Abstract
The currently accepted paradigm for the primary T cell response is that effector T cells commit to autonomous developmental programs. This concept is based on several experiments that have demonstrated that the dynamics of a T cell response is largely determined shortly after antigen exposure and that T cell dynamics do not depend on the level and duration of antigen stimulation. Another experimental study has also shown that T cell responses are robust to variations in antigen-specific precursor frequency.
Various mathematical models have corroborated the first result that programmed T cell responses are insensitive to the level of antigen stimulation. However, this paper proposes that programmed responses do not entirely explain the robustness of T cell dynamics to variations in precursor frequency. This work studies the hypothesis that the dynamics of a T cell response may also be governed by a feedback loop involving adaptive regulatory cells rather than by intrinsic developmental programs.
We formulate two mathematical models based on T cell developmental programs. In one model, effector cells undergo a fixed number of divisions before dying. In the second model, effector cells live for a fixed time during which they may divide. The study of these models suggests that developmental programs are not sufficiently robust as they produce an immune response that directly scales with precursor frequencies. Consequently, we derive a third model based on the principle that adaptive regulatory T cells develop in the course of an immune response and suppress effector cells. Our simulations show that this feedback mechanism responds robustly over a range of at least four orders of magnitude of precursor frequencies.
We conclude that the proliferation program paradigm does not entirely capture the observed robustness of T cell responses to variations in precursor frequency. We propose an alternative mechanism by which the primary T cell response is governed by an emergent group dynamic and not by individual T cell programs.
Keywords: Delay differential equations, Age-structured equations, Adaptive regulatory T cells, T cell response
1. Introduction
In many ways, the adaptive immune response works like a controlled explosion. This can be demonstrated, e.g., by the rapid, yet transient T cell response to a viral infection. Prior to such a response, thousands of potentially-reactive T cells wait in lymph nodes until stimulated by specific signals. Upon stimulation, these cells unleash their latent capacity to proliferate until they stop dividing and undergo apoptosis or enter dormancy as memory cells.
What triggers the initial T cell expansion and what triggers the ensuing contraction? Clearly, both phases must occur in a healthy response. The T cell population must expand quickly and adequately to eliminate the invading pathogen. It must also contract to a normal, resting state to reduce the chance of collateral damage to healthy cells. In recent years, several conjectures have been made to address these issues. The various approaches are predominantly based on the notion of a T cell program.
This novel concept is based on several experimental studies that have demonstrated that the dynamics of a T cell response is largely determined shortly after initial antigen exposure (Kaech and Ahmed, 2001; Mercado et al., 2000; Razvi et al., 1995; Renno et al., 1999; Stipdonk et al., 2003; Yang et al., 1998). One of the implications of early programming is that immune dynamics are highly insensitive to the nature of antigen stimulation, a phenomenon that has also been observed experimentally.
An additional study by Badovinac et al. has shown that T cell dynamics are also robust to variations in the precursor frequency of antigen-specific naïve T cells. In particular, their experiments showed that a 10,000-fold difference in antigen-specific naïve T cell concentrations only led to a 13-fold higher peak in the effector response (Badovinac et al., 2007).
In this paper, we construct mathematical models of two types of T cell proliferation programs: cell division-based programs and time-based programs. We examine the characteristics of both types of programs and argue why neither version can entirely explain the inherent robustness of a normal T cell response with respect to variations in precursor frequency. As an alternative regulatory mechanism, we consider adaptive regulatory T cells, also known as antigen-induced regulatory T cells (iTregs), and devise an extended mathematical model incorporating iTregs to show how they may play a crucial role in inducing a timely and robust contraction of the T cell response. Our hypothesis is that T cell responses are not exclusively, or even primarily, regulated by autonomous programs within each T cell. Rather, they result from the dynamics of several immune cells that interact based on relatively simple rules.
The structure of the paper is as follows: In Section 2, we review several papers discussing experimental results and mathematical models pertaining to the concept of a T cell proliferation program. In Section 3, we outline the basic modeling assumptions for the three models in this paper and compare them to existing models. Then in Section 3.1, we develop a delay differential equation (DDE) model for the cell division-based program. In Section 3.2, we develop a partial differential equation (PDE) model for the time-based program. In Section 3.3, we develop a DDE model for the interaction between effectors and iTregs. In Sections 4.1 and 4.2, we present the results of the cell division-based and time-based programs. In Section 4.3, we provide an argument about why the autonomous program paradigm cannot fully explain the robustness of the T cell response to variations in precursor frequency. In Section 4.4, we provide an argument based on medical and modeling literature supporting a model involving iTregs, and in Section 4.5, we discuss the results of the iTreg model. In Section 5, we provide a closing discussion and propose several possibilities for future work.
2. Background on T cell programs
We review several works on programmed T cell responses, which are then used as a basis for our first two mathematical models. In a key experimental work, Mercado et al. demonstrated that the kinetics of CD8+ T cell expansion and contraction are determined within the first day of infection (Mercado et al., 2000). They tested their hypothesis by infecting mice with L. monocytogenes and showed that the size of CD8+ T cell responses are similar for nearly a 1000-fold range of doses. In another study of CD8+ T cell expansion, Kaech et al. showed that upon antigenic stimulation, naïve CD8+ T cells divide at least 7–10 times and differentiate into functional effector and memory cells even if antigen is removed (Kaech and Ahmed, 2001). They hypothesized that this developmental program is initiated in parent cells and is passed on to daughter cells independently of antigenic stimulation. They suggested that the initial round of 7–10 divisions comprises a “minimal developmental program,” and that effector cells may continue dividing under further stimulation.
An alternative experimental approach by van Stipdonk et al. also focused on CD8+ T cell stimulation (Stipdonk et al., 2001). They showed that naïve CD8+ T cells become activated after only 2 hours of exposure to mature antigen presenting cells (APCs). After activation, these T cells divided and differentiated into effector and eventually memory cells without a need for further antigenic stimulation. By comparing T cell stimulation in vivo and in vitro, they found that in both cases T cell expansion rates were nearly identical at every measured time point. In addition, naïve T cells were primed in vivo and then pulled out to expand overnight in vitro and vice versa. In both cases, cells successfully divided and differentiated into functional effectors. These results show that T cells can carry out their proliferation programs independently and without the aid of accessory cells or other factors. In a subsequent paper, they observed that naïve CD8+ T cells that have been stimulated for 20 h were able to carry out extensive proliferation and cytotoxic activity, characteristic of a fully developed immune response (Stipdonk et al., 2003). They proposed that the fate of a T cell response is governed by a “cell-instrinsic developmental program” that is set even before the first cell division takes place.
A couple of mathematical models of the T cell proliferation program have been developed in parallel to these experiments. Antia et al. devised a mathematical model to investigate whether the program is completely specified by the initial encounter with antigen or whether it can be subsequently modified by the amount of antigen present (Antia et al., 2003). They considered two scenarios, a “strict program” in which activated T cells follow a fixed program of expansion, contraction, and differentiation, and an alternative paradigm in which stimulated T cells undergo a short period of antigen-dependent expansion (up to 2.5 days after the original infection or 1.1 days after stimulation) before entering an antigen-independent program. In both cases, the antigen-independent program can be either modeled as a function of time after activation or a function of the number of T cell divisions. Their results favor the second paradigm in which the T cell population briefly expands in response to the amount of antigen present before committing to a fixed program.
We point out that the antigen-dependent and antigen-independent parts of the model from Antia et al. (2003) occur in the opposite order of the more common notion that a developmental program precedes antigen-dependent proliferation as suggested in Kaech and Ahmed (2001). Furthermore, the time windows for antigen-dependent expansion in Antia et al. (2003) do not agree with the recent experimental results discussed above. In particular, the model from Antia et al. (2003) considers two scenarios: one in which individually stimulated T cells each undergo antigen-dependent proliferation for 1.1 days prior to beginning the antigen-independent program, and one in which the entire population of available T cells undergoes 2.5 days of antigen-dependent expansion before collectively entering the proliferation program. On the contrary, the experiments of van Stipdonk et al. propose a 20 hour “programming” period (Stipdonk et al., 2003), during which no cell divisions take place (Stipdonk et al., 2001, 2003; Veiga-Fernandes et al., 2000). In addition, the experiments of Mercado et al. show that nearly all potentially-reactive T cells are stimulated within 24 hours (Mercado et al., 2000), also before any cell divisions have taken place. Hence, the current experimental evidence supports the notion that T cells typically enter a proliferation program shortly after stimulation, even before undergoing one round of cell division. Thus, in this paper, we only address the scenario in which a minimal developmental program precedes a period of antigen-dependent proliferation.
In a more recent work, Wodarz and Thomsen developed a mathematical model in which activated T cells undergo a fixed number of divisions before differentiating into effectors and then into memory cells (Wodarz and Thomsen, 2005). If infection persists, memory cells recycle back into the effector state and enter another round of divisions, repeating the program as many times as necessary. The purpose of the study by Wodarz and Thomsen was to find the optimal fixed program that could respond effectively to a wide variety of infections. They concluded that the 7–10 divisions observed experimentally represented such an optimum.
Several experimental studies have also focused on the contraction of the T cell response and nearly all researchers propose a mechanism of apoptosis, or programmed cell death. For example, Razvi et al. tested various possible scenarios in vivo and concluded that apoptosis of effector CD8+ T cells in lymph nodes is a principal mediator of T cell contraction (Razvi et al., 1995). They observed that the rate of apoptosis increases in two phases with an early rise at day 3 after infection and a much larger jump at day 11, which coincides with the beginning of T cell contraction. They also concluded that a deprivation of growth factor is not the major cause of apoptosis. A different mechanism must trigger apoptosis, and hence terminate the T cell response.
Yang et al. (1998) infected mice with staphylococcal enterotoxin B (SEB) to study the activation and apoptotic death of naïve CD4+ T cells. Like Razvi et al. (1995), they observed that the rate of apoptosis peaks at the height of the T cell response, indicating that a large number of T cells commit apoptosis approximately at the same time, terminating T cell expansion. They concluded that apoptosis is programmed into T cells and that it is executed after a certain delay following activation. In a similar set up, Renno et al. likewise infected mice with SEB to study programmed cell death in CD4+ T cells (Renno et al., 1999). They concluded that apoptosis takes place after a fixed number of cell divisions rather than after a fixed time. Although these papers differ in their proposed mechanisms, they both postulate more or less intrinsic programs that execute after a certain time has elapsed or after a certain number of cell divisions.
The two mathematical modeling papers discussed above implicitly include contraction programs. In particular, Antia et al. study both time-based and cell division-based programs (Antia et al., 2003), and Wodarz and Thomsen also include both types by default, since for a strict program in which cells undergo a fixed number of divisions, the time and cell division-based programs end up being identical (Wodarz and Thomsen, 2005).
All together the experimental and mathematical modeling papers propose a general paradigm for T cell expansion, which can be stated as follows: Upon stimulation, T cells enter a minimal developmental program of about 7–10 divisions that is followed by a period of antigen-dependent proliferation that terminates after a certain time or after a certain number of cell divisions.
3. Methods
We present three mathematical models for T cell expansion and contraction that we refer to as the cell division-based program, the time-based program, and the iTreg model. In all three models, activated naïve T cells automatically undergo a minimal developmental program. After the minimal developmental program, each of the three models account for different possibilities for further T cell development. The first two models are based on the idea that activated T cells carry a built-in apoptotic mechanism that executes at a certain time or after a certain number of divisions to guarantee a timely end of the proliferation process. In the third model, activated T cells do not possess an inherent apoptotic mechanism, but are shut down based on a simple feedback loop.
For the cell division-based program, effector cells may undergo additional divisions upon antigen stimulation up to a fixed number of divisions. Upon attaining this maximum, effector cells inevitably die without further proliferation. For the time-based program, effector cells live for a fixed time after activation and may divide during this period. These models are in the same spirit as the mathematical models of Antia et al. (2003) and Wodarz and Thomsen (2005), which consider alternative versions of possible T cell programs.
The T cell program presented in Antia et al. (2003) is a fusion of both division-based and time-based programs, because T cells pass through a fixed time window of proliferation at a programmed rate that is followed by a fixed time window of contraction at a programmed rate. In this case, the division-based and time-based mechanisms are indistinguishable since counting the number of divisions is equivalent to measuring time. In our models, we isolate the two mechanisms of cellular control and study them separately.
In the model of Wodarz and Thomsen (2005), T cells cannot undergo variable periods of antigen-dependent proliferation after activation, but must recycle through the full program of divisions upon further stimulation. In such a model, immune responses will tend to jump from small to medium to large as antigen levels increase rather than follow a more gradual increase as observed in our models.
The iTreg model assumes that T cell development depends on feedback from the T cell population itself rather than relying on intrinsic developmental programs. Proliferating effector cells generate the self-regulating feedback by occasionally producing iTregs that suppress effector activity. No individual T cell possesses an internal mechanism to induce a change in behavior from expansion to contraction.
The experimental studies discussed in Section 1 show that the T cell program applies to both CD4+ and CD8+ T cells. Hence, in this paper, we consider one T cell population that includes both CD4+ and CD8+ T cells. This approach allows us to study the general pattern of contraction and expansion of the T cell response without making the modeling equations too complex. Thus, the mathematical models in this paper track concentrations of antigen presenting cells (APCs), T cells, and eventually adaptive regulatory T cells (iTregs).
For additional simplicity, the models only focus on the dynamics of these cells in the lymph node. This is a sensible approach because apart from the initial influx of mature APCs from the site of infection, nearly all subsequent dynamics take place primarily in the lymph node. Indeed, an experimental study shows that the T cell behaviors of interest, namely activation, division, and migration between the lymph node and tissue, only occur for T cells in the lymph node and not for those in the periphery (Harris et al., 2002).
3.1. Cell division-based program
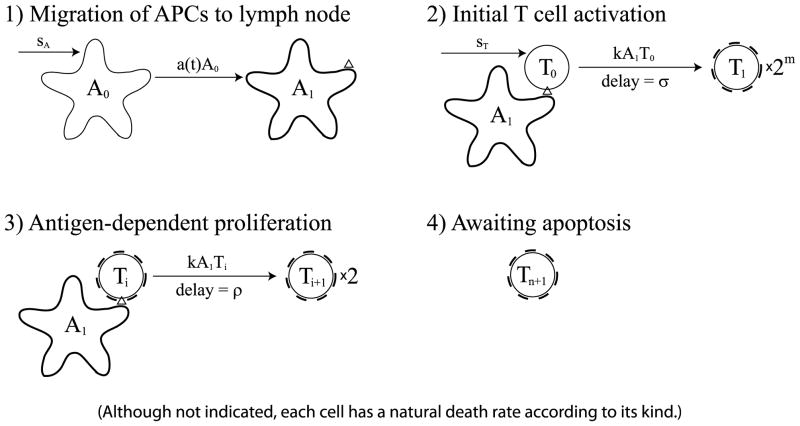
The T cell response begins when APCs mature and migrate from a site of infection, typically in the peripheral tissue, into the draining lymph node. The mature APCs then activate naïve (or memory) T cells that begin to proliferate and eventually emigrate back to the original site of infection. We model this process in four steps (illustrated in Fig. 1):
Fig. 1.
The cell division-based program. (1) Immature APCs pick up antigen at the site of infection at a time-dependent rate a(t). These APCs mature and migrate to the lymph node. (2) Mature antigen-bearing APCs present antigen to naïve T cells causing them to activate and enter the minimal developmental program of m divisions. (3) Activated T cells that have completed the minimal program continue to divide upon further interaction with mature APCs for up to n additional divisions. (4) T cells that have completed the maximal number of divisions stop dividing and wait for apoptosis. Although not indicated, each cell in the diagram has a natural death rate according to its kind.
APCs mature, present relevant target antigen, and migrate from the site of infection to the draining lymph node.
In the lymph node, APCs activate naïve T cells that enter a minimal developmental program of m cell divisions.
T cells that have completed the minimal developmental program become effector cells that can divide in an antigen-dependent manner (i.e., upon further interaction with APCs) up to n additional times.
-
Effector cells that divided the maximum number of times stop dividing.
The cell division-based program is formulated as a system of DDEs as follows:(1) (2)
Here, A0 is the concentration of APCs at the site of infection, and A1 is the concentration of APCs that have matured, started to present target antigen, and migrated to the lymph node. Also, T0 is the concentration of naïve T cells in the lymph node, and Ti+1 is the concentration of effector cells that undergone i antigen-dependent divisions after the minimal developmental program. We measure concentration in units of k/μL, i.e., thousands of cells per microliter (of lymphatic fluid).
The first equation in (1) pertains to APCs waiting at the site of infection. These cells are supplied at a constant rate, sA, and die at a proportional rate, d0. Thus, without stimulation, the population remains at its equilibrium level, sA/d0. The time-dependent coefficient a(t) denotes the rate of stimulation of APCs as a function of time. Although we do not explicitly model the antigen-generation process, the function a(t) can be seen as being proportional to the antigen concentration at the site of infection.
The second equation in (1) pertains to APCs that have matured, started to present relevant antigen, and migrated to the lymph node. The model accounts for the maturation, presentation of antigen, and migration of APCs as one collective event. APCs that only undergo one or two, but not all three processes, are not pertinent to the dynamics of the model since they cannot stimulate relevant T cells. The first term of the equation corresponds to the rate at which these APCs enter the lymph node. The second term is the natural death rate of this population.
The first equation in (2) pertains to naïve T cells. The population is replenished at a constant rate, sT, and dies at a proportional rate, δ0. Without stimulation, the population remains at its equilibrium level, sT/δ0. The third term in this equation is the rate of stimulation of naïve T cells by mature APCs. The bilinear form of this term follows the law of mass action where k is the proportionality constant (or kinetic coefficient).
The second equation in (2) pertains to newly differentiated effector cells that have just finished the minimal developmental program of m divisions. The first term gives the rate at which activated naïve T cells enter the first effector state, T1. This term corresponds to the final term of the previous equation for , except that it has an additional coefficient of 2m and it depends on cell concentrations at time t − σ. The coefficient 2m accounts for the increase in population of naïve T cells after m divisions, and the time delay, σ, is the duration of the minimal developmental program. This term accounts for newly proliferated effector cells that appear in the T1 population σ time units after activation from T0. The second term is the rate at which T1 cells are stimulated by mature APCs for further division. It is based on the law of mass action and is thus of the same form as the final term of the equation for . This term exists in the equation only if the number of possible antigen-dependent divisions, n is not 0. Finally, as shown by the last term, T1 cells continuously die at rate δ1.
For i = 2, …, n, the equations for are analogous to the equation for , except that these cells only divide once after stimulation. Hence, the coefficient of the first term is 2, and the time delay is ρ, which is the duration of a single division. As before, the second term is the rate at which these cells become stimulated for further division, and the final term is the death rate. Note that we use the same death rate, δ1, for all effector cells.
The final equation in (2) pertains to cells that have undergone the maximum number of possible antigen-dependent divisions. These cells do not divide anymore and can only die at rate δ1. We note that in this model we assume that the system begins with APCs that are waiting in the tissue and naïve T cells that are waiting in the lymph node. The model only considers activation of naïve T cells, because memory cells interact differently with APCs (see Belz et al., 2007).
3.1.1. Parameter estimates
The parameter estimates in this section are summarized in Table 1. These estimates represent base values, but we experiment with various parameter ranges in the simulations.
Table 1.
Cell division-based model’s parameters. Concentrations are in units of k/μL, and time is measured in days
| Parameter | Description | Estimate |
|---|---|---|
| A0(0) | Initial concentration of immature APCs | sA/d0 = 10 |
| T0(0) | Initial concentration of naïve T cells | sT/δ0 = 0.04 |
| sA | Supply rate of immature APCs | 0.3 |
| sT | Supply rate of naïve T cells | 0.0012 |
| d0 | Death/turnover rate of immature APCs | 0.03 |
| δ0 | Death/turnover rate of naïve T cells | 0.03 |
| d1 | Death/turnover rate of mature APCs | 0.8 |
| δ1 | Death/turnover rate of effector T cells | 0.4 |
| k | Kinetic coefficient | 20 |
| m | Number of divisions in minimal developmental program | 7 |
| n | Maximum number of antigen-dependent divisions | 3, 10, 16 |
| ρ | Duration of one T cell division | 1/3 |
| σ | Duration of minimal developmental program | 3 |
| a(t) | Rate of APC stimulation | c1[0,b] or (3) |
| b | Duration of antigen availability | 10 |
| c | Level of APC stimulation | 1 |
The initial cell concentrations are obtained from a study by Catron et al. in which they simulated a hypothetical, spherical, skin-draining lymph node of radius 1 mm (Catron et al., 2004). In their paper, they considered a slice of about 1/500 of the total volume and estimated that the slice contains about 1600 T cells and 100 dendritic cells (DCs) (Catron et al., 2004). Such a slice would have a volume of (4π/3)(0.1 cm)3/500 = 8.4 × 10−3 μL, yielding T cell and DC concentrations of approximately 200k/μL and 10k/μL, respectively.
We would like to estimate the subset of T cells in the lymph node that are reactive to a particular antigen. Using the estimate from Catron et al. (2004) that about 1/5,000 naïve T cells are LCMV-specific, we estimate that the concentration of antigen-specific naïve T cells in the lymph node is (200/5000)k/μL = 0.04k/μL. An alternative estimate is provided in Blattman et al. (2002), which states that approximately 1/100,000 naïve T cells are LCMV-specific. This discrepancy might result because more than one clone is responding causing different papers to estimate diverse precursor frequencies. However, in our paper, we are not concerned with a precise estimate for this value, and in our parameter sensitivity analyses, we vary the naïve T cell concentrations over a 10,000-fold range, a range which includes both estimates.
Since we are setting initial conditions for DDEs, we are interested in the history of cell concentrations before time 0, specifically on the time interval [−σ, 0]. We make the assumption that the system was at steady state before an infection and write T0(t) = 0.04 for t ≤ 0.
Since DCs are the primary APCs that stimulate T cells (Janeway et al., 2005, p. 319), we assume that our estimate of the DC concentration is also a good estimate of the APC concentration. We do not know how many APCs reside in a tissue that drains into a particular lymph node, but we assume that it is of the same order of magnitude as the number of APCs in the lymph node. Hence, we estimate that the initial concentration of APCs at the site of infection before time 0 is 10k/μL, i.e., A0(t) = 10 for t ≤ 0. We assume that all other cell concentrations start at 0.
Next, we estimate the death and supply rates of naïve T cells and immature APCs. Since we are dealing with a closed system, we recognize that cells may leave the system due to random circulation or emigration. For convenience, we incorporate these cases into the death rates. T cells are supplied at a rate of approximately 3% per day at steady state (Mohri et al., 2001), so it follows that the death rate is also around 3% per day. Proliferation is less significant at about 0.3% to 0.4% per day (Mohri et al., 2001). This gives a death rate, δ0, of −log(100% − 3%) = 0.03/day and a steady state supply rate, sT, of δ0T0(0) = 0.0012k/μL day−1 for naïve T cells. Not having explicit references for the turnover rates of immature APCs in tissue, we assume they are similar to those of naïve T cells, so we set d0 = 0.03 and sA = d0A0(0) = 0.3k/μL day−1.
The half-life during T cell contraction is 41 hours, so we estimate an effector death rate of δ1 = (ln 2)/41 h−1 ≈ 0.4 day−1 (Boer et al., 2003). Furthermore, the level of antigen presentation following the third day after infection decays with a half-life of around 19 h and 20.4 h (Belz et al., 2007). Hence, using a half-life of 20 h, we obtain a mature APC death rate of d1 = (ln 2)/20 h−1 ≈ 0.8 day−1. We note that these APCs might not actually be dying. Instead, they might be turning over surface molecules and discarding old antigen in the process, but for our purposes, these APCs can be considered dead.
To calculate the kinetic coefficient, k, we use the estimate that in the lymph node slice, one T cell and one DC will have 0.20 ± 0.06 interactions per hour, or 4.8 ± 1.4 interactions per day. Assuming that DCs represent the majority of APCs that stimulate T cells, we obtain an estimate of the kinetic coefficient k0 = 4.8 cell−1 day−1 (Catron et al., 2004). Recalling that the lymph node slice has a volume of 8.4 × 10−3 μL, we obtain the unit conversion
It is unlikely that every antigen-specific T cell-APC interaction leads to T cell stimulation, so we set the probability of stimulation as p = 0.5, and use the estimate k = pk0 = 20 (k/μL)−1 day−1 for the kinetic coefficient.
For the minimal developmental program, we estimate that each T cell initially undergoes m = 7 divisions (Kaech and Ahmed, 2001). Following the minimal developmental program, T cells may undergo up to n additional antigen-dependent divisions. We consider the cases when n = 3 (maximal 1,000-fold expansion), n = 10 (maximal 100,000-fold expansion), and n = 16 (maximal 10 million-fold expansion). The first estimate, n = 3, corresponds to a program that permits between 7–10 divisions (Wodarz and Thomsen, 2005). The second estimate, n = 10, corresponds to a program that allows the T cell population to expand up to five orders of magnitude (Boer et al., 2001). The third estimate, n = 16, corresponds to a program that allows T cells to undergo as many as 23 divisions, which is the maximum number of divisions human T cells can undergo in vitro before reaching senescence, a state of growth arrest (Effros and Pawelec, 1997).
For the time delays, the duration of one division is between 6 to 12 hours (i.e., 2 to 4 times per day) (Janeway et al., 2005, p. 19). In addition, the T cell population doubles approximately every 8 hours during expansion (Boer et al., 2003). We use the intermediate value of ρ = 8 hours, or 1/3 day. The minimal developmental program consists of m divisions, but the first division does not occur until 24 hours after stimulation (Stipdonk et al., 2001; Veiga-Fernandes et al., 2000). Hence, we set the duration of the minimal developmental program to be σ = 1+(m−1) ρ to account for the fact that the first division takes one day while subsequent divisions take ρ days. Using the estimates ρ = 1/3 and m = 7, we obtain σ = 3 days.
For the antigen function a(t), we assume that it starts at 0, stays positive for some time, and eventually returns to 0. For simplicity, we start by setting a(t) = c1[0,b] where b, c > 0, a choice a(t) that is convenient in spite of the discontinuities. Later, when we move to numerical simulations, we switch to a continuous function with support on (0, b). To generate a smooth version of a(t), we let
and set
| (3) |
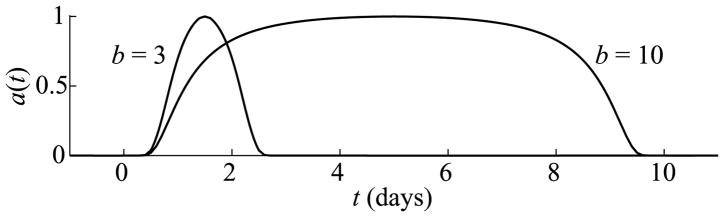
where b, c > 0. The variable t is defined such that mature APCs begin appearing in the lymph node at t = 0, although the infection may have begun slightly earlier. According to Belz et al. (2007), during a primary response, antigen-presentation begins in the lymph node about 1 day after infection and persists up to 12 days after infection, which is 1–2 days after viral clearance from the site of infection. Hence, we estimate that the duration of antigen availability, b, is about 10 days. Not having a good estimate for the level of APC stimulation, we typically set c = 1, which means that about 60% of available APCs mature per day at the peak of antigen availability. (See Fig. 2 for graphs of the smooth versions of a(t) for b = 3 and b = 10 when c = 1.)
Fig. 2.
Graphs of the antigen function a(t) given by (3) for b = 3 and b = 10 when c = 1. The function a(t) represents the time-dependent rate that immature APCs pick up antigen and are stimulated.
All parameter estimates are summarized in Table 1. We use these estimates as base values, but experiment with various parameter ranges in the simulations.
3.2. Time-based program
In the time-based proliferation program, effector cells live τ units of time before committing a programmed cell death. During their life span, they can undergo division cycles. In the time-based program, apoptosis is triggered by a “death” clock and not by a sequence of events. We model this process in four steps. The first two steps are identical to those of the cell division program.
APCs mature, present relevant target antigen, and migrate from the site of infection to the draining lymph node.
In the lymph node, APCs activate naïve T cells that enter a minimal developmental program of m cell divisions.
T cells that have completed the minimal developmental program become effector cells that can divide in an antigen-dependent manner (i.e., upon further interaction with APCs).
Effector cells that have lived τ units of time commit apoptosis.
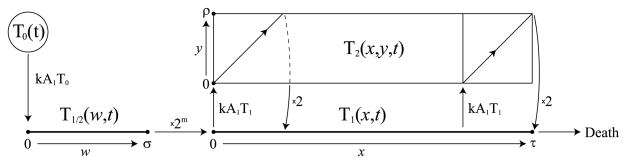
The time-based program is formulated as the following system of PDEs (illustrated in Fig. 3):
| (4) |
and
Fig. 3.
The time-division based program (5)–(6). APCs and naïve cells are modeled in the same way as in the cell division-based program. (1) Naïve T cells, T0, are stimulated by mature APCs and enter the pool of cells undergoing a minimal developmental program, denoted by T1/2. (2) After σ units of time (the duration of the minimal program) cells enter the effector state, T1, after having divided m times. (3) Cells live in the effector state for τ units of time before committing apoptosis. (4) As an ongoing step, effector cells repeatedly get stimulated by mature APCs and enter the dividing population, T2, where they spend ρ units of time (the duration of one cell division) before returning to the effector state after having divided once.
| (5) |
Here, A0, A1, and T0 are concentrations of immature APCs, mature APCs, and naïve T cells, T1/2(w, t) is the population density of newly activated T cells that have spent w units of time in the minimal developmental program, T1(x, t) is the population density of effector cells that have lived for x units of time as effectors, and T2(x, y, t) is the population density of dividing effector cells that have lived as effectors for x units of time and are at time y of the division process. The boundary conditions for (5) are
| (6) |
The variables T1/2(w, t), T1(x, t), and T2(x, y, t) are defined on the intervals w ∈ [0, σ], x ∈ [0, τ], and (x, y) ∈ [0, τ] × [0, ρ], respectively.
The first three equations, (4), are identical to those of the cell division-based program. (Compare with Eqs. (1)–(2) in Section 3.1.)
The first equation in (5) pertains to newly activated T cells, T1/2(w, t), that are undergoing the minimal developmental program before becoming full effectors. The state variable w ∈ [0, σ] denotes the cell’s position in the minimal developmental program. The boundary condition for T1/2(0, t) in (6) is the rate at which naïve T cells become stimulated by mature APCs.
The second equation in (5) pertains to effector cells, T1(x, t). The state variable x ∈ [0, τ] denotes the amount of time a cell has spent in the effector state. The first term on the right-hand side of the equation is the rate of stimulation of effector cells for further division. These stimulated effector cells exit the effector state and enter the dividing state for ρ units of time to complete a division cycle. This term is multiplied by an indicator function, because cells that begin dividing after x = τ − ρ will not finish the division process before the apoptosis clock expires at x = τ. To keep numerics simple, we do not allow such cells to enter the dividing state. The boundary condition for T1(0, t) in (6) is the rate that newly activated T cells complete the minimal developmental program and reenter the system as effectors after having expanded 2m-fold.
The final equation in (5) pertains to dividing effector cells, T2(x, y, t). As before, the state variable x ∈ [0, τ] denotes the amount of time a cell has spent in the effector state, and y ∈ [0, ρ] denotes the cell’s position in the division cycle. The boundary condition for T2(x, 0, t) in (6) is the rate that effector cells get stimulated for further division. The boundary condition for T2(0, y, t) is set to 0, because cells that have just entered the effector state have not yet completed part of the division process.
All parameters in this model are identical to those listed in Table 1, except for τ, which is new to this model. To estimate τ, we note that Yang et al. (1998) reports two increases in the apoptosis rate over the course of an immune response: the first peak occurs at day 3 and the second at day 11. The first peak corresponds to the time most cells finish the minimal developmental program, and the second peak corresponds to the time most cells begin apoptosis at the end of the T cell response. Hence, we obtain the estimate τ = 11 − 3 = 8. Also, in Boer et al. (2003), it is estimated that there is a T cell peak on day 8 after infection, which approximately corresponds to day 7 after antigen appears in the lymph node. Using our estimate that most cells complete the minimal developmental program on day 4, we obtain the estimate τ = 7 − 4 = 3. We use both estimates in our simulations.
3.3. Adaptive regulatory T cell model
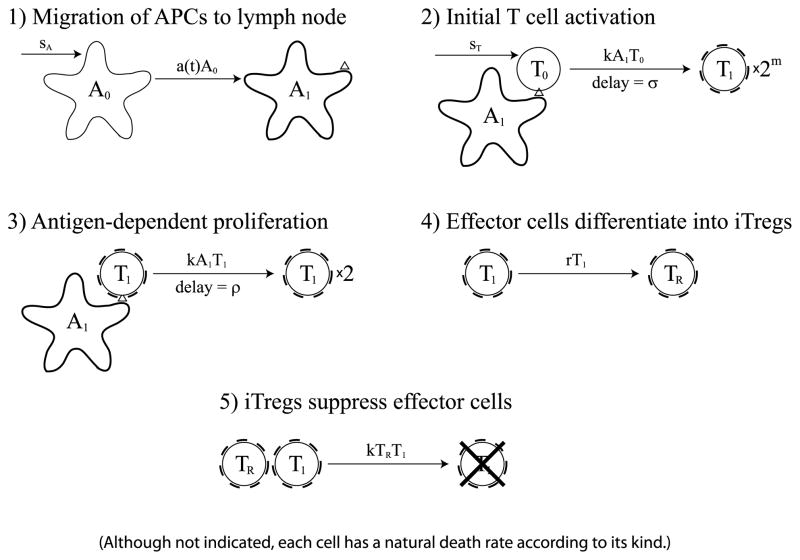
In this model, we propose that iTregs generated from responding effector cells induce a timely contraction of the immune response. In the iTreg model, T cell responses begin in the same way as in the previous two models that were based on developmental programs. Accordingly, newly activated effectors follow the same minimal developmental program. However, contraction initiates differently, since it is mediated by suppression via iTregs and not by a preprogrammed number of divisions or a given life time. We model this process in five steps (illustrated in Fig. 4):
Fig. 4.
Diagram of the iTreg model. The first three steps are identical to those in the cell division-based model that is shown in Fig. 1. In the fourth step, effector cells differentiate into iTregs at rate r. In the fifth step, iTregs suppress effector cells. Although not indicated, each cell in the diagram has a natural death rate according to its kind.
APCs mature, present relevant target antigen, and migrate from the site of infection to the draining lymph node.
In the lymph node, APCs activate naïve T cells that enter a minimal developmental program of m cell divisions.
T cells that have completed the minimal developmental program become effector cells that keep dividing in an antigen-dependent manner as long as they are not suppressed by iTregs.
Effector cells differentiate into iTregs at a constant rate.
The iTregs suppress effector cells upon interaction.
Both CD4+ and CD8+ iTregs differentiate from nonregulatory T cells during a primary response (Cantor et al., 1976; Walker et al., 2005). Although current evidence is unclear about the role of iTregs in maintaining long-term self-tolerance (Sakaguchi et al., 2008), we propose the alternative role that iTregs limit the expansion and duration of a T cell response. It has been hypothesized that iTregs may differentiate from naïve or memory T cell populations (Walker et al., 2005). However, current evidence does not preclude the possibility that iTregs could differentiate from effector cells. Indeed, the iTreg state may represent a further level of effector differentiation, much like the memory state. In this case, a fraction effector cells would differentiate continually into iTregs rather than memory cells. This possibility is supported by the observation that iTregs possess some characteristics of memory cells, such as shortened telomere length (Walker et al., 2005). Nonetheless, the feedback mechanism in this model applies as long as effector cells induce the production of iTregs, even if not by direct differentiation. For example, effectors cells may recruit iTregs from some subset of the T cell population, such as the memory pool, and thus effectively replace themselves with cells having suppressive capability.
The model is formulated as the following system of DDEs:
| (7) |
Here, A0 is the concentration of APCs at the site of infection, A1 is the concentration of APCs that have matured, started to present target antigen, and migrated to the lymph node, T0 is the concentration of naïve T cells in the lymph node, T1 is the concentration of effector cells, and TR is the concentration of iTregs.
The first three equations for APCs and naïve T cells are identical to those in the models from Sections 3.1 and 3.2. The first two terms of the equation for are identical to the first two terms of the corresponding equation in (2) for the cell division based-program. The third term in this equation is the rate that cells that have just finished dividing reenter the effector cell population. Since we are not counting the number of divisions a cell has undertaken, we do not need to have more than one variable for the effector cell population. The fourth term is the rate that effector cells exit the population through death at rate δ1 or differentiation into iTregs at rate r. The final term is the rate that effector cells are suppressed by iTregs. We assume that the rate of iTreg-effector interactions follow the same mass action law as APC-T cell interactions. Note that there is no limit on the number of effector cell divisions or on the duration of the proliferation. The only regulatory mechanism is suppression by iTregs.
The final equation in (7) pertains to iTregs. The first term is the rate at which effector cells differentiate into iTregs, and the second term is the rate at which iTregs die. We assume that iTregs have the same death rate as effector cells.
Although we assume iTregs cause effector cells to “die,” that is, to exit the system, we recognize that in reality, effector cells might not die from interacting with an iTreg. Instead, some cells might turn into memory cells and others might even migrate away from the lymph node and carry out effector functions in the periphery before dying as suggested in Kim et al. (2007). For the purposes of the model, however, suppressed cells are irrelevant to the dynamics, so we consider them as dead.
4. Results
4.1. Cell division-based program
Since the equations in (1) are relatively simple, we can solve them using integrating factors to obtain
| (8) |
and
| (9) |
Note that we are using the equilibrium relation A0(0) = sA/d0. Also, to make the derivation more valid, we should define a continuous function aε (t) that is 0 for t ≤ 0 and t ≥ b, is c for t ∈ [1 + ε, 10 − ε], and is piecewise linear in between. Then we can consider aε (t) for small ε > 0 rather than using a(t). For brevity, we omit these details.
Equations (8)–(9) imply that the concentrations of APCs over time are proportional to the initial/equilibrium concentration of immature APCs. The dependence of the mature APCs, A1(t), on c is more complicated, but there is, more or less, a saturating dependence of the Michaelis–Menten form. The leading coefficient, c/(d0 + c), has a very small Michaelis constant of d0 = 0.03, so it saturates very quickly. On the other hand, the coefficient, c/(d0 − d1 + c), of the second term of the expression has a negative “Michaelis constant” of about −0.8, which means that it saturates for c much greater than 0.8 and has a sign change around 0.8. Note that the second term has a well-defined limit even if the denominator d0 − d1 + c = 0, because of the exponential factor. The dependence on b is also complicated, but because the top expression of (9) decays quickly, there is also a saturating dependence, i.e., very long b’s do not add much to APC dynamics, at least for an acute stimulation. For turnover rates, we assume that the turnover, d0, of immature APCs is low, and hence insignificant, since this rate is dominated by other parameters. The turnover, d1, of mature APCs mostly affects the rate of decay of the mature APC concentration after the peak of antigen-presentation. All together, these parameters influence the dynamics of mature APC concentrations, which in turn directly affect the stimulation levels of T cells.
Due to the sequential nature of the cell division-based program, we can find explicit expressions for all variables. However, because of the complexity of these expressions, and to keep our investigation fruitful, we switch to numerical simulations using the DDE solver “dde23” in Matlab. At this point, we also switch to the continuous version of a(t) given by (3).
Before proceeding to numerical simulations, we observe that the T cell dynamics given by (2) scales with respect to the precursor concentration, T0(0). We can see this by nondimensionalizing the T cell populations by T0(0). Hence, from now on, we consider the relative T cell expansion level, Ttotal(t)/T0(0), rather than the total T cell population, Ttotal(t), which is given by
Note that (2) and Fig. 1 imply that stimulated T cells leave the system during the division process and return ρ time units later. Hence, the total T cell concentration is not only the sum of T cell populations given by Ti (t), but also the populations that are undergoing division, which are given by the integrals in the above expression.
To characterize T cell dynamics, we measure the maximum level of the relative expansion and the time of peak. As mentioned above, due to scaling, the relative expansion and the time of peak are independent of the precursor concentration, so we do not need to vary this parameter.
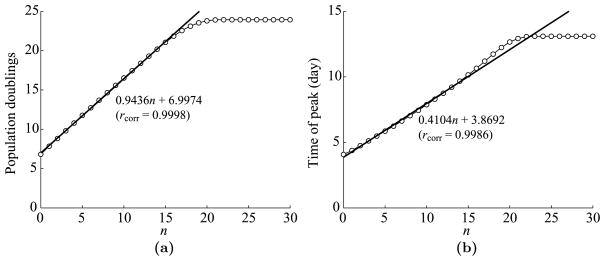
First, we fix m = 7 and examine the dependence of T cell dynamics on the maximum number, n, of antigen-dependent cell divisions after the minimal developmental program. We use the parameters listed in Table 1 except that we vary n from 0 to 30. See Fig. 5 for plots of the maximum expansion level and the time of peak versus n. We measure the expansion level in terms of population doublings, which are given by log2(max(Ttotal/T0(0))).
Fig. 5.
Dependence of T cell dynamics on n, the maximum number of antigen-dependent divisions after the minimal program. (a) Maximum T cell expansion level versus n. The expansion level is measured in population doublings, which is defined by log2(max(Ttotal/T0(0))). The regression coefficient is fit to the first 17 points, i.e., n = 0, …, 16. The linear correlation rcorr = 0.9998. (b) The time of T cell peak versus n. The linear regression is fit to the first 17 points. The linear correlation rcorr = 0.9986.
As apparent from Fig. 5(a), each increase in n roughly leads to one more population doubling until the curve starts to saturate at around n = 17 or 18. From this point, the T cell population does not double much more than 24 times, no matter how many additional antigen-dependent divisions are permitted. We fit a linear regression to the first 17 points, which correspond to n = 0 to 16. Note that n = 16 is the highest estimate for n in Table 1, so the relation is almost linear for reasonable values of n. Furthermore, the slope of the line is 0.9436, meaning that after each increase in n, about 92% of the responding effector cells undergo one additional division. (We obtain this estimate because 20.9436 − 1 = 92%.) In addition, the y-intercept is 6.9974, meaning that about 99.8% of naïve T cells respond to the stimulus and enter the minimal developmental program of 7 divisions. This estimate agrees very closely with the number obtained from the numerical solution of T0(t).
In Fig. 5(b), we see that the dependence of the time of peak on n is also almost linear, at least for n between 0 and 16. As in Fig. 5(a), the linear regression is fit to the first 17 points. The slope of 0.4104 indicates that effector cells continue expanding for about 0.4104 days longer for each increase in n. This extension in expansion time is slightly longer than the duration ρ = 1/3 day of one division. Furthermore, the time of peak at n = 0 is 4.0773 days, which is close to the y-intercept 3.8692 of the linear fit. Hence, most cells complete the minimal developmental program around 4 days after the beginning of antigen-presentation in the lymph node, which means that most naïve T cells take about 1 day for stimulation by APCs followed by σ = 3 days to complete the minimal developmental program. This result is in agreement with (Mercado et al., 2000), which states that nearly all potentially-reactive T cells are stimulated within 24 hours of infection.
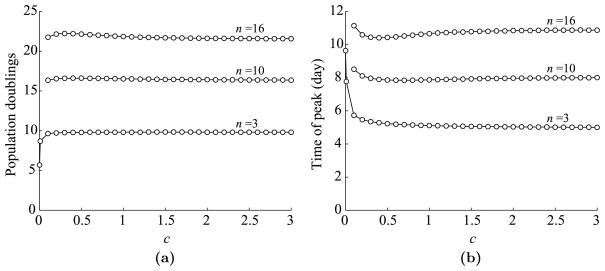
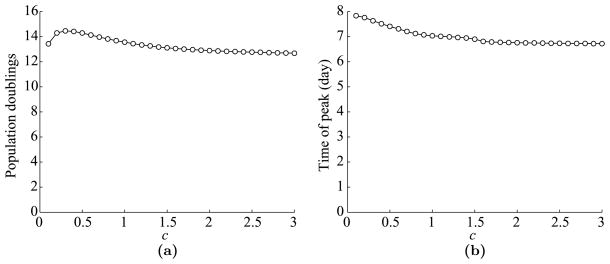
Next, we look at the dependence of T cell dynamics on c, the parameter corresponding to the level of antigen-presentation in a(t). Again, we use the parameters listed in Table 1 and vary c from 0.1 to 3. The maximum expansion level and the time of peak versus c are plotted in Fig. 6. We use all three values (3, 10, and 16) of n and draw three curves in each plot.
Fig. 6.
Dependence of T cell dynamics on c, a parameter corresponding to the level of antigen presentation. (a) Maximum T cell expansion level versus c. Expansion level is measured in population doublings, which is defined by log2(max(Ttotal/T0(0))). Data is shown for three possible values of n: 3, 10, and 16. (b) The time of T cell peak versus c.
As we can see in Fig. 6(a) and (b), T cell dynamics saturate very quickly in relation to c, so much so that the period doubling level is almost constant from as low as c = 0.1 to as high as c = 3. It might seem slightly peculiar that the peak expansion increases slightly for low values of c and then decreases again for high values. This happens because for high c values, almost all available APCs are recruited shortly after infection, vacating the pool of immature APCs at the site of infection. Thus, once the mature APCs arrive in the lymph node, they die at rate d1 = 0.8/day and are not quickly replenished. By continuity, the size of the T cell peak must go to 0 as c decreases, but the drop is very steep. The two extra points shown in the curves for n = 3 correspond to c = 0.001 and c = 0.01. These values correspond to roughly 0.1% and 1% of APCs getting stimulated per day. Hence, even very low stimulation levels result in nearly saturated T cell dynamics.
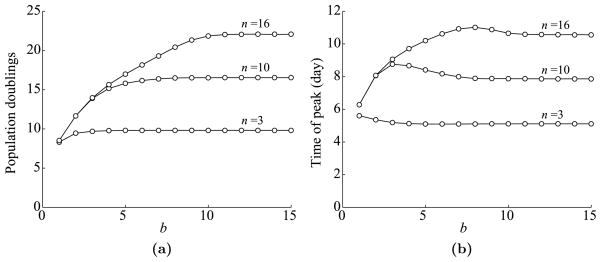
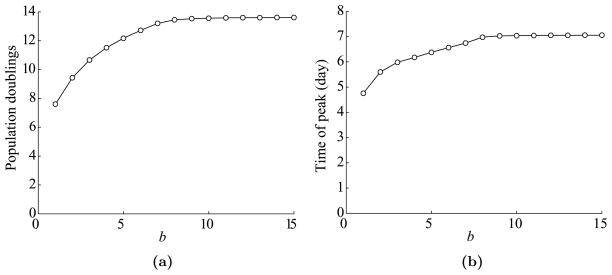
The next issue we explore is the dependence of T cell dynamics on the duration of antigen availability at the site of infection, b. Using the parameters listed in Table 1, we vary b from 1 to 15 days. The plots of the maximum expansion level and the time of peak versus b are shown in Fig. 7.
Fig. 7.
Dependence of T cell dynamics on b, the duration of antigen availability at the site of infection. (a) Maximum T cell expansion level versus b. Expansion level is measured in population doublings, which is defined by log2(max(Ttotal/T0(0))). Data is shown for three possible values of n: 3, 10, and 16. (b) The time of T cell peak versus b.
This figure shows that as before T cell dynamics eventually saturate as b increases, but not as quickly as for c. Hence, the duration of antigen availability is more important than the antigen level. These results make sense, because if an antigen is available longer, effector cells will continue to divide for longer in an antigen-dependent manner.
Although we use three estimates for the maximal length of antigen-dependent proliferation, the true number probably lies between n = 3 and n = 10 (1,000-fold and 100,000-fold expansion) rather than at the high end. The large estimate of n = 16 (10 million-fold expansion) comes from observations in vitro (Effros and Pawelec, 1997), but does not seem typical of T cell expansion in vivo (Boer et al., 2001). Thus, we will focus on the behavior of the bottom two curves in Fig. 7(a) and not on the top one.
The results shown in Fig. 5(b) implied that the minimal time to completion of the minimal developmental program is around 4 days. Hence, even if effector cells were able to complete dividing, emigrate to the site of infection, and decimate infected cells immediately, antigen would still be presented in the lymph node for at least 4 days while T cells undergo the initial activation process. A minimal activation period for a primary T cell response is a fairly standard concept. Indeed, Belz et al. report from their study with influenza virus that cytotoxic activity was not detectable in mice until 5 days after the primary infection (Belz et al., 2007). These results are in a striking agreement with the results from the mathematical model, since Belz et al. count the number of days after infection, while in their experiments antigen does not appear in the lymph node until day 1 (Belz et al., 2007). On the other hand, we define time 0 as the time APCs begin arriving in the lymph node, so there is approximately a one day difference in the definitions of the initial time. The time discrepancy between antigen availability at the site of infection and presentation in the lymph node might be due to the travel time of APCs from the tissue to the draining lymph node. Catron et al. estimate that after a dendritic cell matures it takes about 18 hours for it to detach and appear in the lymph node (Catron et al., 2004).
Concerning the duration of antigen availability, Belz et al. observe that ablation of antigen presentation on day 3 severely inhibited the expansion of the primary T cell response, whereas antigen ablation from day 5 onward had no significant effect (Belz et al., 2007). These observations also coincide well with the mathematical modeling results shown in Fig. 7(a), since for n = 3 and n = 10, T cell expansion levels start to saturate around b = 4 days.
These numbers show that the results pertaining to the T cell proliferation program from Kaech and Ahmed (2001), Mercado et al. (2000), and Stipdonk et al. (2001) agree with independent experiments on antigen presentation from Belz et al. (2007). The turning point of 5 days after infection (t = 4 days in our model) when the T cell response begins to approach a maximal saturation level is probably strongly related to the time required for most cells to complete the minimal developmental program. It seems that stimulating newly activated T cells even briefly after the minimal developmental program is enough to drive them to nearly full potential expansion.
In most cases involving a primary T cell response, it is likely that b will be high enough to saturate T cell expansion, which probably explains why most T cell responses in vivo seem to follow a fixed program. On the other hand, if the immune response eliminates pathogens within 5 days after infection, clearance was probably not mediated by the primary T cell response anyway.
In an unexpected yet interesting way, the findings in this section agree with the model of Antia et al. (2003), although for different reasons. Recall that in Antia et al. (2003), newly activated T cells undergo antigen-dependent proliferation for up to 2.5 days after infection to tune the resulting T cell response. The results from Belz et al. (2007) and the simulation data shown in Fig. 7 also indicate that antigen availability during the 3–4 days following infection is critical to determining the size of the resulting response. In this mathematical model, there is no antigen-dependent expansion prior to the minimal developmental program. Instead, the critical period of 3 or so days after infection represents the time during which most T cells are undergoing an initial activation. The presence of antigen during this period affects whether T cells will complete the minimal number of divisions and stop or whether they will further divide before contracting.
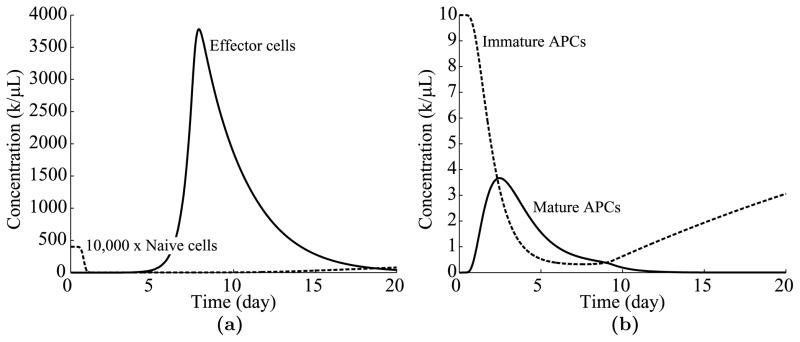
To close the discussion of the cell division-based program, Fig. 8 shows the time evolution of various cell populations when n = 10 and the rest of the parameters are taken from Table 1.
Fig. 8.
Time evolution of immune cell populations over time. (a) The dynamics of naïve and effector cells over 20 days. (b) The dynamics of immature and mature APCs.
Notice from Fig. 8(a) that nearly all available naïve T cells are recruited within a day of antigen presentation, a result corroborated by the experimental data of Mercado et al. (2000). In addition, the effector cell population peaks at day 7.87, near the estimate of Boer et al. (2003) that the T cell peak occurs around day 8 after infection. Also, notice from Fig. 8(b) that the mature APC concentration peaks at day 2.5, which is close to the experimental measurement of a peak at day 2.2 in Belz et al. (2007).1 These results demonstrate a strong correspondence between the mathematical model and the experimental data.
4.2. Time-based program
As in the case of the cell division-based program, we can see by nondimensionalizing the populations that T cell dynamics scale with respect to the precursor concentration, T0(0). We thus consider the relative T cell expansion level, Ttotal/T0(0), where
This expression is obtained by integrating over the two population densities, T1(x, t) and T2(x, y, t), for effector cells.
As before, we characterize T cell dynamics based on the maximum expansion level and on the peak time. First, we fix the parameters listed in Table 1 and vary τ from 1/3 to 8 days. See Fig. 9 for plots of the expansion level and the peak time versus the lifespan, τ, of effector cells.
Fig. 9.
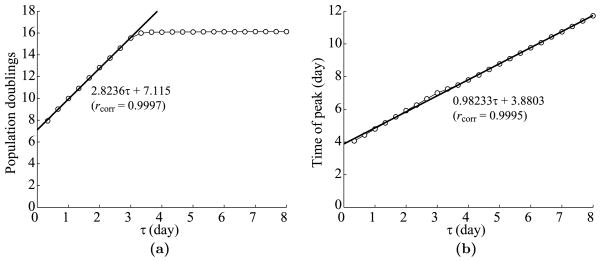
Dependence of T cell dynamics on the effector lifespan, τ. (a) Maximum T cell expansion level versus τ. The linear regression is fit to the first 9 points, i.e., τ = 1/3, 2/3, …, 3. The linear correlation rcorr = 0.9997. (b) The time of T cell peak versus τ. There is no saturating phenomenon, so the linear regression is fit to all 24 points. The linear correlation rcorr = 0.9995.
Analogously to the cell division-based program, each increase in τ by 1 leads to approximately 3 more populations doublings, since each cell division takes 1/3 of a day. The estimated y-intercept of about 7 corresponds to the number of divisions in the minimal developmental program. Also, as in the cell division-based program, the increase in expansion levels saturates, in this case around τ = 3 days. See Fig. 9(a). In contrast to the cell division-based program, the time of the T cell peak depends linearly on τ without saturating. This linear dependence happens because effector cells live τ units of time after completing the minimal developmental program regardless of whether they are dividing or not. The slope of the linear regression is about 1, because one added day of T cell life postpones the beginning of contraction by one day. In addition, the y-intercept of about 3.9 days corresponds to the duration of the minimal developmental program. See Fig. 9(b).
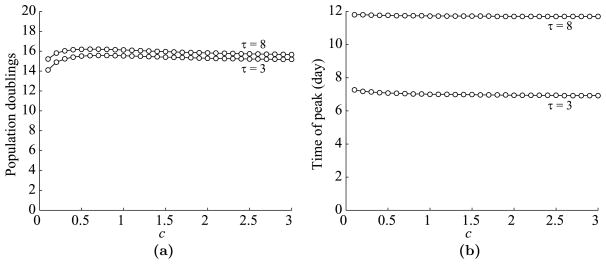
Next, we look at the dependence of the T cell dynamics on c, the parameter corresponding the level of antigen presentation in a(t). See Fig. 10 for plots of the maximum expansion level and the time of peak versus c. We plot curves for τ = 3 and τ = 8 days.
Fig. 10.
Dependence of T cell dynamics on c, a parameter corresponding to the level of antigen presentation. (a) Maximum T cell expansion level versus c. Data is shown for τ = 3 and 8 days. (b) The time of T cell peak versus c.
As in the cell division-based program, T cell dynamics saturate quickly with respect to c, so much so that the expansion level is almost constant from as low as c = 0.1. See Fig. 10(a). The time of peak is almost independent of c and depends far more directly on τ as discussed in the preceding paragraph. See Fig. 10(b). Next, we look at the dependence of T cell dynamics on b, the duration of antigen availability at the site of infection. We vary b from 1 to 15 days. See Fig. 11 for plots of the maximum expansion level and the time of peak versus b.
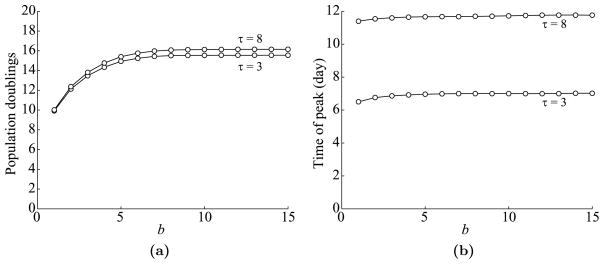
Fig. 11.
Dependence of T cell dynamics on b, the duration of antigen availability at the site of infection. (a) Maximum T cell expansion level versus b. Data is shown for τ = 3 and 8 days. (b) The time of T cell peak versus b.
As in the cell division-based program, T cell dynamics saturates as b increases, although not as quickly as for c. Indeed, as before, the expansion levels start saturating around b = 4. See Fig. 11(a). Again, the time of peak is almost independent of b and depends more directly on τ. See Fig. 11(b).
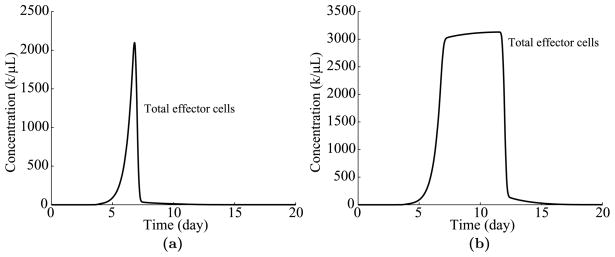
To close the discussion of the time-based program, Fig. 12 shows graphs of the time evolution of T cell populations for τ = 3 and τ = 8, where the remaining parameters are taken from Table 1. As shown in Fig. 12, the T cell response ramps up in much the same way as in the cell division-based program 8(a). However, in the time-based program, effector cells linger until the end of their lifespan, τ, even if they are not dividing. Once the lifespan of most effectors is reached at approximately τ = τ + 4 (see the linear regression in Fig. 9(b)), the response contracts rapidly.
Fig. 12.
Time evolution of T cell populations over time. (a) The dynamics of effector cells for τ = 3 days. (b) Dynamics for τ = 8 days.
We note that the dramatic drops observed in Fig. 12 do not reflect the more gradual contractions observed in real life. One likely cause of this discrepancy is that the lifespans of effector cells are probably not all the same, but are distributed over a wide range of values. We could incorporate this variability to obtain a more accurate model, but as will be discussed in Section 4.3, this adjustment is not important or even relevant for the main point of this paper.
In conclusion, T cell dynamics of the time-based program are similar to those of the cell division-based program, especially for low values of τ. The main difference between both programs is that in the time-based program, effector cells can linger around for longer without dividing when τ is large.
4.3. Is the autonomous program paradigm complete?
The paradigm that T cells follow an autonomous development and differentiation program has one automatic strength. It sets a firm restriction on the duration and expansion of the T cell response, guaranteeing that T cells will not proliferate excessively. But can it guarantee robustness with respect to precursor frequency?
Autonomous programs must scale with respect to the T cell precursor frequency as shown in Sections 3.1 and 3.2. For example, if a lymph node begins with 10 times as many reactive precursors as estimated, it will give rise to a response that is fully 10 times higher. This may be a little excessive, although it will probably still do the job. More significantly, if a lymph node begins with 1/10 or 1/100 times as many precursors, the resulting immune response will scale similarly, a potentially serious shortcoming. Furthermore, the results of Badovinac et al. show that a 10,000-fold difference in precursor frequencies drastically reduces to a 13-fold difference in peak effector levels during a T cell response (Badovinac et al., 2007).
An alternative possible feedback is early antigen elimination. However, as discussed in Section 4.1 and shown in Belz et al. (2007), T cell-mediated clearance does not begin earlier than 5 days after infection, and hence antigen elimination that occurs faster than 5 days probably involves immune agents other than T cells. On the other hand, antigenic stimulation that elicits T cell intervention most likely lingers for at least 5 days, and as seen in Fig. 7, T cell expansion has saturated by then.
In short, the paradigm of cell division-based and time-based programs of T cell expansion and contraction have several strengths: they guarantee a foolproof and timely endings of T cell proliferation, and the saturating phenomena discussed in Sections 3.1 and 3.2 render the response robust with respect to certain key parameters, in particular to the level and the duration of antigen availability during infection. However, one fundamental problem still remains: the dynamics of autonomous programs scale with respect to precursor frequencies.
Before proposing an alternative hypothesis, we put forward two central questions: What internal mechanisms induce an effector cell to die after a certain number of divisions or after a certain amount of time and can these mechanisms be reversed? The answer to the first question is answered by several papers proposing that programmed cell death is induced by Fas-FasL interaction and certain apoptosis genes expressed within the cell (Razvi et al., 1995; Renno et al., 1999; Yang et al., 1998). The answer to the second question has also been well demonstrated, at least in principle. Indeed, T cells can be expanded in vitro well beyond normally observed proliferation levels up to senescence (i.e., the Hayflick Limit) (Effros and Pawelec, 1997). This result implies that there are means of inhibiting apoptosis and allowing T cells to continue dividing. If there were an intrinsic contraction program in each cell, it should also be observable in vitro in the same way as the initial proliferation program. The discrepancy between T cell behavior in vivo and in vitro strongly suggests that they respond to an external signal to induce the contraction process, albeit an external signal that seems to be almost always present during a normal immune response in vivo.
Taking a step back, let us consider what we expect to observe in most T cell responses. A variety of sources (e.g. Boer et al., 2003; Mercado et al., 2000; Yang et al., 1998), report similar magnitudes and timings of T cell peaks under a variety of stimulatory conditions. In addition, T cell dynamics appear to behave robustly with respect to a wide range parameters, presumably including initial naïve T cell concentrations. This highly uniform behavior may look like a “program,” but perhaps it could be instead that a simple feedback network is set up so that T cell dynamics consistently end up entrained into a highly stable and robust attractor, such as a fixed point, regardless of initial conditions.
4.4. The basis for an adaptive regulatory T cell-mediated contraction
Since programmed responses do not seem to be sufficiently robust with respect to precursor frequencies, we consider a simple alternative based on negative feedback. The next model incorporates the notion of adaptive regulatory T cells (iTregs). Like thymus-derived, naturally-occurring regulatory T cells (nTregs), these cells are stimulated in an antigen-specific manner and suppress immune cells in an antigen-nonspecific manner (Taams et al., 2002; Walker et al., 2003, 2005). The main difference between iTregs and nTregs is, however, that iTregs do not emerge from the thymus, but are generated from nonregulatory T cells in the periphery (Taams et al., 2002; Walker et al., 2003, 2005).
Suppression of immune cells by iTregs primarily functions by cell contact rather than by cytokines (Walker et al., 2003). Furthermore, iTregs can appear in a cell culture of originally nonregulatory T cells within 10 days of persistent stimulation (Walker et al., 2005). This period of less than 10 days for iTreg generation implies that iTregs differentiate from effectors during the course of a normal primary immune response, which generally peaks 8 to 9 days after the initial stimulation (Boer et al., 2003). These observations suggest that iTregs may play a key role in a primary T cell response and that suppression by iTregs might even be the central mechanism that induces T cell contraction.
Along this line, the experiments of Haribhai et al. show that conventional and regulatory T cells expand and contract together during a primary immune response (Haribhai et al., 2007). From these results, they propose that regulatory T cells play a key role in regulating the primary immune response. However, they conclude that nTregs, and not iTregs, play the primary role in this regulation. In a similar manner, a mathematical model we published the same year proposed that foreign-reactive nTregs exist and serve to induce a timely contraction of the primary T cell response (Kim et al., 2007). On the other hand, we also commented that robust behavior is highly dependent on the initial conditions. In particular, the system requires a narrow balance between the reactivities of the responding effector and of the regulatory T cells (Kim et al., 2007). Even though initial conditions may be chosen to make the immune response robust, any explanation relying heavily on initial conditions must also describe how these conditions could be set prior to the immunogenic challenge. According to Kim et al. (2007), an nTreg-induced contraction of the primary T cell response is, for this reason, hard to explain.
Several other mathematical models have been developed for naturally occurring regulatory T cells (Burroughs et al., 2006; Carneiro et al., 2005; León et al., 2000, 2001, 2003, 2004, 2007a, 2007b) and adaptive regulatory T cells (Fouchet and Regoes, 2008), but these papers focus on the function of regulatory T cells in inducing and maintaining immune tolerance to certain targets. In this paper, we do not address how regulatory T cells induce tolerance, but in how they govern the dynamics of a primary immune response against infection.
4.5. The adaptive regulatory T cell model
Due to the negative feedback from iTregs in (7), T cell dynamics do not scale with respect to precursor frequencies as in the other two models. Thus, it is informative to look at the total effector cell population, given by
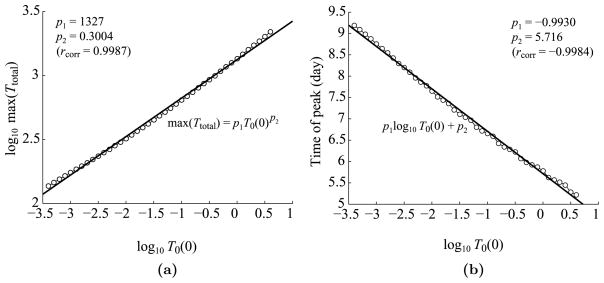
Figure 13 displays log-log plots of the maximum expansion level and time of peak versus the initial naïve T cell concentration, T0(0), which is varied from 4 × 10−4 to 4k/μL, a range 100 times lower and 100 times higher than the estimated value in Table 1. As shown in Fig. 13(a), the simulated data fits a power law of exponent 0.3004, meaning that the expansion roughly scales to the cubed root of the initial naïve cell concentration. In other words, to obtain a T cell response that is 10 times higher (or lower) than normal, the system would need to start with a reactive precursor concentration that is 1,000 times higher (or lower) than normal, an event that probably rarely happens in real life. Hence, the dynamics of the feedback loop automatically compensates for a shortage or abundance of precursors. As shown in Fig. 13(b), the running time of the “program” also increases or decreases based on where the system starts. From these results, we see that the simple feedback between conventional and regulatory T cells provides a more flexible response than a built-in program.
Fig. 13.
Log–log plots of the dependence of T cell dynamics on T0(0), the initial concentration of naïve T cells. (a) Maximum T cell expansion level versus T0(0). The linear regression shows that the maximum expansion level is roughly proportional to T0(0)1/3. The linear correlation rcorr = 0.9987. (b) The time of T cell peak versus T0(0). The linear correlation rcorr = −0.9984.
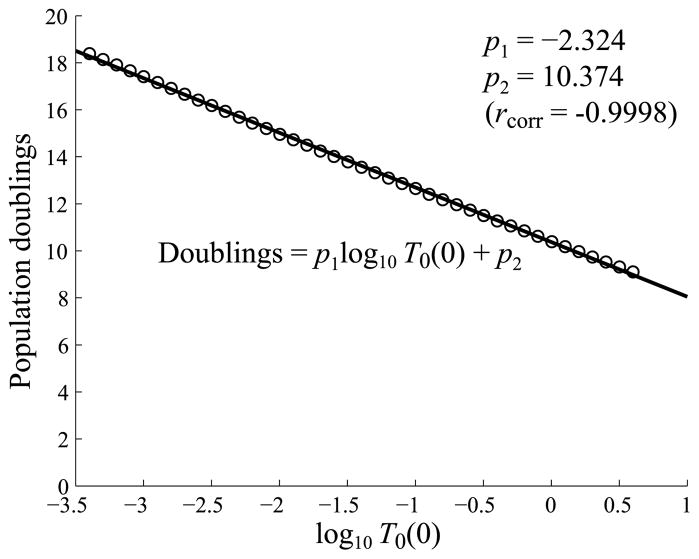
As in Figs. 5 and 9, it is also convenient to express the relative level of expansion in terms of population doublings. We show a plot of population doublings versus the log of the initial T cell concentration in Fig. 14. As discussed above, this plot also shows that the iTreg model compensates for an abundance or shortage of precursors by inducing a fewer or greater number of cell divisions.
Fig. 14.
Plots of the number of T cell population doublings, defined by log2(max(Ttotal/T0(0))), versus log10 T0(0). The plot shows that if the system starts with a higher precursor concentration, effector cells divide fewer times on average. Likewise, if the system starts with a lower precursor concentration, T cells divide a greater number of times to compensate. The linear correlation rcorr = −0.9998.
Returning to the more standard sensitivity analyses conducted for the program-based models, we see in Figs. 15 and 16 that the dynamics of the iTreg model exhibits similar saturating behavior with respect to the level and to the duration of antigen stimulation, given by c and b, respectively.
Fig. 15.
Dependence of T cell dynamics on c, a parameter corresponding to the level of antigen presentation. (a) Maximum T cell expansion level versus c. (b) The time of T cell peak versus c.
Fig. 16.
Dependence of T cell dynamics on b, the duration of antigen presentation. (a) Maximum T cell expansion level versus b. (b) The time of T cell peak versus b.
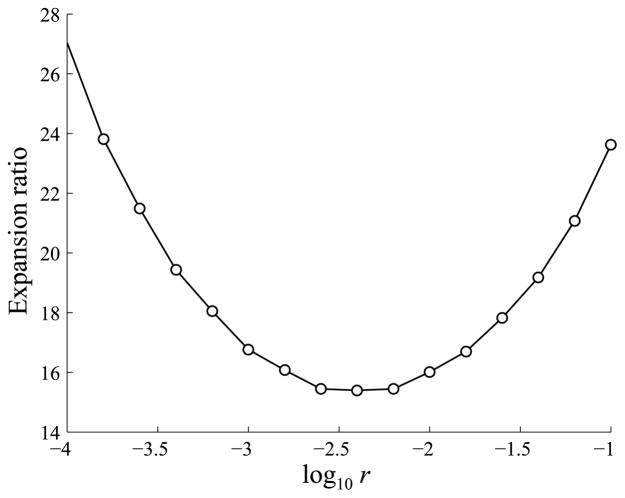
From the simulations in Fig. 13, the ratio of T cell peaks from the minimum to the maximum precursor concentrations (4 × 10−4 to 4k/μL) is 16.01, which is near the experimental result from Badovinac et al. (2007) that a 10,000-fold difference in precursor frequencies only resulted in a 13-fold difference in T cell expansion levels. This ratio depends in part on the parameter r, the rate at which effector T cells differentiate into iTregs, and as shown in Fig. 17, the ratio falls between 15 and 20 for values of r ranging from approximately 10−3.5 to 10−1.5. Hence, the ratio falls near the experimentally observed value of 13 for a wide range of values of r.
Fig. 17.
The effect of the parameter r, representing the rate at which effector cells differentiate into iTregs, on the ratios between T cell peaks for precursor concentrations of 4 and 4 × 10−4k/μL.
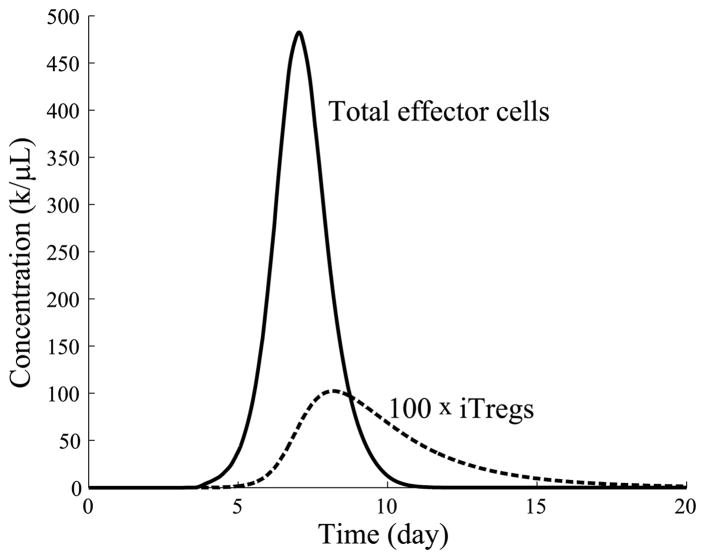
Figure 18 shows the time evolution of the effector and iTreg populations when r = 0.01/day and all other parameters are taken from Table 1. The figure indicates that the iTreg concentration peaks around the same time as the T cell response, but lingers a while longer ensuring a full contraction of the T cell population. In this example, the naïve T cell population begins at 0.04k/μL and peaks at 482k/μL, corresponding to an expansion level of 13.6 divisions on average.
Fig. 18.
Time evolution of effector and iTreg populations over time. The peak of the iTreg response roughly coincides with the peak of the T cell response, but the iTreg response decays more slowly.
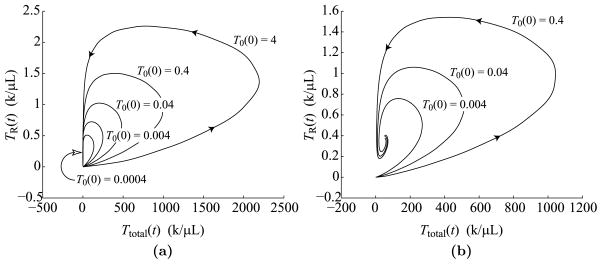
Figure 19(a) displays phase portraits of the iTreg versus the effector population for initial naïve cell concentrations of 0.0004, 0.004, 0.04, 0.4, and 4k/μL. The five curves correspond to population doublings of 18.4, 15.9, 13.6, 11.3, and 9.1, respectively, showing that every 10-fold increase or decrease in precursor concentrations corresponds to approximately 2.2 fewer or 2.2 additional divisions that adjust the difference. Thus, larger initial conditions lead to larger T cell responses, but not at the level of sensitivity exhibited by the two program-based models. All phase portraits exhibit similar shapes and return to the resting state in a timely fashion. The phase portraits give dynamics up to 20 days as in Fig. 18.
Fig. 19.
Phase portraits of iTreg versus effector dynamics over 20 days. (a) Phase portraits for five different precursor frequencies, T0(0) = 0.0004, 0.004, 0.04, 0.4, and 4k/μL. The curve for T0(0) = 0.04 shows the same dynamics as Fig. 18. (b) Phase portraits for three different precursor frequencies, T0(0) = 0.004, 0.04, and 0.4k/μL, corresponding to T cell dynamics under persistent antigen stimulation, i.e., b = 1,000 days.
Figure 19(b) shows similar phase portraits as in Fig. 19(a), except that the duration, b, of antigen presentation is set to 1,000 days so that antigen is chronically presented. As shown in this figure, the effector and iTreg populations spiral in to a stable fixed point. Elongated, teardrop shapes of the phase portraits form as a result of the rapid increase in the level of antigen presentation by mature APCs over the first few days after infection before decaying to a steady level several days later. (See Fig. 8b for a sense of mature APC behavior, but note that this figure corresponds to b = 10 rather than to b = 1, 000.) The brief burst of mature APC levels in the lymph node allows the effector concentration to expand rapidly for a brief time before being pulled back into the stable fixed point.
5. Discussion
The main purpose of this paper is to examine several existing models and propose a new mathematical model for the primary T cell response. The model is based on a simple feedback loop between effectors and iTregs that have differentiated during the course of the immune response. We also put forward the possibility that the accepted paradigm of a T cell proliferation program followed by programmed cell death does not entirely explain the robustness of the T cell response to precursor frequency as observed in Badovinac et al. (2007).
The theory of the T cell developmental program states that stimulated naïve T cells undergo several rounds of antigen-independent division, followed by a period of antigen-dependent proliferation that is terminated by programmed cell death. Programmed cell death limits the extent of T cell proliferation either by constraining the maximum number of divisions a cell can undergo or by initiating apoptosis at a certain time. The death program can also be a hybrid version of the two programs, since the scaling argument in Section 4.3 still applies for any signal that is induced internally in each cell rather than by external stimulation. Hence, this possibility does not affect the main argument of the paper.
The paradigm of T cell developmental programs was primarily formulated around a series of experimental studies investigating T cell expansion (Kaech and Ahmed, 2001; Mercado et al., 2000; Stipdonk et al., 2001, 2003) and contraction (Razvi et al., 1995; Renno et al., 1999; Yang et al., 1998). These experiments observed that T cell responses exhibit very similar dynamics for a broad range of antigen presentation levels and durations. These observations led experimentalists to conclude that T cells must follow a mostly antigen-independent developmental program. Although some parts of the program might be antigen dependent, the key transitions (such as the duration of the minimal developmental program and the initiation of contraction) are antigen-independent. Based on these results, two mathematical models were developed to analyze the dynamics of T cell developmental programs (Antia et al., 2003; Wodarz and Thomsen, 2005).
On the other hand, as shown in this work, both developmental approaches imply that the resulting dynamics scale directly with respect to precursor frequencies. This feature provides the main thrust of the argument of the paper. We would like to stress that this direct scaling is not a particular consequence of our formulations of developmental programs in Sections 3.1 and 3.2. It is an integral consequence of the developmental program paradigm.
If the paradigm of developmental programs were true, the high sensitivity of the T cell dynamics (i.e., directly scaled to the precursor frequencies of reactive naïve T cells) would have to be explained. Since naïve T cells are generated with random antigen specificities (at least with regard to nonself antigen), it is expected that antigen-specific precursor frequencies will be highly variable, particularly within a specific lymph node.
One way of circumventing this difficulty is that limited availability of resources prevents T cells from proliferating excessively. A couple of likely candidates of limited resources are positive growth signal, such as IL-2, and available space on antigen presenting cells. Indeed, the limited availability of these resources are typically thought of as possible control mechanisms, but is this a sufficiently good explanation for controlling the primary T cell response? The experiments of van Stipdonk et al. discussed in Section 1 demonstrate that naïve T cells can proliferate and differentiate in the absence of exogenous IL-2, meaning that they produce whatever is needed in an autocrine loop (Stipdonk et al., 2001). Furthermore, the experiments of Mercado et al. indicate that nearly all potentially-reactive T cells become activated within 24 hours of infection (Mercado et al., 2000), meaning that there are enough APCs to satisfy every T cell. In any case, we recognize that the limited resource hypothesis is plausible. Yet, as discussed, the limiting factor might not be a lack of positive growth signal or space on APCs.
An appealing alternative is considered by Allan et al. (2004). This paper considers four distinct mechanisms for T cell regulation, and most of the scenarios serve to render the T cell response insensitive to the nature of antigen stimulation, a stated goal of the paper. As a result, these mechanisms do not necessarily lead to robustness with respect to precursor frequency (an issue which is not studied in Allan et al., 2004). We examined the models proposed in Allan et al. (2004) and concluded that, as presented, they are not robust with respect to precursor frequency. On the other hand, the model in which T cells regulate each other via contact interactions can be made robust by choosing parameters that are different from those in Allan et al. (2004). With the original choice of parameters, this model predicts a power law relation with exponent 0.79 between antigen-specific naïve T cell concentrations and the heights of T cell peaks. Hence, a 10,000-fold difference between precursor frequencies reduces to a 1,445-fold difference between T cell peaks, almost a 10-fold reduction from direct scaling. A different choice of parameters (on which we do not elaborate here), can be made to render this model more robust with respect to precursor frequency. It is important to note that our hypothesis of iTreg-induced T cell contraction can work in conjunction with such a mechanism. Focused experimental studies are needed to differentiate between the two possibilities. In particular, one could examine whether de novo generated Tregs contribute significantly to T cell contraction in vivo.
In summary, we propose an alternative hypothesis to the T cell program paradigm that primary T cell expansion may be controlled by the appearance of adaptive regulatory T cells later in the immune response. The resulting behavior would be governed by emergent group dynamics rather than by autonomous programs. Our study shows that this approach produces a primary T cell response that is robust to antigen stimulation and precursor frequency. In particular, the maximal number of cells no longer scales with the precursor frequencies. At the same time, our simulations predict that the number of cell divisions in such a complex response still falls within the experimentally observed range, namely between 9 to 20 divisions (approximately between 103 to 106-fold expansions).
When it comes to future work, there are several possible directions. In this paper, one of our main goals was to suggest a potential role of adaptive regulatory cells. Our next step will be to consider the contributions of CD4+ and CD8+ T cells separately.
In addition, the other possible control mechanisms mentioned above and our iTreg model fall into the general category of resource competition models. Each model most likely produces dynamics that could be distinguished experimentally. For example, the mechanisms involving competition for growth signal or APC space might give rise to T cell dynamics that first increase linearly and then saturate with respect to precursor concentrations, the mechanism involving contact-dependent suppression between T cells could result in logistic-like dynamics that are largely independent of precursor frequency (although that is not what was obtained in Allan et al., 2004), and as shown in the paper, the iTreg model results in dynamics that are fairly robust, yet influenced by variations in precursor concentration. A thorough consideration of various competition models is a direction for future work.
One of our model assumptions is that the primary role of regulatory cells is to suppress primed T cells. This assumption is supported by the experimental results of Sakaguchi et al. (1995). Other experiments have also shown that regulatory cells can suppress dendritic cells (Chang et al., 2002). Hence, we plan to consider the case where regulatory cells suppress APCs as well.
According to Belz et al. (2007), stimulated memory cells can kill APCs, which would provide more rapidly acting feedback loop than the generation of iTregs. We could consider this alternative feedback control system to see how it compares to the iTreg model for primary immune responses.
In the current model, we only consider the lymph node compartment and exclude the dynamics of T cell migration and target elimination in the peripheral tissue. We could extend the model to include a tissue compartment. Indeed, as observed in the mathematical model of Kim et al. (2007), the T cell response may operate in a two-phase cycle during which T cells start by proliferating in the lymph node and then suddenly and collectively emigrating to the tissue.
It is likely that more than one scheme functions to ensure a timely T cell contraction. For example, iTreg-induced contraction and a cell division-based program might work simultaneously and whichever scheme initiates first mediates the contraction. A multi-faceted contraction signal could work to make T cell dynamics even more stable than what was considered in this paper. Finally, the behavior shown in Fig. 19 suggests that the iTreg model could be further simplified, perhaps into only two differential equations, and analyzed for fixed points and stability.
Acknowledgments
The work of PSK was supported in part by the NSF Research Training Grant and the Department of Mathematics at the University of Utah. The work of DL was supported in part by the joint NSF/NIGMS program under Grant Number DMS-0758374. This work was supported by a Department of Defense Era of Hope grant to PPL. The work of DL and of PPL was supported in part by Grant Number R01CA130817 from the National Cancer Institute. The content is solely the responsibility of the authors and is does not necessarily represent the official views of the National Cancer Institute or the National Institute of Health.
Footnotes
Belz et al. (2007) actually reports a peak at day 3.2 following infection, which ends up being about 2.2 days after antigen begins to appear in the lymph node.
References
- Allan MJ, Callard R, Stark J, Yates A. Comparing antigen-independent mechanisms of T cell regulation. J Theor Biol. 2004;228(1):81–95. doi: 10.1016/j.jtbi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Antia R, Bergstrom CT, Pilyugin SS, Kaech SM, Ahmed R. Models of CD8+ responses: 1. What is the antigen-independent proliferation program. J Theor Biol. 2003;221(4):585–598. doi: 10.1006/jtbi.2003.3208. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26(6):827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Zhang L, Lay MD, Kupresanin F, Davenport MP. Killer T cells regulate antigen presentation for early expansion of memory, but not naïve, CD8+ T cell. Proc Natl Acad Sci USA. 2007;104(15):6341–6346. doi: 10.1073/pnas.0609990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific cd8 t cells. J Exp Med. 2002;195(5):657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs NJ, de Oliveira BMPM, Pinto AA. Regulatory T cell adjustment of quorum growth thresholds and the control of local immune responses. J Theor Biol. 2006;241(1):134–141. doi: 10.1016/j.jtbi.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976;143:1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro J, Paixão T, Milutinovic D, Sousa J, Leon K, Gardner R, Faro J. Immunological self-tolerance: Lessons from mathematical modeling. J Comput Appl Math. 2005;184(1):77–100. [Google Scholar]
- Catron DM, Itano AA, Pape KA, Mueller DL, Jenkins MK. Visualizing the first 50 hr of the primary immune response to a soluble antigen. Immunity. 2004;21(3):341–347. doi: 10.1016/j.immuni.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, Lederman S, Colonna M, Cortesini R, Dalla-Favera R, Suciu-Foca N. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3(3):237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- De Boer RJ, Oprea M, Antia R, Murali-Krishna K, Ahmed R, Perelson AS. Recruitment times, proliferation, and apoptosis rates during the CD8(+) T-cell response to lymphocytic choriomeningitis virus. J Virol. 2001;75(22):10663–10669. doi: 10.1128/JVI.75.22.10663-10669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer RJ, Homann D, Perelson AS. Different dynamics of CD4+ and CD8+ T cell responses during and after acute lymphocytic choriomeningitis virus infection. J Immunol. 2003;171(8):3928–3935. doi: 10.4049/jimmunol.171.8.3928. [DOI] [PubMed] [Google Scholar]
- Effros RB, Pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today. 1997;18(9):450–454. doi: 10.1016/s0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- Fouchet D, Regoes R. A population dynamics analysis of the interaction between adaptive regulatory t cells and antigen presenting cells. PLoS ONE. 2008;3(5):e2306. doi: 10.1371/journal.pone.0002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178(5):2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med. 2002;195(3):317–326. doi: 10.1084/jem.20011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Jr, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 6. Garland, New York: 2005. [Google Scholar]
- Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2(5):415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PS, Lee PP, Levy D. Modeling regulation mechanisms of the immune system. J Theor Biol. 2007;246(1):33–69. doi: 10.1016/j.jtbi.2006.12.012. [DOI] [PubMed] [Google Scholar]
- León K, Peréz R, Lage A, Carneiro J. Modelling T-cell-mediated suppression dependent on interactions in multicellular conjugates. J Theor Biol. 2000;207(2):231–254. doi: 10.1006/jtbi.2000.2169. [DOI] [PubMed] [Google Scholar]
- León K, Peréz R, Lage A, Carneiro J. Three-cell interactions in T cell-mediated suppression? a mathematical analysis of its quantitative implications. J Immunol. 2001;166(9):5356–5365. doi: 10.4049/jimmunol.166.9.5356. [DOI] [PubMed] [Google Scholar]
- León K, Lage A, Carneiro J. Tolerance and immunity in a mathematical model of T-cell mediated suppression. J Theor Biol. 2003;225(1):107–126. doi: 10.1016/s0022-5193(03)00226-1. [DOI] [PubMed] [Google Scholar]
- León K, Faro J, Lage A, Carneiro J. Inverse correlation between the incidences of autoimmune disease and infection predicted by a model of T cell mediated tolerance. J Autoimmunity. 2004;22(1):31–42. doi: 10.1016/j.jaut.2003.10.002. [DOI] [PubMed] [Google Scholar]
- León K, Lage A, Carneiro J. How regulatory CD25+CD4+ T cells impinge on tumor immunobiology? on the existence of two alternative dynamical classes of tumors. J Theor Biol. 2007a;247(1):122–137. doi: 10.1016/j.jtbi.2007.01.029. [DOI] [PubMed] [Google Scholar]
- León K, Lage A, Carneiro J. How regulatory CD25+CD4+ T cells impinge on tumor immunobiology: the differential response of tumors to therapies. J Immunol. 2007b;179(9):5659–5668. doi: 10.4049/jimmunol.179.9.5659. [DOI] [PubMed] [Google Scholar]
- Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165(12):6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- Mohri H, Perelson AS, Tung K, Ribeiro RM, Ramratnam B, Markowitz M, Kost R, Hurley A, Weinberger L, Cesar D, Hellerstein MK, Ho DD. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J Exp Med. 2001;194(9):1277–1287. doi: 10.1084/jem.194.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razvi ES, Jiang Z, Woda BA, Welsh RM. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr, and Bcl-2-transgenic mice. Am J Pathol. 1995;147(1):79–91. [PMC free article] [PubMed] [Google Scholar]
- Renno T, Attinger A, Locatelli S, Bakker T, Vacheron S, MacDonald HR. Cutting edge: apoptosis of superantigen-activated T cells occurs preferentially after a discrete number of cell divisions in vivo. J Immunol. 1999;162(11):6312–6315. [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor a-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory t cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Taams LS, Vukmanovic-Stejic M, Smith J, Dunne PJ, Fletcher JM, Plunkett FJ, Ebeling SB, Lombardi G, Rustin MH, Bijlsma JW, Lafeber FP, Salmon M, Akbar AN. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32(6):1621–1630. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2(5):423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4(4):361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1(1):47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J Clin Invest. 2003;112(9):1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25− cells. Proc Natl Acad Sci USA. 2005;102(11):4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz D, Thomsen AR. Effect of the CTL proliferation program on virus dynamics. Int Immunol. 2005;17(9):1269–1276. doi: 10.1093/intimm/dxh303. [DOI] [PubMed] [Google Scholar]
- Yang Y, Kim D, Fathman CG. Regulation of programmed cell death following T cell activation in vivo. Int Immunol. 1998;10(2):175–183. doi: 10.1093/intimm/10.2.175. [DOI] [PubMed] [Google Scholar]