Abstract
Purpose
We evaluated the associations of statins and serum cholesterol with PSA to understand whether the inverse associations of statins and low cholesterol with aggressive prostate cancer are explained by detection bias.
Methods
We analyzed data from 2,574 men aged ≥40 years without prostate cancer in The National Health and Nutrition Examination Survey 2001–2004. We estimated multivariable-adjusted geometric mean PSA by statin use and cholesterol quintiles. To limit the influence of correlates of statin use and cholesterol on PSA, we stratified by comorbidities.
Results
Statin users had a non-statistically significantly lower PSA than nonusers (0.89 vs 0.95 ng/mL, p=0.22), especially in men without comorbidities (n=1,680; 0.86 vs 0.99 ng/mL p=0.02). In men with comorbidities, statin users had a non-statistically significantly higher PSA than nonusers (0.91 vs 0.83 ng/mL, p=0.14). Men with lower cholesterol had lower PSA (bottom vs top quintile: 0.92, 1.02 ng/mL, p-trend=0.06).
Conclusion
Statin users and men with lower cholesterol may have lower PSA. If so, the probability of detecting asymptomatic prostate cancer might be lower at present, but these cases might be more likely to be diagnosed at an advanced stage in the future. Thus, PSA-associated bias is unlikely to explain the inverse association of statins with advanced prostate cancer.
Keywords: prostatic neoplasms, hydroxymethylglutaryl-CoA reductases, cholesterol, prostate-specific antigen, cross-sectional studies
Introduction
Observational studies suggest that statin drugs (HMG-CoA reductase inhibitors), a class of commonly prescribed drugs used to lower cholesterol, may protect against advanced and possibly high-grade prostate cancer [1–6]. The mechanism by which statins may influence prostate cancer is unknown, but may involve their cholesterol-lowering properties or their influence on other pathways [7, 8]. Indeed, studies of the association between serum cholesterol and prostate cancer risk have found that men with low cholesterol were less likely to develop high-grade prostate cancer [9–11].
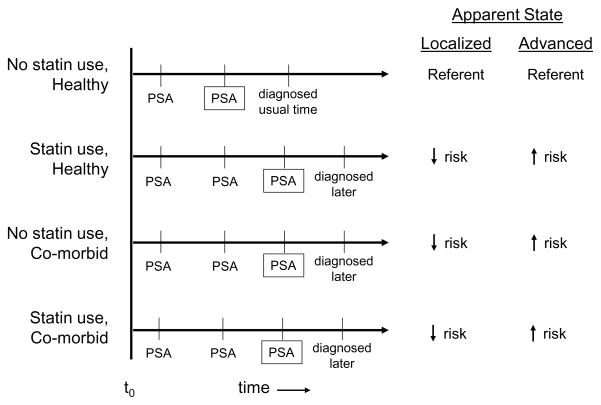
Few studies have investigated the influence of statin drugs or serum cholesterol on PSA concentration in men free of a prostate cancer diagnosis. Because PSA is widely used as a screening test for prostate cancer, an effect of statins or cholesterol on PSA level independent of any biological influence on prostate cancer could create a detection bias; that is, a different accuracy of detection of asymptomatic prostate cancer between statin users and nonusers or between men with lower and higher serum cholesterol (Figure 1). For such a bias to make statin drugs appear to be protective for advanced prostate cancer, as has been observed in the literature [1–6], use of a statin would have to cause or otherwise be associated with an increase in PSA concentration. If this were true, then statin users would reach the threshold for biopsy referral earlier in the natural history of prostate cancer than non-users. Thus, statin users with asymptomatic prostate cancer could have their tumors detected at an earlier stage, which might make statin use appear to be protective for advanced prostate cancer and appear to increase the risk of localized disease, as has been observed in some studies [4–6] (Figure 1). On the other hand, if statin use caused or were otherwise associated with a decrease in PSA concentration and follow-up were short, statin drug users with asymptomatic prostate cancer might be less likely to have their cancer detected than non-users making statin use appear to be protective for prostate cancer at any stage of diagnosis (Figure 1). A similar detection bias could also be present among men with low serum cholesterol independent of their use of cholesterol-lowering drugs.
Figure 1.
This figure illustrates the asociations that would be observed between statin use and localized and advanced stage prostate cancer if statin use either increased or decreased PSA concentration.
t0 = Development of asymptomatic tumor at same stage and grade for all scenarios.
PSA = PSA screening test
 = PSA screening test when PSA concentration reached the level for referral for biopsy
= PSA screening test when PSA concentration reached the level for referral for biopsy
At this time the mechanisms by which statin drugs or cholesterol level might influence PSA concentration are not known. Nevertheless, two observational studies [12, 13] observed a small decrease in PSA level after starting on a statin; only one was statistically significant [13]. In that latter study population, serum total and LDL cholesterol cross-sectionally were positively associated with PSA concentration before starting a statin [13], and declines in total and LDL cholesterol comparing after starting on a statin to before were associated with a decrease in PSA concentration [13]. One cross-sectional study found no association between circulating cholesterol and PSA concentrations [14]. One randomized controlled trial of statins and benign prostatic hyperplasia (BPH) in which PSA level was measured as a BPH-associated outcome, found no influence of statin drugs on PSA [15]. Thus, the small number of prior studies suggests that statin drug use and cholesterol concentration either have no influence or may cause a small decrease in circulating PSA concentration.
We hypothesized that statin use or low serum cholesterol concentration may influence PSA concentration in men without a diagnosis of prostate cancer in the general population. The presence of such an association could create a detection bias in observational studies of these exposures and prostate cancer. To test this hypothesis, we conducted an analysis in the National Health and Nutrition Examination Survey (NHANES) 2001–2004, a large, cross-sectional study that is representative of the United States population.
Materials and Methods
Study Population
NHANES is a cross-sectional study undertaken by the National Center for Health Statistics. The study uses a stratified multistage probability design to represent the total United States civilian, noninstitutionalized population over 2 months of age. To make more precise estimates in certain subgroups of the population, Mexican-Americans, non-Hispanic blacks, and the elderly are over-sampled.
Our analysis included data from male participants in the 2001–2002 and 2003–2004 NHANES cycles. Serum PSA concentration was measured in men aged ≥40 years who had never been diagnosed with prostate cancer and who had not recently had a prostate biopsy, examination, or infection (n=2,574).
PSA and Cholesterol Measurements
Serum total PSA concentration was measured as a component of NHANES using the Hybritech method on the Beckman Access (Fullerton, CA). Serum total cholesterol concentration was measured enzymatically. Serum HDL cholesterol concentration was measured by a heparin-Mn precipitation method or, when sample volume was small, direct immunoassay. In the fasting subsample, triglycerides concentration was measured enzymatically; total cholesterol, HDL cholesterol, and triglycerides were used to estimate LDL cholesterol concentration using the Friedewald formula. The details of NHANES laboratory and quality control methods are reported elsewhere [16, 17].
Assessment of Statin Use
Information on statin use was collected in two ways: 1) Interviewers asked participants whether they were currently taking prescribed medication to lower their blood cholesterol. Participants were not asked which specific medication they were taking during this segment of the interview. However, based on the information described in part 2 below, 91% of the cholesterol-lowering drug users took a statin; and 2) During a different segment of the interview, participants were asked whether they had taken any prescription medications in the previous 30 days. If they answered “yes”, the interviewer asked to see their prescription bottles and recorded the medications they were taking. Participants were also asked how long they had been taking each medication. Prescriptions categorized as statin drugs were those with standard generic ingredient codes corresponding to the following medications: atorvastatin calcium, cerivastatin sodium, fluvastatin sodium, lovastatin, pravastatin sodium, and simvastatin. Prescriptions were categorized as other cholesterol-lowering medications if the standard generic ingredient name was one of the following: cholestyramine, colestipol hydrochloride, ezetimibe, gemfibrozil, niacin, or cholesterol lowering drug-unspecified. We analyzed information on medication use from both parts of the interview and we considered statin drugs separately from other cholesterol-lowering medications when using information recorded from prescription labels.
Statistical Analysis
All analyses were conducted using SUDAAN v 9.0 software (Research Triangle Park, NC) as implemented in SAS v 9.2 (Cary, NC), to account for the unequal probabilities of selection, over-sampling, and non-response that were part of the complex NHANES sampling design [18]. We estimated age (continuous) and multivariable-adjusted geometric mean PSA concentrations and 95% confidence intervals by statin and cholesterol-lowering drug use and for each quintile of serum total cholesterol concentration using linear regression. We repeated these analyses for HDL cholesterol and, in the fasting subsample (n=1,101), LDL cholesterol. We transformed PSA concentration using the natural logarithm because it was not normally distributed. We also estimated geometric mean PSA concentration by deciles and clinical cutpoints of total cholesterol to determine whether modeling according to quintile cutpoints accurately captured the shape of the association between total cholesterol and PSA concentration. The inferences were similar using each of the three sets of cutpoints, so we report the results by quintiles of total cholesterol.
We included in the multivariable models factors that were known or that we hypothesized would be associated with both PSA concentration and either statin use or cholesterol concentration: race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, other Hispanic, other), measured body mass index (cutpoints based on World Health Organization categories [19] with finer categorization: <22.5, 22.5–<25, 25–<27.5, 27,5–<30, 30–<32.5, 32.5–<35, and ≥35 kg/m2), and cigarette smoking (never, current, former). Information on cigarette smoking was collected by interview. Further adjustment for serum cotinine, serum C-reactive protein concentration, waist circumference, skinfold measurements, education level, poverty index ratio, dietary intake of total fat, protein, and carbohydrate, serum cholesterol concentration (in analyses of statin use), and aspirin use (2001–2002 only) did not change these results (no point estimate changed by >0.01); thus these variables were not included in the final model.
Subanalyses were conducted to limit the possible confounding influence of health status by stratifying by presence or absence of major comorbidities (cancer, myocardial infarction, stroke, angina, congestive heart failure, coronary heart disease, diabetes); information on comorbidities was self-reported during the interview. Within strata of health status we further adjusted for liver and kidney function, as defined using methods previously described [20, 21], because we hypothesized that impaired function could influence PSA clearance. We also conducted analyses stratified by age (40–<50, 50–<60, 60–<70, 70–<80, ≥80 years) and BMI (<25, 25–<30, ≥30 kg/m2). Further, we conducted analyses excluding men who were taking finasteride (n=40). To be able to efficiently control for confounding and account for possible interactions among the confounders, we generated a propensity score [22] using the following: age, race/ethnicity, BMI, waist circumference, cigarette smoking status, alcohol consumption, hypertension, diabetes, kidney function, and liver function. We then adjusted for quintiles of the propensity score using indicator variables. Statistical interaction was assessed by including interaction terms between the potential effect modifiers as categorized above and statin use or quintiles of cholesterol concentration in the multivariable model and estimating its statistical significance using the Wald test.
All protocols for the implementation of NHANES 2001–2004 were approved by the Institutional Review Board of the National Center for Health Statistics, Centers for Disease Control and Prevention; informed consent was obtained for all participants.
Results
Cholesterol-lowering Drugs, including Statins, and PSA
The weighted prevalence of current cholesterol-lowering drug use in men ≥40 was 18.8% ± 0.97%. Characteristics of the study population by current use of cholesterol-lowering drugs are shown in Table 1. Men who reported current use of these drugs tended to be older (Table 1). After adjustment for age, men using cholesterol-lowering drugs were more likely to be non-Hispanic white and had a slightly higher BMI. Cigarette smoking prevalence also varied by use of cholesterol-lowering drugs (Table 1).
Table 1.
Age-adjusteda weighted characteristics of men 40 years and older by cholesterol-lowering drug use, NHANES 2001 – 2004
| Cholesterol-Lowering Drug Useb | ||
|---|---|---|
| No | Yes | |
| N | 2,076 (81.2%) | 498 (18.8%) |
| Age (years) | ||
| mean (se) | 53.4 (0.3) | 60.0 (0.5) |
| Race % (se) | ||
| Non-Hispanic white | 79.5 (2.2) | 84.9 (2.4) |
| Non-Hispanic black | 8.9 (1.0) | 5.8 (1.0) |
| Mexican-American | 5.2 (1.1) | 2.5 (0.6) |
| Other Hispanic | 3.9 (1.2) | 4.3 (1.8) |
| Other | 2.6 (0.6) | 2.5 (0.7) |
| Body Mass Index (kg/m2) | ||
| mean (se) | 28.3 (0.1) | 29.8 (0.5) |
| Cigarette Smoking % (se) | ||
| never | 37.5 (1.6) | 37.8 (2.5) |
| former | 38.8 (1.2) | 43.6 (2.2) |
| current | 23.6 (1.1) | 18.6 (1.8) |
Standardized to 2000 Census US age distribution
91% of users took a statin
After multivariable adjustment, men who reported currently using cholesterol-lowering drugs had a slightly lower PSA concentration compared with nonusers, although the difference was not statistically significant (Table 2). Multivariable-adjusted results were similar to those obtained when adjusting for age alone (Table 2). No statistically significant interactions were observed between cholesterol-lowering drug use and age (p-interaction=0.50) or BMI (p-interaction=0.53). The results were unchanged when men using finasteride, a drug that is known to lower PSA, were excluded (n=40), or when stratified by time of day of blood collection (data not shown).
Table 2.
Serum PSA concentration in men 40 years and older by cholesterol-lowering drug use, NHANES 2001 – 2004
| N | Geometric Mean PSA concentration ng/mL (95% CI) |
||
|---|---|---|---|
| Age Adjusted* | Multivariable Adjusted† | ||
| Current Use of Cholesterol-lowering Drugs§ | |||
| No | 2,076 | 0.96 (0.92 – 1.00) | 0.95 (0.91 – 0.99) |
| Yes | 498 | 0.88 (0.81 – 0.95) | 0.90 (0.83 – 0.97) |
| p-value‡ | 0.09 | 0.22 | |
| Cholesterol-lowering Drugs in the Past Month** | |||
| No | 2,044 | 0.95 (0.92 – 0.99) | 0.95 (0.91 – 0.99) |
| Yes | 530 | 0.89 (0.83 – 0.95) | 0.91 (0.86 – 0.97) |
| p-value‡ | 0.11 | 0.31 | |
| <1 year | 133 | 0.87 (0.76 – 0.99) | 0.88 (0.77 – 1.00) |
| 1 – <5 years | 260 | 0.99 (0.86 – 1.13) | 1.01 (0.88 – 1.16) |
| ≥ 5 years | 127 | 0.74 (0.65 – 0.85) | 0.77 (0.67 – 0.88) |
| p trend‡ | 0.004 | 0.02 | |
| Statins in the Past Month** | |||
| No | 2,091 | 0.95 (0.92 – 0.99) | 0.95 (0.91 – 0.99) |
| Yes | 483 | 0.88 (0.82 – 0.94) | 0.90 (0.85 – 0.96) |
| p-value‡ | 0.06 | 0.20 | |
| <1 year | 117 | 0.87 (0.76 – 0.99) | 0.88 (0.78 – 1.01) |
| 1 – <5 years | 236 | 0.97 (0.84 – 1.12) | 0.99 (0.86 – 1.14) |
| ≥ 5 years | 122 | 0.74 (0.65 – 0.86) | 0.77 (0.61 – 0.89) |
| p trend‡ | 0.003 | 0.02 | |
Adjusting for age in years (continuous)
Adjusting for age in years (continuous), race/ethnicity, body mass index, and cigarette smoking status
p-values calculated modeling the natural logarithm transformed PSA
Participants were asked if they were currently taking medications to lower cholesterol; 91% of the users took a statin.
Participants were asked whether they had taken any prescription medications in the previous 30 days. Interviewers recorded type of medication as well as how long participants had been taking each medication.
When the more detailed information on medication use during the past 30 days was used, the inferences were similar for both total cholesterol-lowering drug use and statin drug use (Table 2). When information from both questions was combined to create more sensitive (reported cholesterol-lowering drug use on either question) or more specific (reported cholesterol-lowering drug use on both questions) definitions, our results were unchanged (data not shown). For both total cholesterol-lowering drug use (p-trend=0.02) and statin drug use (p-trend=0.02), there was a significant decrease in serum PSA concentration with increasing duration of use (Table 2).
Serum Cholesterol and PSA
After multivariable adjustment, PSA concentration appeared to increase slightly across quintiles of serum cholesterol concentration, although a U-shaped association could not be ruled out (Table 3). Further adjustment for cholesterol-lowering drug use did not influence these results, nor did adjustment for serum high sensitivity C-reactive protein concentration, waist circumference, skinfold measurements, education level, poverty index ratio, or aspirin use (2001–2002 only) (data not shown). The results were similar and were statistically significant (p-trend=0.02) when men using cholesterol-lowering drugs were excluded from the analysis (Table 3). No trend was observed for serum cholesterol among men who were using cholesterol-lowering drugs (n=617; p-trend=0.65) (data not shown). No statistically significant interactions were observed between serum cholesterol and age (p-interaction=0.22) or BMI (p-interaction=0.19). The results were unchanged when stratified by time of day of blood collection or by fasting status (data not shown).
Table 3.
Serum PSA concentration in men 40 years and older by quintiles of serum cholesterol, NHANES 2001 – 2004
| Geometric Mean PSA in ng/mL (95% CI) | ||||
|---|---|---|---|---|
| N | Age Adjusteda | Multivariable Adjusted b | Multivariable Adjusted and Excluding Cholesterol-lowering Drug Users c | |
| Quintiles of Serum Cholesterol (mg/dL) | ||||
| <169 | 509 | 0.92 (0.85 – 1.00) | 0.92 (0.85 – 1.00) | 0.86 (0.76 – 0.98) |
| 169 – <191 | 506 | 0.96 (0.88 – 1.04) | 0.95 (0.88 – 1.04) | 0.92 (0.84 – 1.01) |
| 191 – <210 | 506 | 0.84 (0.76 – 0.94) | 0.83 (0.75 – 0.92) | 0.83 (0.74 – 0.93) |
| 210 – <234 | 523 | 0.98 (0.91 – 1.06) | 0.98 (0.91 – 1.06) | 0.95 (0.87 – 1.04) |
| ≥ 234 | 530 | 1.01 (0.94 – 1.08) | 1.02 (0.95 – 1.09) | 1.01 (0.94 – 1.08) |
| p trendd | 0.10 | 0.06 | 0.02 | |
Adjusted for age in years (continuous)
Adjusted for age in years (continuous), race/ethnicity, body mass index, and smoking status
Adjusted for age in years (continuous), race/ethnicity, body mass index, smoking status, excluding cholesterol-lowering drug users (sensitive definition, N=617)
P-values calculated modeling the natural logarithm transformed PSA
HDL cholesterol was not associated with PSA (within quintiles of HDL: 0.91, 1.04, 0.92, 0.82, 0.96 ng/mL, p-trend=0.98). In men who were fasting, the shape of the association for LDL cholesterol and PSA (within quintiles of LDL: 0.84, 0.93, 0.87, 0.94, 1.04 ng/mL, p-trend=0.04) was similar to total cholesterol.
Stratification by Comorbidity Status
When the analysis was limited to the 1,680 men without major comorbidities, the inverse association between current use of cholesterol-lowering drugs and PSA concentration was more pronounced and was statistically significant (Table 4). In contrast, among men with major comorbidities, cholesterol-lowering drug users had a non-significantly higher PSA concentration than nonusers (Table 4); adjustment for each individual comorbid condition did not change these results (data not shown). It did not appear that any one comorbidity was driving the result in men with comorbidities; stratification by each comorbid condition separately yielded similar results (data not shown). We hypothesized that even within strata of comorbidities, differences could exist between men taking and not taking a statin on kidney and liver function, which could affect PSA clearance; after further adjusting for kidney and liver function the results were unchanged (data not shown). Further adjustment for serum high sensitivity C-reactive protein concentration, waist circumference, skinfold measurements, education level, poverty index ratio, aspirin use (2001–2002 only), or alcohol intake also did not change these results (data not shown). Finally, adjustment for all of these factors using propensity score analysis yielded the same results (data not shown). Unlike statin drug use, there was no statistically significant interaction between serum total cholesterol and health status (p-interaction=0.63).
Table 4.
Serum PSA concentration (geometric mean and 95% confidence intervals) in men 40 years and older by cholesterol-lowering drug use and stratified by health status, NHANES 2001 – 2004
| No Co-morbiditiesc | Co-morbiditiesc | p interactionb | |||
|---|---|---|---|---|---|
| N | Multivariable Adjusteda | N | Multivariable Adjusteda | ||
| Current Use of Cholesterol-lowering Drugsd | |||||
| No | 1,488 | 0.99 (0.95 – 1.04) | 588 | 0.83 (0.75 – 0.91) | 0.003 |
| Yes | 192 | 0.86 (0.77 – 0.96) | 306 | 0.91 (0.82 – 1.01) | |
| p-valueb | 0.02 | 0.14 | |||
| Cholesterol-lowering Drug Use in the Past Monthe | |||||
| No | 1,503 | 0.99 (0.94 – 1.04) | 541 | 0.83 (0.76 – 0.90) | 0.14 |
| Yes | 177 | 0.91 (0.80 – 1.02) | 353 | 0.90 (0.80 – 1.01) | |
| p-value‡ | 0.19 | 0.25 | |||
| <1 year | 49 | 0.83 (0.71 – 0.98) | 84 | 0.91 (0.72 – 1.17) | 0.09 |
| 1 – <5 years | 93 | 0.99 (0.82 – 1.19) | 167 | 1.01 (0.83 – 1.23) | |
| ≥ 5 years | 31 | 0.80 (0.52 – 1.21) | 96 | 0.73 (0.65 – 0.83) | |
| p trendb | 0.32 | 0.32 | |||
| Statin Drug Use in the Past Monthe | |||||
| No | 1,518 | 0.99 (0.94 – 1.04) | 573 | 0.84 (0.77 – 0.91) | 0.16 |
| Yes | 162 | 0.90 (0.80 – 1.01) | 321 | 0.89 (0.79 – 1.01) | |
| p-value‡ | 0.14 | 0.41 | |||
| <1 year | 44 | 0.83 (0.70 – 1.00) | 73 | 0.92 (0.71 – 1.18) | 0.16 |
| 1 – <5 years | 86 | 0.96 (0.80 – 1.15) | 150 | 1.00 (0.80 – 1.24) | |
| ≥ 5 years | 29 | 0.82 (0.54 – 1.25) | 93 | 0.72 (0.64 – 0.81) | |
| p trendb | 0.34 | 0.26 | |||
Adjusted for age in years (continuous), race/ethnicity, body mass index, and cigarette smoking status
P-values calculated modeling the natural logarithm transformed PSA
Men with co-morbidities have one or more of the following: cancer, myocardial infarction, stroke, angina, coronary heart disease, congestive heart failure, or diabetes.
Participants were asked if they were currently taking medications to lower cholesterol; 91% of users took a statin.
Participants were asked whether they had taken any prescription medications in the previous 30 days. Interviewers recorded type of medication as well as how long participants had been taking each medication.
Discussion
This is the first report of the association between statin drug use and PSA concentration in men without a prostate cancer diagnosis in a nationally representative sample of US men. We observed that men ≥40 years old who were taking statin drugs had a slightly lower PSA concentration compared with men who were not taking a statin, especially in longer-term (≥5 years) users of statin drugs and in men without major comorbid conditions. Compatible with the statin findings, we also observed that men with a lower compared with higher serum cholesterol concentration had a lower PSA concentration, although we cannot rule out a U-shaped association. The findings for LDL cholesterol and PSA were similar to those for total cholesterol, whereas HDL cholesterol was not associated with PSA concentration.
Our findings on statin drug use and PSA concentration are consistent with those from two previous longitudinal observational studies on this topic [12, 13], which found a small decrease in PSA after statin use. One of these included only 15 statin drug users [12]. The other study, which included 1,545 men, observed a close correlation between decline in serum total and LDL cholesterol concentrations after starting on a statin and decline in PSA concentration; for every 10% decrease in LDL, PSA concentration declined by 1.5% (p=0.001) [13].
Detection bias due to the possible association between use of a statin and PSA concentration as an explanation for the finding of inverse association between statin drug use and advanced prostate cancer in several recent studies [1–6] requires consideration. If, as we observed, statins are associated with lower PSA, then the probability of detecting asymptomatic prostate cancer might be lower among statin users, and if a tumor were present then, in the future, users might be more likely to be diagnosed at a higher stage than nonusers (Figure 2). Thus, our data suggest that detection bias due to statin users having a lower PSA concentration does not explain the inverse association between statin use and advanced prostate cancer that has been reported in the literature.
Figure 2.
In this scenario, we assume that statin drug use has no influence on the development of advanced prostate cancer. Note that if comorbid statin users were compared to comorbid men who do not use a statin (as in Table 3), the apparent state might be different.
t0 = Development of asymptomatic tumor at same stage and grade for all scenarios.
PSA = PSA screening test
 = PSA screening test when PSA concentration reached the level for referral for biopsy
= PSA screening test when PSA concentration reached the level for referral for biopsy
We observed that the inverse association between statin use and PSA concentration was stronger and statistically significant when the potential influence of comorbid conditions was removed. One possible explanation for this finding is that men who have major comorbidities who are taking a statin may be the least healthy men in the study population and some aspect of their poor health status may be contributing to their higher serum PSA. Therefore, it is only among the healthier men that a direct influence of statin drugs on serum PSA concentration can be observed. Although in our analysis stratified by comorbid status we used several methods to control for many potential confounding factors, we cannot rule out the potential influence of residual confounding on these results.
We observed that men who had lower serum cholesterol also had lower circulating PSA concentration, especially when we restricted to men who were not taking cholesterol-lowering drugs. This indirectly suggests that one mechanism by which statins may influence PSA concentration is through cholesterol-lowering, although other mechanisms are possible. However, the fact that even after controlling for cholesterol level, statin users tended to have a lower PSA, suggest there may be non-cholesterol mediated mechanisms as well. This is consistent with the prior observational study, which observed associations between statin drug use and PSA after adjustment for change in cholesterol, but observed the strongest association among men who experienced the largest decline in LDL cholesterol with statin use; these results suggested both cholesterol and non-cholesterol mechanisms through which statins lower PSA levels [13]. Our findings are different from those from one previous study, which reported no correlation between circulating cholesterol and PSA concentrations in a group of older male athletes [14]. However, this result was not adjusted for any potentially confounding factors such as age [14].
Although these results support that detection bias due to an association between use of a statin and PSA concentration is unlikely to account for the results of observational studies of statins and advanced prostate cancer risk, the inverse association between statin use and PSA concentration may have implications for prostate cancer screening. For example, if statins were to preferentially decrease PSA concentration in men without prostate cancer, then the specificity of the PSA test for prostate cancer screening might be improved in men taking a statin. However, if statins were to decrease PSA production by a prostate tumor or alter the ability of tumor-derived PSA to enter circulation, then it might be necessary to adjust the PSA threshold for biopsy referral. Other explanations for our observed associations would have different implications for PSA screening. Thus, further study is needed to determine the mechanism by which statins may influence PSA concentration and the influence this may have on prostate cancer screening.
Our large sample size and nationally representative data are strengths of this study. We were able to perform adjusted and restricted analyses to take into account many potential confounding factors, and examine potential interactions. However, because NHANES is a cross-sectional study, we were unable to longitudinally examine change in PSA concentration before and after statin use in the same man.
In conclusion, in this nationally representative sample statin users and men with lower cholesterol had lower PSA. If true, the probability of detecting asymptomatic prostate cancer would be lower at present, but these cases would be more likely to be diagnosed at an advanced stage in the future. Thus, PSA-associated bias is unlikely to explain the inverse association of statins with advanced prostate cancer.
Acknowledgments
Financial Support: Dr. Mondul was supported by a National Research Service Award (T32 CA009314) from the National Cancer Institute, National Institutes of Health. Dr. Selvin was supported by a grant (K01 DK076595) from the National Institutes of Digestive, Diabetes, and Kidney Diseases, National Institutes of Health.
Footnotes
Other Notes: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Shannon J, Tewoderos S, Garzotto M, et al. Statins and prostate cancer risk: a case-control study. American Journal of Epidemiology. 162(4):318–25. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- 2.Platz EA, Leitzmann MF, Visvanathan K, et al. Statin Drugs and Risk of Advanced Prostate Cancer. Journal of the National Cancer Institute. 98(24):1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 3.Flick ED, Habel LA, Chan KA, et al. Statin use and risk of prostate cancer in the California Men's Health Study cohort. Cancer epidemiology, biomarkers & prevention. 16(11):2218–25. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs EJ, Rodriguez C, Bain EB, et al. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer epidemiology, biomarkers & prevention. 16(11):2213–7. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 5.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer epidemiology, biomarkers & prevention. 16(11):2226–32. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 6.Friedman GD, Flick ED, Udaltsova N, et al. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361 859 recipients. Pharmacoepidemiology and drug safety. 17(1):27–36. doi: 10.1002/pds.1507. [DOI] [PubMed] [Google Scholar]
- 7.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 5(12):930–42. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 8.Solomon KR, Freeman MR. Do the cholesterol-lowering properties of statins affect cancer risk? Trends in endocrinology and metabolism. 19(4):113–21. doi: 10.1016/j.tem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. International journal of cancer. 123(7):1693–8. doi: 10.1002/ijc.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platz EA, Till C, Goodman PJ, et al. Men with low serum cholesterol have a lower risk of high grade prostate cancer in the Prostate Cancer Prevention Trial. Cancer epidemiology, biomarkers & prevention. doi: 10.1158/1055-9965.EPI-09-0472. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association of Plasma Total Cholesterol Concentration with Incident Prostate Cancer in the CLUE II Cohort. Cancer causes and control. 2009 Oct 6; doi: 10.1007/s10552-009-9434-8. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cyrus-David MS, Weinberg A, Thompson T, Kadmon D. The effect of statins on serum prostate specific antigen levels in a cohort of airline pilots: a preliminary report. The Journal of urology. 2005:1923–5. doi: 10.1097/01.ju.0000158044.94188.88. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 100(21):1511–8. doi: 10.1093/jnci/djn362. [DOI] [PubMed] [Google Scholar]
- 14.Merrill RM, Perego UA, Heiner SW. Age, lifestyle, health risk indicators, and prostate-specific antigen scores in men participating in the world senior games. Urologic oncology. 7(3):105–9. doi: 10.1016/s1078-1439(01)00176-4. [DOI] [PubMed] [Google Scholar]
- 15.Mills IA, Crossland A, Patel A, Ramonas H. Atorvastatin Treatment for Men with Lower Urinary Tract Symptoms and Benign Prostatic Enlargement. European urology. 52:503–9. doi: 10.1016/j.eururo.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Report. Hyattsville, MD: National Institutes of Health; 2008. Total Prostate-Specific Antigen in Serum NHANES 2003–2004. [Google Scholar]
- 17.Report. Hyattsville, MD: National Institutes of Health; 2008. Total Cholesterol, Direct HDL, Precipitated HDL, Triglycerides, and LDL NHANES 2003–2004. [Google Scholar]
- 18.Analytic and Reporting Guidelines: The National Health and Nutrition Examination Survey (NHANES) Hyattsville, MD: National Center for Health Statistics; 2006. [Google Scholar]
- 19.Consultation WHOE. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva, Switzerland: 2000. [PubMed] [Google Scholar]
- 20.Lazo M, Selvin E, Clark JM. Brief Communication: clinical implications of short-term variablilty in liver function test results. Annals of Internal Medicine. 148(5):348–52. doi: 10.7326/0003-4819-148-5-200803040-00005. [DOI] [PubMed] [Google Scholar]
- 21.Yi S, Selvin E, Rohrmann S, et al. Endogenous sex steroid hormones and measures of chronic kidney disease in a nationally representative sample of men. Clinical Endocrinology. 71(2):246–52. doi: 10.1111/j.1365-2265.2008.03455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. Journal of the American Statistical Association. 79(387):516–24. [Google Scholar]