Abstract
BBZDR/Wor rat is a new model of type II diabetes with spontaneous obesity and clinical characteristics close to human diabetes. In this study the time-course of cerebroarterial dysfunction was characterized. Posterior cerebral arteries from BBZDR/Wor rats and their age-matched lean controls were pressurized to 70mmHg in an arteriograph. Effects of intraluminal pressure and different pharmacological agents on myogenic tone were evaluated. Pressure-myogenic tone curves in diabetic arteries were similar to that in non-diabetic arteries at pre-diabetic age, showed leftward shift at 4 weeks and were significantly different with higher myogenic tone at 5 and 8 months of diabetes. Age-dependent decrease in myogenic tone was observed in non-diabetic arteries. Dilation to histamine was similar to that in non-diabetic arteries at pre-diabetic and at 4 weeks but significantly reduced at 5 and 8 months of diabetes. Bradykinin-mediated dilation was significantly reduced in early and chronic diabetes, whereas (±)-S-nitroso-N-acetylpenicillamine (SNAP)-mediated dilation was decreased modestly at 8 months of diabetes. Sensitivity and constriction to 5-hydroxytryptamine were increased in early and chronic diabetes. Responses to bradykinin and 5-hydroxytryptamine were decreased and increased, respectively. Myogenic tone was significantly less sensitive to (lower pIC50) U-73122 than normal arteries at 4 weeks and 8 months of diabetes suggesting an increased activation of phospholipase C (PLC). This study shows that pressure-mediated autoregulation of cerebral arteries in type II diabetes operates at higher resistance. Endothelium-dependent dilation was decreased with chronic diabetes with increased sensitivity to constrictor agonist. Endothelium-independent dilation was modestly affected. Arterial hyper-reactivity to pressure and constrictor agonist were likely due to increased PLC activation.
Keywords: BBZDR/Wor rats, type II diabetes, cerebral arteries, myogenic tone
1. Introduction
Non-insulin dependent diabetes mellitus is characterized by insulin resistance, altered glucose and fatty acid metabolism and moderate hypertension (Ganne et al., 2007; Kashyap and Defronzo, 2007). In many cases, it follows clinically apparent obesity (McGillis Bindler, 2007). Diabetes is known to be associated with macro and microvascular disease resulting in life threatening cardiovascular events such as myocardial ischaemia and stroke (Chyun and Young, 2006). Mechanisms underlying diabetic vascular damage in clinical setting are heterogeneous and multifactorial, and recent work by Brownlee (Brownlee, 2001) showed a unifying hypothesis for cellular damage by hyperglycaemia.
Microvasculature determines and regulates blood flow to organs by the virtue of myogenic tone, constriction in response to intraluminal pressure, and therefore ensures smooth blood flow to organs despite changes in systemic haemodynamics (Johnson, 1986). Arteriolar hypo- and hyper-responsiveness to endogenous vasodilators and constrictors have been reported in conduit and resistance arteries in diabetic models but very few studies have been focused on myogenic tone (Pieper, 1998). Increased myogenic tone was observed in cerebral arteries from streptozotocin-induced type I diabetic rats (Zimmermann et al., 1997), in mesenteric arteries of db/db mice (Lagaud et al., 2001) and in skeletal muscle arterioles of obese Zucker rats (Frisbee et al., 2002) and streptozotocin-induced type I diabetic rats (Ungvari et al., 1999).
Microvasculature in type II diabetes was not extensively studied, probably due to lack of availability of appropriate animal models. BBZDR/Wor rat is a new animal model of type II diabetes (Tirabassi et al., 2004), produced by crossing the Zucker fatty rat and non-diabetic BB/Wor rat (also known as BBDR/Wor rat, which serves as control for BBDP/Wor type I diabetic rat (Mordes et al., 2004)). Lean non-diabetic heterozygous littermates serve as control for the obese diabetic BBZDR/Wor rats.
Clinical characteristics of diabetic syndrome in BBZDR/Wor rats include hyperglycaemia, hyperinsulinaemia, hyperlipidemia and they tend to develop moderate hypertension, polyneuropathy, retinopathy and erectile dysfunction (Guberski et al., 1993; Vernet et al., 1995; Sima et al., 2000; Tirabassi et al., 2004) and these characteristics make this model more closer to the clinical diabetes in human than any other existing models. Macro and microvascular function in this model are not yet studied, therefore the use of this model to study microvascular complications needs to be validated. In the present study we characterized cerebroarterial dysfunction in early and chronic diabetic stages in BBZDR/Wor rat. Pressure-dependent autoregulation of myogenic tone and reactivity to contractile and dilatory agonists were evaluated. At the same time age-dependent changes in these arterial properties were evaluated.
2. Methods
Animal procedures have been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of University of Florida. Male BBZDR/Wor and their age-matched lean non-diabetic rats were obtained from Biomedical Research Models Inc. (Worcester, MA). Rats obtained were of pre-diabetic age (<10 weeks) and 4 weeks, 5 months and 8 months after the onset of diabetes (ages of the three diabetic groups were 14 weeks, 7 and 11 months, respectively). Blood was collected for the analysis of glucose and insulin from left ventricle after anesthetizing rats as described below. Blood glucose was analysed by One Touch Ultra glucometer (detection limits 20–600 mg/dL, Lifescan, Johnson & Johnson, Miltipas, CA, USA). Plasma was obtained by collecting the blood in vacutainer tubes (BD, NJ, USA) and plasma insulin was measured by radioimmunoassay as described earlier (detection limits 0.1–100 ng/L) (Guberski et al., 1993). Collection of blood samples and sacrificing rats were always taken place between 9–10 am.
2.1. Preparation of cerebral arteries, measurement of arterial diameter
Rats were anesthetized by an intraperitoneal injection of pentobarbital sodium (60 mg/kg) and killed by decapitation. The brain was removed and placed in an ice-cold oxygenated physiological saline solution (PSS, see below for composition). Posterior cerebral arteries were quickly isolated and dissected free of connective tissue. Secondary or tertiary branches were isolated and transferred to an arteriograph (Danish MyoTechnology (DMT), Aarhus, Denmark) filled with ice-cold PSS, cannulated with glass pipettes and secured with nylon thread as described previously (Jarajapu and Knot, 2002). The arteriograph was then placed on the stage of an inverted microscope, visualized with a monochrome CCD camera for the continuous measurement of arterial diameter and the data was acquired by Myoview software (DMT). The arteries were slowly pressurized to 70 mm Hg under no flow conditions using pressure myograph system (P110, DMT), with PSS bubbled with 21% O2, 5% CO2, 74% N2 (pH 7.35–7.40 in the bath) maintained at 37°C.
2.2. Experimental protocol
After an equilibration period of ~20 minutes, arteries showed stable myogenic tone at 70 mm Hg. Arteries were assessed for receptor-independent contraction to 60 mM KCl to check their viability. Afterwards, the effect of different pharmacological agents on myogenic tone was evaluated. Concentration response curves to different agents were obtained by cumulative addition. The maximal dilated diameter/passive diameter was obtained in calcium-free PSS at the end of the experiment. All experiments were performed in endothelium-intact arteries.
Pressure-dependent changes in arterial diameter were evaluated by increasing the intraluminal pressure in 10 mm Hg steps from 10–200 mm Hg. After exposure to the highest, the intraluminal pressure was lowered down to 10 mm Hg and the artery and pressure-dependent changes in diameter were obtained in the presence of calcium-free PSS. Myogenic tone was calculated by the following equation:
| (1) |
where Da is the active diameter of the artery with myogenic tone and Dp is the passive diameter in the presence of calcium-free PSS at a particular intraluminal pressure. Experiments evaluating myogenic tone over a range of intraluminal pressures were performed in arteries that were not used for any other protocols in this study.
2.3. Data analysis and statistics
Results were expressed as mean ± S.E.M; n indicates the number of independent experiments, which equals the number of animals used for experimentation. Means were compared by Student’s t-test and pressure-myogenic tone curves were compared by one-way ANOVA. A ‘P’ value < 0.05 was considered as statistically significant. The potency of the contractile agonist and enzyme inhibitors was expressed as pEC50 or pIC50 (negative logarithm of the concentration of the agonist or the inhibitor to produce the 50% of the maximum effect) as calculated by the software program GraphPad Prism that fits the data to a four-parameter logistic equation given below:
| (2) |
where minimum and maximum indicate the smallest and the highest responses produced by an agonist, X is the logarithm of the molar concentration of an agonist, Y is the response at a concentration of X and P is the Hill slope.
2.4. Drugs, chemicals and solutions
Histamine, U-73122 (1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole-2,5-dione) and ethylene glycol-bis(β-aminoethyl ether)-N, N, N′, N′-tetraacetic acid (EGTA) were purchased from Sigma (St. Louis, MO, USA), bradykinin and 5-hydroxytryptamine (5-HT) (serotonin HCl) was purchased from Fluka (Steinheim, Germany) and (±)-S-nitroso-N-acetylpenicillamine (SNAP) was purchased from Calbiochem (San Diego, CA, USA). Stock solutions (10 mM) of U-73122 was prepared in DMSO and those of all the other substances were prepared in distilled water. The composition of PSS (mM): NaCl (120), KCl (3), NaHCO3 (24), NaH2PO4. H2O (1.2), CaCl2 (2.5), MgSO4.7H2O (1.2) and glucose (4). PSS with 60 mM KCl was prepared by replacing NaCl with an equimolar quantity of KCl. Calcium free PSS was prepared by replacing CaCl2 with an equimolar quantity of MgSO4.7H2O with 2 mM EGTA.
3. Results
Body weights of BBZDR/Wor rats were significantly higher (P<0.01) at 4 weeks and 5 months after the onset of diabetes but not at the pre-diabetic age and 8 months of diabetes (Table 1). BBZDR/Wor rats showed decline in body weights at 5 months of diabetes and no significant difference was found at 8 months of diabetes when compared with the age-matched controls. Blood glucose levels of BBZDR/Wor and lean rats were similar at the pre-diabetic age but significantly higher (P<0.01) in BBZDR/Wor rats at different stages of diabetes (Table 1). Blood glucose levels in pre-diabetic groups appeared higher than usual but at this stage they were aglycosuric with normal insulin levels and glucose tolerance. Plasma insulin levels were significantly higher in all three diabetic groups compared to the age-matched lean group as well as pre-diabetic BBZDR/Wor group (Table 1).
Table 1.
Body weight, blood glucose and plasma insulin of obese diabetic BBZDR/Wor rats and their age-matched lean control rats.
| Pre-diabetic |
Diabetic |
|||||||
|---|---|---|---|---|---|---|---|---|
| BBZDR | Control | 4 weeks | 5 months | 8 months | ||||
| BBZDR | Control | BBZDR | Control | BBZDR | Control | |||
| Body Weight (g) | 307±15 | 279±13 | 624±23a | 367±60 | 714±18a | 395±7b | 540±21 | 503±33c |
| Blood Glucose1 Immunoreactive | 175±17 | 177±9 | 418±52a | 159±9 | 479±19a | 149±4 | 554±25a | 165±13 |
| Insulin (ng/L) | 1.0±0.4 | 0.8±0.2 | 11±2d | 1±0.2 | 17±2d | 1±0.3 | 19±2d | 1.2±0.2 |
Data shown were Mean±S.E.M.
mg/dl;
P<0.05 compared to the age-matched lean group.
P<0.01 and
P<0.001 (Students ‘t’-test) compared to the body weights of pre-diabetic lean group.
Significantly higher (P<0.001, Students ‘t’-test) in all three diabetic groups compared to the age-matched lean group as well as pre-diabetic BBZDR/Wor group.
3.1. Characteristics of posterior cerebral arteries
Passive diameters of posterior cerebral arteries (at an intraluminal pressure of 70 mm Hg) from BBZDR/Wor rats were not significantly different from that of their age-matched controls (Table 2). Constriction to 60% KCl was similar in arteries from BBZDR/Wor rats compared to that from the age-matched control rats (n=10 for pre-diabetic group and n=5 for the other three groups) (Table 2).
Table 2.
Properties and the effect of different vasoactive agents in posterior cerebral arteries of obese diabetic BBZDR/Wor and their age-matched lean control rats.
| Pre-diabetic |
Diabetic |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 weeks | 5 months | 8 months | |||||||
| BBZDR | Control | BBZDR | Control | BBZDR | control | BBZDR | Control | ||
| Active diameter1 | 101±3 | 103±4 | 84±4 | 90±11 | 91±4 | 97±7 | 98±4 | 102±2 | |
| Passive diameter1 | 154±4 | 167±8 | 132±7 | 142±10 | 137±8 | 155±8 | 151±11 | 163±2 | |
| Constriction to 60 mM KCl2 | 165±4 | 157±2 | 152±6 | 157±6 | 168±7 | 159±3 | 162±16 | 158±9 | |
| Histamine | pEC50 | 6±0.1 | 5.7±0.2 | 6±0.1 | 5.7±0.1 | 6±0.2 | 6±0.1 | 6±0.2 | |
| Emax3 | 87±4 | 78±7 | 82±5 | 81±8 | 53±15b | 82±5 | 95±3 | ||
| Bradykinin | pEC50 | 7.3±0.1 | 7.2±0.1 | 7.1±0.1 | 7.4±0.1 | 6.7±0.2 | 6.9±0.1 | ||
| Emax3 | 89±1 | 82±3 | 70±3a | 82±2 | 50±3b | 70±4d | |||
| SNAP | pEC50 | 6.7±0.1 | 6.4±0.1 | 6.5±0.1 | 6.8±0.1 | 6.2±0.1 | 6.4±0.1 | ||
| Emax3 | 74±5 | 79±4 | 68±4a | 78±4 | 51±5b | 74±3 | |||
| 5HT | pEC50 | 7.6±0.2 | 7.7±0.1 | 8.6±0.3 | 8.7±0.2b | 7.5±0.1 | 9.3±0.1c | 8.1±0.2 | |
| Emax4 | 46±6 | 50±5 | 54±3 | 67±8a | 57±5 | 78±4a | 67±6d | ||
| U-73122 | pIC50 | 6.2±0.2 | 6.2±0.1 | 5.5±0.1a | 6±0.1 | 5.5±0.1a | 6±0.1 | ||
| Emax3 | 96±2 | 92±3 | 83±3 | 92±1 | 87±3 | 97±1 | |||
Data shown were Mean±S.E.M.
microns;
%MT;
%Decrease in MT;
%KCl (60mM) response.
P<0.05/0.01,
P<0.001,
P<0.0001 (Students ‘t’-test) significantly different from corresponding control values.
P<0.05 (Students ‘t’-test) significantly different from control, pre-diabetic age group.
3.2. Pressure-induced constriction
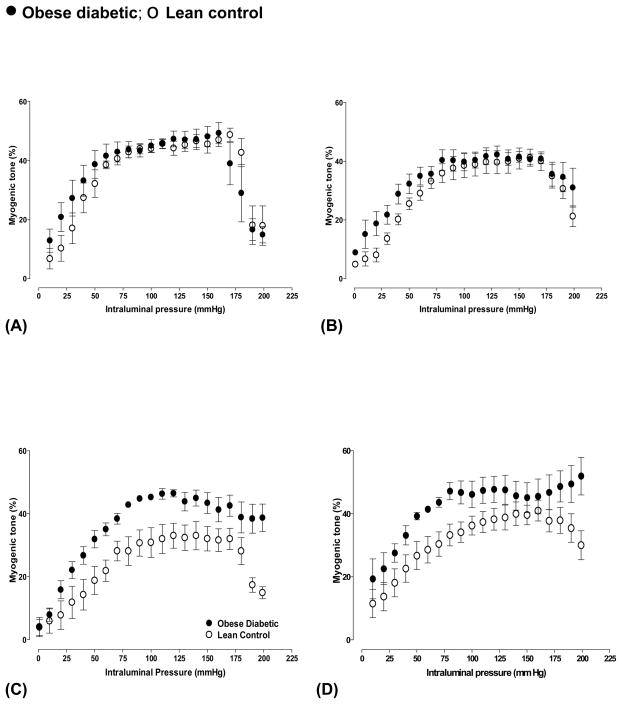
Arteries from both BBZDR/Wor and control rats at pre-diabetic age showed typical autoregulatory properties in response to intraluminal pressure (Fig 1A). Increase in pressure up to 40 mm Hg increased internal diameter resulting in less than 25% myogenic tone. From 60 to 160 mm Hg the diameter remained same, showing similar myogenic tone throughout the range of pressures. Pressures higher than160 mm Hg resulted in forced dilatation. Pressure-myogenic tone curves in diabetic arteries were similar to that in control arteries at pre-diabetic age. Similar autoregulatory response was observed in arteries at 4 weeks of diabetes and their age-matched control rats except that at pressures <60 mm Hg higher myogenic tone was observed in diabetic arteries (Fig 1B) with a significant leftward shift (P<0.05, two-way ANOVA, n=5) suggesting increased sensitivity to intraluminal pressure.
Fig 1.
Pressure-mediated autoregulatory curves in cerebral arteries from obese diabetic BBZDR/Wor and age-matched lean control rats at (A) pre-diabetic age and at different durations of diabetes ((B) 4 weeks, (C) 5 months and (D) 8 months of diabetes). Pressure-myogenic tone curves in 4 weeks diabetic arteries were significantly shifted left-ward (P<0.05, n=5). In (C) and (D), pressure-myogenic tone curves are significantly different between diabetic and control arteries (P<0.01, n=4). Data shown were Mean±S.E.M.
Arteries from 5 months diabetic rats showed higher myogenic tone at pressures higher than 30 mm Hg compared to that from the age-matched controls (Fig 1C). Similar behavior was also observed with the arteries from rats that were diabetic for 8 months (Fig 1D). Arteries from these chronic diabetic rats did not show forced dilatation at pressures >160 mm Hg. Pressure-myogenic tone curves were significantly different (P<0.01, two-way ANOVA, n=4) in diabetic and normal arteries at 5 and 8 months of diabetic age.
Age-dependent differences were observed in pressure-myogenic tone curves in arteries from either control or diabetic rats. In control rats at 7 and 11 months of age, pressure-myogenic tone curves in arteries were significantly different (P<0.001, two-way ANOVA) and myogenic tone was lower in the active pressure range i.e. 50–110 mm Hg compared to that of 10 weeks of age. In contrast, pressure-myogenic tone curves in diabetic arteries at 7 and 11 months of age were significantly different from that of 10 weeks or pre-diabetic group (P<0.01) with increased constriction at higher pressures and with no forced dilation, while no difference was observed at 14 weeks.
3.3. Arterial dilation
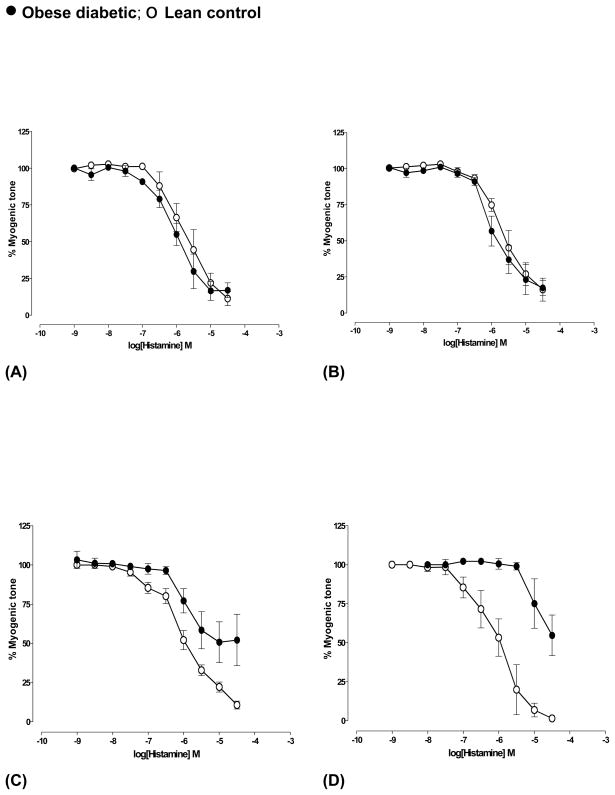
Maximum dilation and sensitivity of the arteries to histamine were similar in arteries from both BBZDR/Wor and lean control rats at pre-diabetic and at 4 weeks of diabetes (Fig 2A and 2B). Maximum dilation was significantly reduced (P<0.001, n=5) in arteries at 5 months of diabetic age compared to that in arteries from age-matched controls (Fig 2C, Table 2). Arteries from rats with 8 months of diabetes showed dilatory responses to histamine only at higher concentrations (≥10 μM) (n=4) suggesting significantly decreased sensitivity, whereas arteries from their age-matched controls showed maximum dilation (n=5) (Fig 2D). Age-dependent changes in dilation to histamine were not observed.
Fig 2.
Histamine-mediated dilation of cerebral arteries from obese diabetic BBZDR/Wor and age-matched lean control rats at (A) pre-diabetic age and at different durations of diabetes ((B) 4 weeks, (C) 5 months and (D) 8 months of diabetes). Arteries were pressurized at 70 mm Hg. In (C) and (D), dilation to histamine was significantly reduced (P<0.001, Students ‘t’-test, n=5). Data shown were Mean±S.E.M.
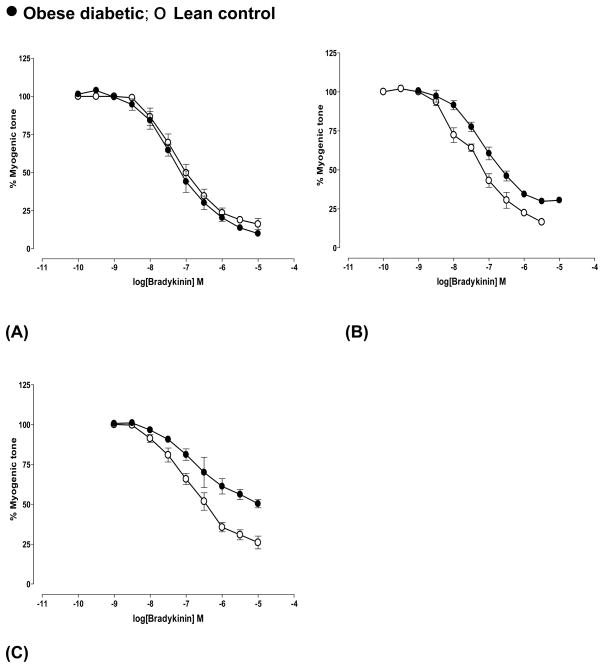
Concentration response curves to bradykinin were similar in arteries from diabetic and control rats at pre-diabetic age (Fig 3A). Maximum dilation was reduced in diabetic arteries compared to age-matched controls at 4 weeks of diabetes (P<0.05, n=5, Table 2) (Fig 3B) and at 5 months of diabetes (P<0.001, n=4) (Fig 3C, Table 2). Dilation to bradykinin accompanied extensive vasomotion (changes in diameter) in arteries from 8 months diabetic rats with no consistent response (data not shown). Age-dependent decrease in bradykinin-mediated dilation was observed in controls of 7 months age (P<0.05, n=5).
Fig 3.
Bradykinin-mediated dilation of cerebral arteries from obese diabetic BBZDR/Wor and age-matched lean control rats at (A) pre-diabetic age and at different durations of diabetes ((B) 4 weeks and (C) 5 months of diabetes). Arteries were pressurized at 70 mm Hg. Bradykinin-mediated dilation was significantly decreased in 4 weeks (P<0.05, n=5) and 5 months (P<0.001, n=4) diabetic arteries according to Students ‘t’-test. Data shown were Mean±S.E.M.
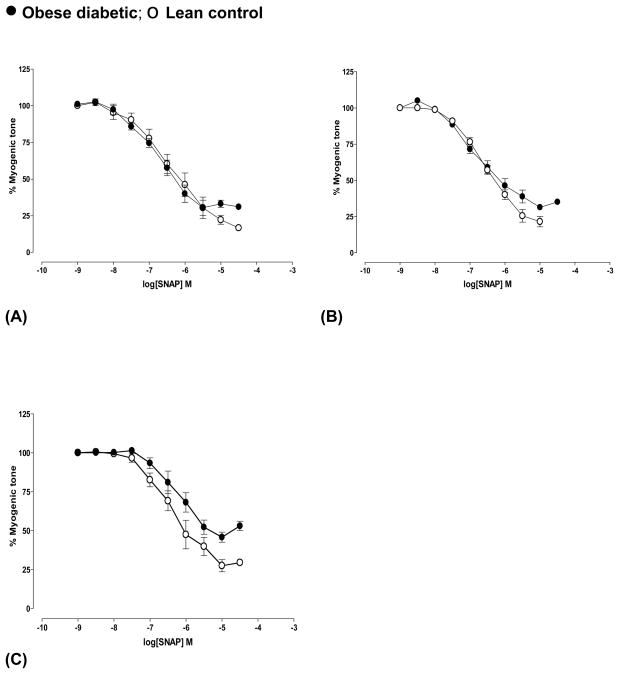
SNAP-mediated dilation was similar in arteries from both diabetic and control rats at pre-diabetic (Fig 4A) and 4 weeks (data not shown) of diabetes. Dilation was not affected in arteries from 5 months diabetic rats (Fig 4B, n=4) but significantly decreased in those from 8 months diabetic rats (Fig 4C) (P<0.01, n=3) (Table 2). Dilation to SNAP was not altered with age in controls.
Fig 4.
SNAP-mediated dilation of cerebral arteries from obese diabetic BBZDR/Wor and age-matched lean control rats at (A) pre-diabetic age and at different durations of diabetes ((B) 5 months and (C) 8 months of diabetes). Arteries were pressurized at 70 mm Hg. See text and table 2 for details. Dilation to SNAP was significantly decreased in 8 months diabetic arteries (P<0.01, Students ‘t’-test, n=3). Data shown were Mean±S.E.M.
3.4. Arterial constriction
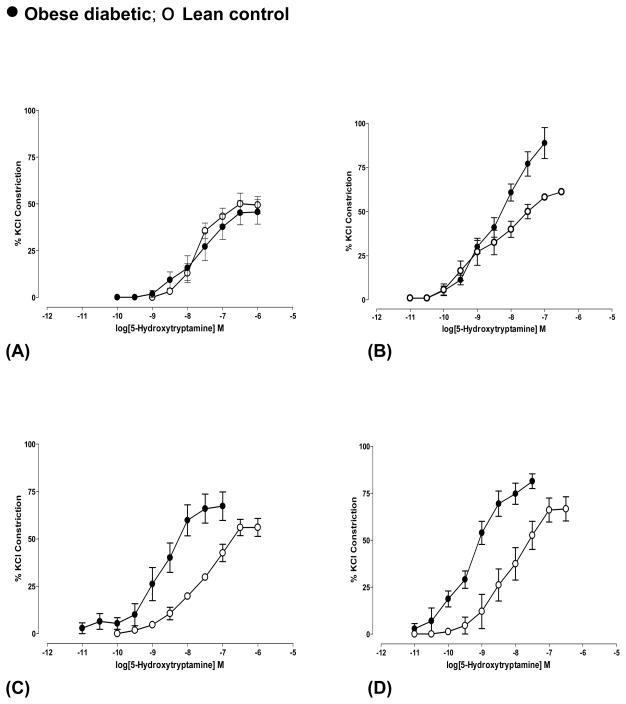
Constriction to 5HT was expressed as percent of 60 mM KCl response and was similar in diabetic and control arteries at pre-diabetic age (Fig 5A, Table 2). At 4 weeks of diabetes, arteries showed higher (P<0.0001, n=5) responses to 5HT compared to the age-matched controls with (Fig 5B, Table 2). At 5 and 8 months of diabetic age, arteries showed higher sensitivity (higher pEC50, see Table 2) to 5HT with significantly higher responses (P<0.01, n=5) (Fig 5C and 5D). Age-dependent increase in 5HT- constriction was observed in arteries from controls only at 11 months of age (P<0.05, n=5) compared to that in arteries from 10 week old.
Fig 5.
5-Hydroxytryptamine-induced constriction of cerebral arteries from obese diabetic BBZDR/Wor and age-matched lean control rats at (A) pre-diabetic age and at different durations of diabetes ((B) 4 weeks, (C) 5 months and (D) 8 months of diabetes). Arteries were pressurized at 70 mm Hg. Constriction was expressed as percent response of 60 mM KCl response. 5HT-mediated constriction was significantly increased in diabetic arteries (B) P<0.0001, n=5, (C) P<0.01, n=5 and (D) P<0.01, n=4 (Students ‘t’-test) with higher sensitivity in (C) and (D) (see Table 2 for details). Data shown were Mean±S.E.M.
3.5. Sensitivity of myogenic tone to U-73122
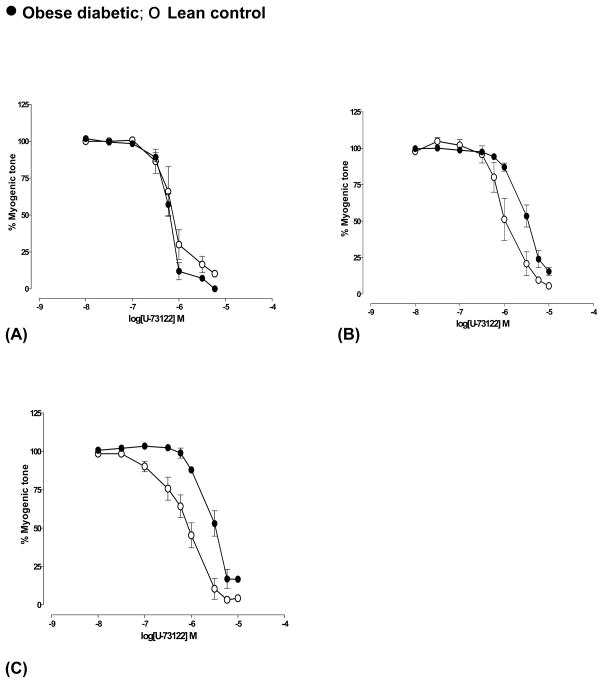
Maximum dilation produced by U-73122, an inhibitor phosphatidyl inositol-specific phospholipase C (PLC) was similar in both diabetic and age-matched control arteries from all four groups. The inhibitory potency (pIC50) of U-73122 was similar in arteries from diabetic and control rats at pre-diabetic age but lowered in diabetic arteries by 3-fold (P<0.01, n=6) at 4 weeks and at 8 months (P<0.01, n=4) of diabetes (Fig 6) (Table 2) compared to their respective controls. Similar difference was observed at the diabetic age of 5 months (n=2, data not shown).
Fig 6.
U-73122-mediated decrease in myogenic tone of cerebral arteries from obese diabetic BBZDR/Wor and BBZDR/Wor lean control rats at (A) pre-diabetic age and at different durations of diabetes ((B) 4 weeks and (C) 8 months of diabetes). Arteries were pressurized at 70 mm Hg. See text and table 2 for details. Significant right-ward shift was observed in concentration-myogenic tone curves in 4 weeks (P<0.01, n=6) and 8 months (P<0.01, n=4) diabetic arteries according to Students ‘t’-test. Data shown were Mean±S.E.M.
4. Discussion
The main focus of this study was to evaluate cerebroarterial function in this relatively new model of type II diabetes, BBZDR/Wor rat, thereby validating the use of this model to study and understand the underlying mechanisms of vascular complications in type II diabetes. This is the first study to evaluate pressure-mediated autoregulation of cerebral arterial tone over the course of type II diabetes in an experimental model.
4.1. Pressure-mediated autoregulation of cerebral arterial tone
Myogenic arteries constrict in response to physiological pressures and dilate at physiologically lower pressures thereby autoregulate blood flow to organs/tissues (Johnson, 1986). The present findings suggest that the pressure-dependent autoregulation in cerebral arteries is lost. In early diabetic stage, arteries showed increased sensitivity to pressure i.e. leftward shift in pressure-myogenic tone curves. At chronic stages of diabetes, arteries exhibited higher tone to pressure compared to that observed in arteries from age-matched lean control rats and showed resistance to forced dilatation.
However, age-dependent variations were observed in myogenic tone in control rats. Significant decrease was observed in arteries from control rats of 7 and 11 months of age while forced dilatory properties were unchanged. Inverse relationship between age and myogenic tone was also observed earlier in human ciliary artery (Nyborg and Nielsen, 1990), mesenteric arteries of male and female C57BL/6 mice (Gros et al., 2002) and human brachial artery (Lott et al., 2004). In contrast, arteries from obese diabetic rats showed either similar or higher myogenic tone with the advanced age and acquired the ability to withstand higher pressures with no forced dilation, resulting in a defective pressure-mediated autoregulation of blood flow to the brain. Enhanced myogenic constriction observed in this model is consistent with the available few reports in type II diabetes, namely, in mesenteric arteries from mouse model of type II diabetes (Lagaud et al., 2001) and skeletal muscle arterioles from Zucker rat model of obesity-induced type II diabetes (Frisbee et al., 2002). In contrary, we observed decreased pressure-induced in the ophthalmic artery of BBZDR/Wor rat and the decrease was endothelium-dependent (Ito et al., 2006), suggesting vascular bed-dependent variation in the effect of type II diabetes on pressure-dependent autoregulation.
4.2. Endothelium-dependent and -independent dilation of cerebral arteries
We used three different dilators, histamine, bradykinin and SNAP, with different mechanisms of dilation. Bradykinin is a frequently used endothelium-dependent dilator and in rat posterior cerebral arteries the dilation to bradykinin is solely mediated by nitric oxide (NO) (Lagaud et al., 1999). Histamine-mediated dilation is either endothelium-dependent or independent depending on the vascular bed being studied. In mouse and rat cerebral arteries histamine-mediated dilation is endothelium-independent (Rosenblum et al., 1990; Jarajapu et al., 2006). SNAP is also a widely used endothelium-independent dilator that spontaneously releases NO in aqueous solutions (Ignarro et al., 1988).
Alterations in dilatory responses to different agonists varied with the duration of diabetes. Consistent with the notion that endothelium-mediated dilation is the first mechanism to be affected in diabetes (Pieper, 1998), dilation to bradykinin was significantly affected at an early stage of diabetic syndrome in BBZDR/Wor rat and further aggravated with the duration of diabetes. Histamine-mediated dilation was affected with longer durations of diabetes and SNAP-mediated dilation was modestly affected only at 8 months of diabetes, suggesting that the effect of diabetic syndrome on arterial dilation varies with mechanisms involved in the dilatory effect. Spontaneous NO donor is probably the strongest stimulus to produce smooth muscle relaxation and decreased response to NO donors indicate an altered intracellular signaling in smooth muscle involving NO-cGMP-MLCK pathway and function of different potassium channels (Schubert and Nelson, 2001; Munzel et al., 2003).
Studies carried out in experimental models of type II diabetes are few and provide no clear consensus regarding endothelial dysfunction. Endothelium-dependent dilation in mesenteric arteries was shown to be unaffected in obese Zucker rat (Bohlen and Lash, 1995) and decreased in mesenteric (Lagaud et al., 2001) and cerebral arteries (Didion et al., 2005) of db/db mice. Almost with no exception, studies that have shown impaired endothelium-dependent relaxation in experimental diabetes, have found normal relaxation to nitrovasodilators or agents that release nitric oxide spontaneously. In contrast, in human type II diabetes, both unaltered and attenuated responses to nitrovasodilators was reported (Pieper, 1998).
In the present model also endothelium-dependent dilation was decreased with age while endothelium-independent dilation was unaffected. This is consistent with earlier observations in different arteries (coronary (Csiszar et al., 2002), mesenteric (Sullivan and Davison, 2001), skeletal muscle (Muller-Delp et al., 2002; Woodman et al., 2003) arteries of rat and human forearm resistance arteries (Gerhard et al., 1996)).
4.3. Responses to vasoconstrictor and role of phospholipase C
Consistent with the changes observed in pressure-mediated myogenic tone, agonist-induced constriction was also affected. Enhanced constriction to 5HT with higher sensitivity was observed in the early and chronic stages of diabetes in this model. Impaired endothelium-mediated dilation or hyperreactivity of smooth muscle or both could be the underlying mechanism depending on the duration of diabetes. Earlier studies have shown that experimental diabetes results in protein kinase C (PKC) activation (King et al., 1997) that promotes smooth muscle contraction by calcium-sensitization of contractile proteins (Lee and Severson, 1994) or by inhibiting the activity of potassium channels in smooth muscle membrane (Bonev et al., 1997; Armstead, 2001). Now evidence is accumulating showing the importance of PKC-mediated effects in smooth muscle via NAD(P)H oxidase-production of reactive oxygen species-potassium channel inhibition. Consistent with this notion, studies in retina in this rat model showed time course of increased NAD(P)H, an important source of reactive oxygen species that coincided with the onset of type II diabetes (Ellis et al., 2002).
Experiments with the pharmacological inhibitor of PLC, U-73122 (Muller-Decker, 1989; Smith et al., 1990), showed evidence for an elevated PLC-activation in arteries in response to pressure. This is in accordance with the earlier supposition that in diabetic arterial wall, PKC activation is higher via PLC-mediated production of diacylglycerol (DAG) that activates PKC (Inoguchi et al., 2003). In the present study, the inhibitory potency (pIC50) of PLC inhibitor, U-73122, was taken as a measure of PLC-activation involved in the myogenic tone. We earlier reported that development of myogenic tone in rat cerebral arteries involves activation of PLC and is completely reversed by the treatment of PLC inhibitor U-73122 with no non-specific effects of dilation (Jarajapu and Knot, 2002). Sensitivity to the PLC inhibitor decreased in the early diabetic stage as well as in the chronic diabetic stage with no change in the maximum dilation or reversal of myogenic tone suggesting that activation of PLC involved in the development of myogenic tone was increased with diabetes and remained elevated over the course of diabetes.
In conclusion, this study characterized cerebroarterial dysfunction in BBZ/Wor rat showing that pressure-dependent autoregulation of cerebral blood flow in type II diabetes operates at higher resistance. Endothelium-dependent dilation was greatly attenuated and endothelium-independent dilation was modestly affected with chronic diabetes. Sensitivity to pressure- and agonist-induced contraction was increased over the course of diabetes. Age-dependent changes in the arterial reactivity need to be considered when studying vascular complications in diabetic models with different durations of diabetes.
Acknowledgments
Supported by National Eye Institute Grants EY12601 and EY007739, and Juvenile Diabetes Research Foundation International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Armstead WM. Vasopressin-induced protein kinase C-dependent superoxide generation contributes to atp-sensitive potassium channel but not calcium-sensitive potassium channel function impairment after brain injury. Stroke. 2001;32:1408–1414. doi: 10.1161/01.str.32.6.1408. [DOI] [PubMed] [Google Scholar]
- Bohlen HG, Lash JM. Endothelial-dependent vasodilation is preserved in non-insulin-dependent Zucker fatty diabetic rats. Am J Physiol. 1995;268:H2366–H2374. doi: 10.1152/ajpheart.1995.268.6.H2366. [DOI] [PubMed] [Google Scholar]
- Bonev AD, Jaggar JH, Rubart M, Nelson MT. Activators of protein kinase C decrease Ca2+ spark frequency in smooth muscle cells from cerebral arteries. Am J Physiol. 1997;273:C2090–C2095. doi: 10.1152/ajpcell.1997.273.6.C2090. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Chyun DA, Young LH. Diabetes mellitus and cardiovascular disease. Nurs Clin North Am. 2006;41:681–6ix. doi: 10.1016/j.cnur.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Didion SP, Lynch CM, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of Rho-kinase in cerebral arterioles in type II diabetes. Stroke. 2005;36:342–347. doi: 10.1161/01.STR.0000152952.42730.92. [DOI] [PubMed] [Google Scholar]
- Ellis EA, Guberski DL, Hutson B, Grant MB. Time course of NADH oxidase, inducible nitric oxide synthase and peroxynitrite in diabetic retinopathy in the BBZ/WOR rat. Nitric Oxide. 2002;6:295–304. doi: 10.1006/niox.2001.0419. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Maier KG, Stepp DW. Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;283:H2160–H2168. doi: 10.1152/ajpheart.00379.2002. [DOI] [PubMed] [Google Scholar]
- Ganne S, Arora SK, Dotsenko O, McFarlane SI, Whaley-Connell A. Hypertension in people with diabetes and the metabolic syndrome: pathophysiologic insights and therapeutic update. Curr Diab Rep. 2007;7:208–217. doi: 10.1007/s11892-007-0033-3. [DOI] [PubMed] [Google Scholar]
- Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- Gros R, Van Wert R, You X, Thorin E, Husain M. Effects of age, gender, and blood pressure on myogenic responses of mesenteric arteries from C57BL/6 mice. Am J Physiol Heart Circ Physiol. 2002;282:H380–H388. doi: 10.1152/ajpheart.2002.282.1.H380. [DOI] [PubMed] [Google Scholar]
- Guberski DL, Butler L, Manzi SM, Stubbs M, Like AA. The BBZ/Wor rat: clinical characteristics of the diabetic syndrome. Diabetologia. 1993;36:912–919. doi: 10.1007/BF02374472. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Buga GM, Byrns RE, Wood KS, Chaudhuri G. Endothelium-derived relaxing factor and nitric oxide possess identical pharmacologic properties as relaxants of bovine arterial and venous smooth muscle. J Pharmacol Exp Ther. 1988;246:218–226. [PubMed] [Google Scholar]
- Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003;14:S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- Ito I, Jarajapu YP, Guberski DL, Grant MB, Knot HJ. Myogenic tone and reactivity of rat ophthalmic artery in acute exposure to high glucose and in a type II diabetic model. Invest Ophthalmol Vis Sci. 2006;47:683–692. doi: 10.1167/iovs.05-1012. [DOI] [PubMed] [Google Scholar]
- Jarajapu YP, Knot HJ. Role of phospholipase C in development of myogenic tone in rat posterior cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;283:H2234–H2238. doi: 10.1152/ajpheart.00624.2002. [DOI] [PubMed] [Google Scholar]
- Jarajapu YP, Oomen C, Uteshev VV, Knot HJ. Histamine decreases myogenic tone in rat cerebral arteries by H2-receptor-mediated KV channel activation, independent of endothelium and cyclic AMP. Eur J Pharmacol. 2006;547:116–124. doi: 10.1016/j.ejphar.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Johnson PC. Autoregulation of blood flow. Circ Res. 1986;59:483–495. doi: 10.1161/01.res.59.5.483. [DOI] [PubMed] [Google Scholar]
- Kashyap SR, Defronzo RA. The insulin resistance syndrome: physiological considerations. Diab Vasc Dis Res. 2007;4:13–19. doi: 10.3132/dvdr.2007.001. [DOI] [PubMed] [Google Scholar]
- King GL, Ishii H, Koya D. Diabetic vascular dysfunctions: a model of excessive activation of protein kinase C. Kidney Int Suppl. 1997;60:S77–S85. [PubMed] [Google Scholar]
- Lagaud GJ, Masih-Khan E, Kai S, van Breemen C, Dube GP. Influence of type II diabetes on arterial tone and endothelial function in murine mesenteric resistance arteries. J Vasc Res. 2001;38:578–589. doi: 10.1159/000051094. [DOI] [PubMed] [Google Scholar]
- Lagaud GJ, Skarsgard PL, Laher I, van Breemen C. Heterogeneity of endothelium-dependent vasodilation in pressurized cerebral and small mesenteric resistance arteries of the rat. J Pharmacol Exp Ther. 1999;290:832–839. [PubMed] [Google Scholar]
- Lee MW, Severson DL. Signal transduction in vascular smooth muscle: diacylglycerol second messengers and PKC action. Am J Physiol. 1994;267:C659–C678. doi: 10.1152/ajpcell.1994.267.3.C659. [DOI] [PubMed] [Google Scholar]
- Lott ME, Herr MD, Sinoway LI. Effects of age on brachial artery myogenic responses in humans. Am J Physiol Regul Integr Comp Physiol. 2004;287:R586–R591. doi: 10.1152/ajpregu.00612.2003. [DOI] [PubMed] [Google Scholar]
- McGillis Bindler RC. A cascade of events -- obesity, metabolic syndrome, and type 2 diabetes mellitus in youth. Nurs Clin North Am. 2007;42:29–42. vi. doi: 10.1016/j.cnur.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Mordes JP, Bortell R, Blankenhorn EP, Rossini AA, Greiner DL. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILARJ. 2004;45:278–291. doi: 10.1093/ilar.45.3.278. [DOI] [PubMed] [Google Scholar]
- Muller-Decker K. Interruption of TPA-induced signals by an antiviral and antitumoral xanthate compound: inhibition of a phospholipase C-type reaction. Biochem Biophys Res Commun. 1989;162:198–205. doi: 10.1016/0006-291x(89)91981-5. [DOI] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Munzel T, Feil R, Mulsch A, Lohmann SM, Hofmann F, Walter U. Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase [corrected] Circulation. 2003;108:2172–2183. doi: 10.1161/01.CIR.0000094403.78467.C3. [DOI] [PubMed] [Google Scholar]
- Nyborg NC, Nielsen PJ. The level of spontaneous myogenic tone in isolated human posterior ciliary arteries decreases with age. Exp Eye Res. 1990;51:711–715. doi: 10.1016/0014-4835(90)90056-z. [DOI] [PubMed] [Google Scholar]
- Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998;31:1047–1060. doi: 10.1161/01.hyp.31.5.1047. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI, Nelson GH, Weinbrecht P. Histamine elicits competing endothelium-dependent constriction and endothelium-independent dilation in vivo in mouse cerebral arterioles. Stroke. 1990;21:305–309. doi: 10.1161/01.str.21.2.305. [DOI] [PubMed] [Google Scholar]
- Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- Sima AA, Zhang W, Xu G, Sugimoto K, Guberski D, Yorek MA. A comparison of diabetic polyneuropathy in type II diabetic BBZDR/Wor rats and in type I diabetic BB/Wor rats. Diabetologia. 2000;43:786–793. doi: 10.1007/s001250051376. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- Sullivan JC, Davison CA. Effect of age on electrical field stimulation (EFS)-induced endothelium-dependent vasodilation in male and female rats. Cardiovasc Res. 2001;50:137–144. doi: 10.1016/s0008-6363(01)00193-6. [DOI] [PubMed] [Google Scholar]
- Tirabassi RS, Flanagan JF, Wu T, Kislauskis EH, Birckbichler PJ, Guberski DL. The BBZDR/Wor rat model for investigating the complications of type 2 diabetes mellitus. ILAR J. 2004;45:292–302. doi: 10.1093/ilar.45.3.292. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Pacher P, Kecskemeti V, Papp G, Szollar L, Koller A. Increased myogenic tone in skeletal muscle arterioles of diabetic rats. Possible role of increased activity of smooth muscle Ca2+ channels and protein kinase C. Cardiovasc Res. 1999;43:1018–1028. doi: 10.1016/s0008-6363(99)00106-6. [DOI] [PubMed] [Google Scholar]
- Vernet D, Cai L, Garban H, Babbitt ML, Murray FT, Rajfer J, Gonzalez-Cadavid NF. Reduction of penile nitric oxide synthase in diabetic BB/WORdp (type I) and BBZ/WORdp (type II) rats with erectile dysfunction. Endocrinology. 1995;136:5709–5717. doi: 10.1210/endo.136.12.7588327. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Selected Contribution: Aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol. 2003;95:2164–2170. doi: 10.1152/japplphysiol.01073.2002. [DOI] [PubMed] [Google Scholar]
- Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]