid with a m.p. of 43–46 °C should be appropriate candidates for demonstrating temperature-regulated drug release systems due to their water immiscibilities, relatively good biocompatibility, and melting points close to the human body temperature. Below their melting points, both PCMs are in a solid state with a white color. When heated beyond their melting points, they exhibit a rapid response to temperature, transforming into a transparent liquid phase. We have fabricated PCM-based drug delivery constructs in the forms of spheres and blocks to demonstrate on-demand drug release as regulated by temperature. In our approach, a variety of colloidal particles containing drugs can be encapsulated in the PCM matrix. The particles serve as a diffusive barrier for controlling the release kinetic of the drugs while the PCM serves as a container of the particles and a stopper for the drug release. The rationale for this approach is as follows: in the case of an elevated body temperature due to fever, inflammation, or other diseases, the particles containing the drugs will leach out from the PCM matrix by melting the PCM and the drugs will be subsequently released from the colloidal particles. As soon as the drug-loaded colloidal particles have escaped from the PCM matrix, the drug release should be controlled by the diffusion of drug molecules through the colloidal particles and/or the degradation profile of the colloidal particles. Therefore, the melting of PCM should have no influence on the release of the encapsulated drug. Both the rate and initiation temperature for drug release can be controlled by selecting a proper material for the particles and a PCM, respectively. To the best of our knowledge, this work represents the first demonstration of PCMs for fabricating a temperature-regulated drug delivery system.
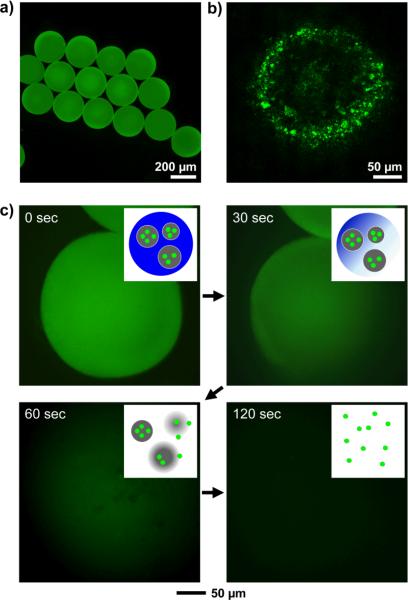
We produced gelatin particles (0.3–2 μm in size) containing fluorescein isothiocyanate-dextran (FITC-dextran) using a typical emulsification method with a homogenizer (Figure S1). Uniform 1-tetradecanol beads containing the gelatin particles were fabricated using a simple fluidic device based on oil-in-water emulsion (Figure S2). Melted 1-tetradecanol containing the gelatin particles was used as the discontinuous phase while an aqueous poly(vinyl alcohol) (PVA, 2 wt%) solution served as the continuous/collection phases in the fluidic device. The temperatures of both phases were kept at 60 °C using a heating tape. PCM droplets pinched off at the tip of the needle, flew along the glass capillary to the collection phase (kept at room temperature to solidify the PCM droplets), and eventually evolved into uniform PCM beads containing the gelatin particles. Figure 1a shows fluorescence micrograph of the resultant 1-tetradecanol beads with a uniform size of 238 ± 13 μm. As shown in Figure 1b, the confocal micrograph confirmed the presence of FITC-dextran-loaded gelatin particles in the PCM bead. To examine the melting of PCM beads and release behavior of FITC-dextran, the PCM beads were attached to the bottom of a Petri dish using an epoxy adhesive and 10 mL of room-temperature water was poured into the Petri dish. Some warm water (at 60 °C) was then added into the Petri dish and fluorescence micrographs were taken every second. As shown in Figure 1c, the 1-tetradecanol beads exhibited their original solid form with green fluorescence prior to the addition of warm water. Upon rising of temperature, it was observed that the PCM beads began to melt and the gelatin particles containing FITC-dextran leached out from the beads and eventually FITC-dextran was released into the warm water. The insets in Figure 1c schematically depict the three steps involved in the release of FITC-dextran from the PCM beads: i) melting of PCM, ii) escaping of the gelatin particles, and iii) release of FITC-dextran from the gelatin particles. In addition to the beads >100 μm in diameter, we could fabricate smaller PCM microspheres with sizes in the range of 10–30 μm by homogenizing melted PCM containing the gelatin particles in a warm PVA solution, followed by cooling the emulsion to solidify the PCM (Figure S3).
Figure 1.
a) Fluorescence and b) confocal micrographs of uniform 1-tetradecanol beads containing gelatin particles loaded with FITC-dextran. c) Time-lapse fluorescence micrographs showing melting of the 1-tetradecanol bead and release of FITC-dextran from the gelatin particles as the temperature was gradually increased by adding warm water (60 °C) under gentle stirring. The insets are schematic diagrams showing the three major steps involved in the release of FITC-dextran: melting of 1-tetradecanol beads, leaching out of gelatin particles, and release of FITC-dextran as gelatin is being dissolved.
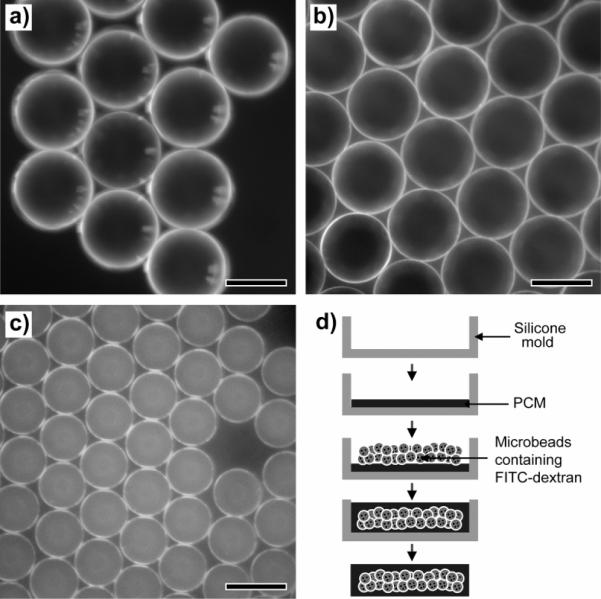
We also fabricated and examined PCM blocks in the shape of a rectangular rod, containing uniform FITC-dextran-loaded microbeads. In this case, a silicone mold with dimensions of 14 × 3.5 × 2.4 mm3 was used. Gelatin, chitosan, and PLGA were selected as the materials for microscale beads loaded with FITC-dextran because these materials have quite different solubilities in water: gelatin is highly soluble in water; chitosan is soluble only in an acidic aqueous solution; and PLGA is insoluble in water. Figure 2, a–c, shows optical micrographs of the three different types of uniform microbeads made of gelatin, chitosan, and PLGA.[8] The microbeads had a uniform diameter of 132 ± 6 μm for gelatin, 125 ± 10 μm for chitosan, and 85 ± 7 μm for PLGA. All the microbeads were prepared at different concentrations for the discontinuous phase using the same fluidic device: 10 wt% for gelatin, 2 wt% for chitosan, and 5 wt% for PLGA. The difference in diameter among the samples can be attributed to the different viscosity, density, and interfacial tension, as well as the concentration of the discontinuous phase.[8] Figure 2d shows a schematic diagram for the fabrication of a PCM-based drug release block with a silicone mold. In a typical procedure, the silicone mold was half filled with a melted PCM and then the PCM was cooled down to room temperature for solidification. The uniform microbeads dispersed in a solvent (e.g., methanol for gelatin and chitosan, and water for PLGA) were placed on top of the solidified PCM and then the solvent was allowed to evaporate. More melted PCM was poured to fill the mold and cooled down again to room temperature. In this case, the PCM-based block exhibited the shape of a rectangular rod due to the shape of the mold. It is expected that the shape and dimensions of the PCM-based block can all be easily varied by changing the silicone mold.
Figure 2.
Optical micrographs of a) gelatin, b) chitosan, and c) PLGA microbeads containing FITC-dextran. d) Schematic diagram for fabricating a PCM-based block containing the uniform microbeads loaded with FITC. The scale bars are 100 μm.
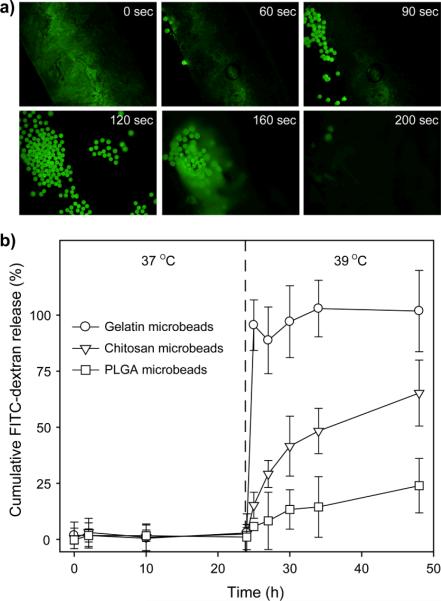
The 1-tetradecanol block containing the gelatin microbeads was attached to the bottom of a Petri dish and a fluorescence micrograph were taken every second as warm (60 °C) water was added to the Petri dish (Movie S1). Figure 3a shows time-lapse fluorescence micrographs of the PCM-based block. As the temperature increased, the PCM block began to melt and some of the gelatin microbeads containing FITC-dextran leached out from the matrix after t=60 s. More gelatin microbeads were observed at 90 s and subsequently dissolved in the warm water at 160 s. The PCM-based block was fully melted after 200 s. The melted PCM floated on the surface of the aqueous medium due to its low density and hydrophobic nature (0.824 g/cm3 at 25 °C). Before leaching out from the PCM-based block, the gelatin microbeads could preserve their own spherical shape without any shape change because gelatin has a glass transition temperature of around 60 °C and an even higher melting temperature.[10] It took less than 5 min for all FITC-dextran molecules to be fully released from the PCM-based block, suggesting that this new system had a faster response to temperature variation than the conventional thermosensitive systems.[11]
Figure 3.
a) Time-lapse fluorescence micrographs showing the release of FITC-dextran from gelatin microbeads encapsulated in a 1-tetradecanol block. The temperature was gradually increased by adding warm water (60 °C) under gentle stirring. b) Release profiles at 37 and 39 °C for FITC-dextran from gelatin, chitosan, and PLGA microbeads encapsulated in the 1-tetradecanol blocks (n = 3). Cumulative FITC-dextran release (%) was defined as the ratio of released amount of FITC-dextran at a given time divided by the theoretical initial amount encapsulated.
Figure 3b shows release profiles of FITC-dextran from the gelatin, chitosan, and PLGA microbeads encapsulated in 1-tetradecanol blocks at 37 and 39 °C. In all cases, FITC-dextran could not be released at 37 °C because the microbeads were fully surrounded by 1-tetradecanol that has a m.p. of 38–39 °C. Our evaluation of the amount of released FITC-dextran at 37 °C for two weeks revealed that there was no significant release of FITC-dextran unlike other conventional systems showing a noticeable amount of released drugs at an initial stage. This feature allows for the precise control over the amount of released drugs. At 39 °C (beyond the m.p. of the 1-tetradecanol), FITC-dextran was instantly released from the gelatin microbeads in a step-function manner. By contrast, FITC-dextran encapsulated in the chitosan microbeads was released in a rather sustained manner than in the gelatin microbeads due to the insolubility of chitosan in water at neutral pH. The PLGA microbeads showed a more sustained release pattern than the chitosan microbeads due to the hydrophobic nature of PLGA. These results confirmed that drug release could be suppressed below the m.p. and initiated just beyond the m.p. of the used PCM. In addition, the release rate could be controlled by the selection of microbead materials, depending on their water-solubility and degradation property. On the other hand, the release patterns of drugs in an “on-off” manner were often demonstrated in the conventional thermosensitive systems. However, our approach based on a PCM is limited to a single release event because the PCM-based constructs cannot restore their original forms once they melt.
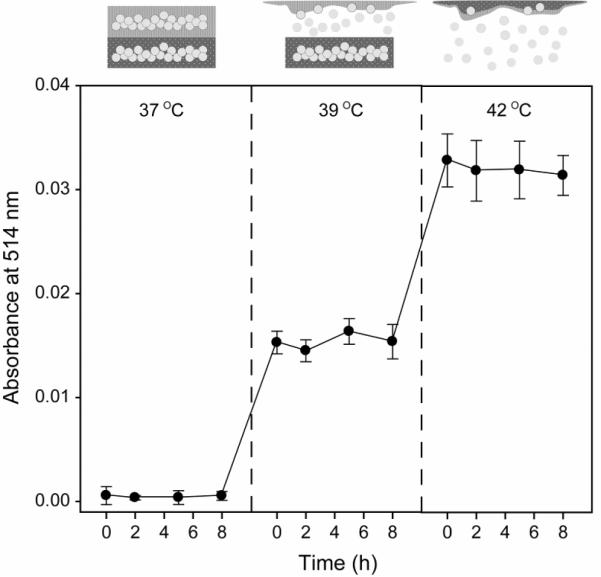
Furthermore, in order to demonstrate a dual temperature-regulated drug release, we incorporated one more PCM, dodecanoic acid (m.p. of 43–46 °C), into the 1-tetradecanol matrix. Both the 1-tetradecanol and dodecanoic acid are generally known to have relatively low toxicities in small doses because they are originally derived from natural fats and oils and thus are often used as ingredients for food and pharmaceuticals.[12] Figure 4 shows the release profile of FITC-dextran from the gelatin microbeads encapsulated in the dual PCM-based matrix at 37, 39, and 44 °C. It was found that FITC-dextran was released from the gelatin microbeads in the 1-tetradecanol matrix at 39 °C and then from the microbeads in the dodecanoic acid matrix at 44 °C. There seems to be little chance of human body temperature to naturally increase beyond 40 °C. However, such a temperature can be intentionally and/or locally induced by exposing the implanted PCM-based device to a warm probe or environment, resulting in onset of drug release.
Figure 4.
Release profiles (n = 3) at 37, 39, and 44 °C for FITC-dextran from gelatin microbeads encapsulated in a dual PCM block fabricated from 1-tetradecanol and dodecanoic acid.
In summary, we have successfully demonstrated a temperature-regulated drug release system based on PCMs. This new system has a number of attractive features: the simplicity in terms of fabrication procedure; no drug release below the m.p. of the PCM; rapid response time to an ambient temperature, precise control over the amount of released drugs, and no need of additional triggering device. In this approach, the initiation temperature and release pattern of drugs can be tailored by selecting a proper combination of PCM and drug-encapsulating material, respectively. Besides FITC-dextran with a high molecular weight, we have also encapsulated FITC (which has a much lower molecular weight then FITC-dextran) and protein conjugates (e.g., FITC-BSA). The encapsulation process should have nothing to do with the molecular weight as long as the drug can be dissolved in a properly selected PCM. The PCM-based drug release system could find use in situations involving elevation of body temperature due to fever, inflammation, and among others. In this work, we have mainly focused on PCM-based devices with relatively large dimensions (>100 μm) that can be implanted into body through surgical operation. Our recent study suggests that the drug-loaded PCM can also be processed as nanoscale particles for easy injection with a syringe.
Experimental Section
Materials
Gelatin (Type A, from porcine skin, Sigma), sorbitan monooleate (Span® 80, Sigma), fluorescein isothiocyanate-dextran (FITC-Dextran, Mw≈20,000), chitosan (low Mw, Aldrich), poly(D, L-lactide-co-glycolide) (PLGA, 75:25, Mw≈66,000–107,000, Sigma), poly(vinyl alcohol) (PVA, Mw≈13,000–23,000, 98% hydrolyzed, Aldrich), dichloromethane (DCM, ≥99.5%, Sigma), and toluene (99.8 %, Sigma-Aldrich) were used for producing colloidal particles and uniform microbeads. 1-tetradecanol (97%, Aldrich) and dodecanoic acid (98%, Aldrich) were employed as the PCMs for fabricating thermosensitive constructs. The water used in all experiments was obtained by filtering through a set of Millipore cartridges (Epure, Dubuque, IA).
Fabrication of uniform PCM-based beads
Gelatin colloidal particles (0.3–2 μm in size) containing FITC-dextran were prepared using a typical emulsification and solvent evaporation method in a homogenizer. Three grams of an aqueous gelatin (3 wt%) solution containing FITC-dextran (0.05 wt%) was emulsified in 50 g of toluene solution containing Span® 80 (3 wt%) using a homogenizer at 23,000 rpm for 3 min and the solvent in the water-in-oil emulsion was allowed to evaporate overnight under stirring. The resultant gelatin colloidal particles were washed with methanol three times. Uniform PCM-based beads containing the gelatin colloidal particles were fabricated using a fluidic device constructed from a poly(vinyl chloride) (PVC) tube (1/16 in. i.d. × 2/16 in. o.d.), a microcapillary tube (0.4 mm i.d.), and a 30 G needle.[8] Dried gelatin particles containing FITC-dextran were dispersed in a melted PCM phase heated to 50 °C using a sonicator and a voltex mixer. The melted PCM phase containing the gelatin particles was introduced into the fluidic device as the discontinuous phase using syringe pumps (KD100, KD Scientific), while an aqueous PVA solution (2 wt%) served as both continuous and collection phases (Figure S2). The syringes for both the phases were heated to 60 °C using heating tapes while the temperature of the collection phase was kept at room temperature.
To fabricate PCM-based thermosensitive microspheres, 1 g of melted 1-tetradecanol containing the gelatin particles was emulsified in 10 g of a warm aqueous PVA solution heated to 60 °C using a homogenizer at 23,000 rpm for 30 s and the resultant oil-in-water emulsion was immediately poured into water at room temperature to solidify the PCM. The presence of the gelatin particle (containing FITC-dextran) in the PCM-based particles was confirmed using a laser scanning confocal microscope (LSCM, Nikon).
Fabrication of PCM-based blocks containing uniform microbeads
Uniform gelatin and chitosan microbeads were produced using the fluidic device based on water-in-oil emulsion. For the uniform gelatin microbeads, an aqueous gelatin solution (10 wt%) with FITC-dextran (0.05 wt%) and a toluene solution containing Span® 80 (3 wt%) were used as the discontinuous and continuous/collection phases, respectively. A syringe filled with the gelatin solution was heated to 45 °C using a heating tape to prevent gelation, and the collection phase was cooled with an ice bath. As for the chitosan microbeads, an aqueous chitosan solution (2 wt%) in 500 mM acetic acid was used as the discontinuous phase without heating. For the PLGA microbeads, the dried gelatin particles prepared by the emulsification method with the homogenizer were dispersed in DCM using sonication and voltex mixing. A PLGA solution (5 wt%) in DCM containing the gelatin particles served as the discontinuous phase and an aqueous PVA solution (2 wt%) served as the continuous/collection phases. After solvent evaporation, the uniform gelatin (or chitosan) microbeads were washed with methanol and the PLGA microbeads were washed with water three times. In all cases, the flow rates for the discontinuous and continuous phases were kept at 0.05 and 1 mL/min, respectively. The average diameter and standard deviation of the microbeads were calculated from optical micrographs by randomly measuring over 100 microbeads for each sample.
A silicone mold (14 × 3.5 × 2.4 mm in thickness, Ladd Research Industries) was used for fabricating the PCM-based block containing the uniform microbeads. The silicone mold was half filled with melted PCM and cooled down to room temperature for 30 min. Each kind of the microbeads of gelatin, chitosan, and PLGA was placed on top of the solidified PCM. After solvent evaporation, more melted PCM was poured into the mold to fill the remaining space and cooled down to room temperature. For a dual PCM-based block, a dodecanoic acid matrix containing the uniform gelatin microbeads was fabricated first and then a 1-tetradecanol matrix was prepared on top of the dodecanoic acid matrix in the same silicone mold.
Release of FITC-dextran from the PCM-based microbeads and blocks
The PCM-based beads (or blocks) were attached to the bottom of a Petri dish using an epoxy adhesive (or carbon tape) and then 10 mL of water (at room temperature) was poured into the Petri dish. To monitor the melting of the PCM and release behavior of FITC-dextran, warm water (60 °C) was added into the Petri dish, fluorescence micrographs were taken every second using a QICAM Fast Cooled Mono 12-bit camera (Q Imaging, Burnaby, BC, Canada) attached to an Olympus microscope with Capture 2.90.1 (Olympus). To obtain release profiles of FITC-dextran, the PCM-based blocks containing each type of the uniform microbeads (gelatin, chitosan, and PLGA) were soaked in 3 mL of PBS hosted in vials. After closing the cap, the vials were placed in a water bath at 37, 39, and 44 °C. One milliliter of solution was sampled from each vial for measuring UV absorbance (Cary 50, Varian) at different time points.
Acknowledgments
This work was supported in part by an NIH Director's Pioneer Award (5DP1 OD000798) and startup funds from Washington University in St. Louis. Part of the work was conducted at the Nano Research Facility (NRF), a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the NSF under award no. ECS-0335765. S.W.C. was also partially supported by a postdoctoral fellowship award from Korea Research Foundation funded by the Korean Government (KRF-2007-357-D00080). S.W.C. wants to thank Prof. Jin-Ho Choi for an idea related to this work.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/.
References
- [1].Qiu Y, Park K. Adv. Drug Deliv. Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- [2].Wu J-Y, Liu S-Q, Heng PW-S, Yang Y-Y. J. Control. Release. 2005;102:361–372. doi: 10.1016/j.jconrel.2004.10.008. [DOI] [PubMed] [Google Scholar]
- [3].(a) Jeong B, Bae YH, Kim SW. J. Control. Release. 2000;63:155–163. doi: 10.1016/s0168-3659(99)00194-7. [DOI] [PubMed] [Google Scholar]; (b) Sosnik A, Cohn D. Biomaterials. 2005;26:349–357. doi: 10.1016/j.biomaterials.2004.02.041. [DOI] [PubMed] [Google Scholar]
- [4].Kang GD, Song S-C. Int. J. Pharm. 2008;349:188–195. doi: 10.1016/j.ijpharm.2007.08.010. [DOI] [PubMed] [Google Scholar]
- [5].Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M. J. Control. Release. 2005;103:609–624. doi: 10.1016/j.jconrel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- [6].(a) Ruel-Gariépy E, Chenite A, Chaput C, Guirguis S, Leroux J-C. Int. J. Pharm. 2000;203:89–98. doi: 10.1016/s0378-5173(00)00428-2. [DOI] [PubMed] [Google Scholar]; (b) Hoare T, Santamaria J, Goya GF, Irusta S, Lin D, Lau S, Padera R, Langer R, Kohane DS. Nano Lett. 2009;9:3651–3657. doi: 10.1021/nl9018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].(a) Ichikawa H, Fukumori Y. J. Control. Release. 2000;63:107–119. doi: 10.1016/s0168-3659(99)00181-9. [DOI] [PubMed] [Google Scholar]; (b) Kikuchi A, Okano T. Adv. Drug Deliv. Rev. 2002;54:53–77. doi: 10.1016/s0169-409x(01)00243-5. [DOI] [PubMed] [Google Scholar]; (c) Alexander C. Nat. Mater. 2008;7:767–768. doi: 10.1038/nmat2281. [DOI] [PubMed] [Google Scholar]; (d) Ehrbar M, Schoenmakers R, Christen EH, Fussenegger M, Weber W. Nat. Mater. 2008;7:800–804. doi: 10.1038/nmat2250. [DOI] [PubMed] [Google Scholar]
- [8].Choi S-W, Cheong IW, Kim J-H, Xia Y. Small. 2009;5:454–459. doi: 10.1002/smll.200801498. [DOI] [PubMed] [Google Scholar]
- [9].Kaygusuz K, Alkan C, Sari A, Uzun O. Energ. Sources Part A. 2008;30:1050–1059. [Google Scholar]
- [10].Dai C-A, Chen Y-F, Liu M-W. J. Appl. Polym. Sci. 2006;99:1795–1801. [Google Scholar]
- [11].Matsuo ES, Tanaka T. J. Chem. Phys. 1988;89:1695–1703. [Google Scholar]
- [12].Kinderlerer JL. Int. Biodeter. Biodegrad. 1994;33:345–354. [Google Scholar]