Abstract
Female sexual dysfunction (FSD) is a prevalent problem, afflicting approximately 40% of women and there are few treatment options. FSD is more typical as women age and is a progressive and widespread condition. Common symptoms associated with FSD include diminished vaginal lubrication, pain and discomfort upon intercourse, decreased sense of arousal and difficulty in achieving orgasm. Only a small percentage of women seek medical attention. In comparison to the overwhelming research and treatment for erectile dysfunction in males, specifically with the development of phosphodiesterase type 5 inhibitors, significantly less has been explored regarding FSD and treatment is primarily limited to psychological therapy. Several cardiovascular diseases have been linked with FSD including atherosclerosis, peripheral arterial disease and hypertension, all of which are also pathological conditions associated with aging and erectile dysfunction in men. Using animal models, we have expanded our understanding of FSD, however a tremendous amount is still to be learned in order to properly treat women suffering from FSD. The aim of this review is to provide the most current knowledge on FSD, advances in basic science addressing this dysfunction, and explore developing therapeutic options.
Keywords: Female sexual dysfunction, pudendal arteries, clitoris, vagina
INTRODUCTION
Human sexual function is an essential component of life, both in species propagation as well as quality of life. Sexual dysfunction can lead to reduced quality of life and potentially procreative advancement. Male sexual dysfunction, especially erectile dysfunction, has been extensively studied and effective therapies are available for men with this disorder. However, female sexual dysfunction (FSD) is more complicated and significantly less is understood in comparison to male sexual dysfunction. Therefore, the present review focuses on therapies available or in development as well as challenges faced by investigators in the study of FSD. Other recent reviews articles may be useful for understanding additional aspects of FSD [1–3].
Current Knowledge of Female Sexual Dysfunction
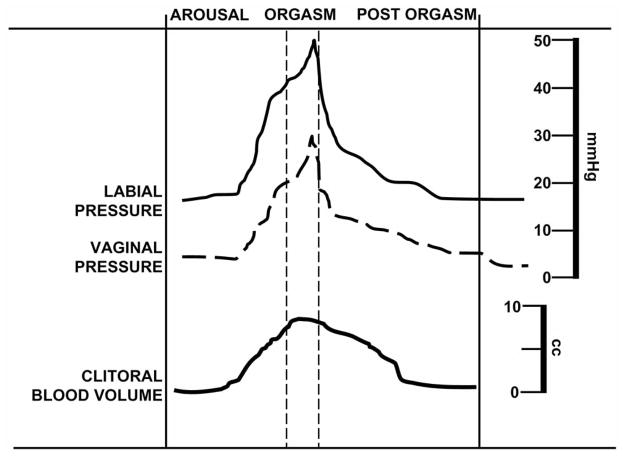
Sexual function results from a complex neurovascular process that is controlled by psychological and hormonal inputs. Like any coordinated physiological response, multiple systems are involved in this function. In respect to proper vaginal and clitoral function, a sufficient blood supply is required for a satisfying sexual experience. Fig. 1 demonstrates relative pressure and volume values from the vagina and labia as well as the clitoris, respectively, during a period of arousal, orgasm and post-orgasm. Vaginal and labial data were derived from partial oxygen pressures detected at each tissue site [4] while magnetic resonance imaging of the clitoris measured changes in volume during neutral and stimulating visual imagery [5]. Fig. 1 represents a typical sexual response in women, however, the complexity and unique individual response allows for numerous sites of dysregulation, which can lead to sexual dysfunction. FSD is defined by the World Health Organization as ‘the various ways in which a woman is unable to participate in a sexual relationship as she would wish’ [6]. FSD is more complex and difficult to categorize due to a woman’s perception about sex when compared to males. FSD is a multifaceted disorder, comprising anatomical, psychological, physiological, as well as social-interpersonal components. With several existing FSD definitions, the most descriptive encompasses FSD as the persistent/recurring decrease in sexual desire or arousal, the difficulty/inability to achieve an orgasm, and/or the feeling of pain during sexual intercourse [7].
Fig. (1).
The psychogenic and hemodynamic events of the normal female sexual cycle. Psychosexual responses from arousal, orgasm and post orgasm frame approximate vaginal and labial pressures as well as clitoral volume. Increasing arousal that culminates in orgasm demonstrates increases in vaginal and labial pressures and filling of the clitoris. Data are compiled from several sources referenced in the text.
Appreciating the uniqueness of each FSD facet is critical in our understanding and potential treatment of FSD in general terms. Hypoactive sexual desire disorder (HSDD) is the persistent or recurrent absence of sexual fantasies/thoughts and/or desire for sexual activity leading to personal distress [1]. Female sexual arousal disorders (FASD) can be defined as a recurrent inability to attain, or maintain until completion, sexual activity. An example of this is an adequate lubrication/swelling response of sexual excitement. The arousal response consists of vasocongestion in the pelvis, vaginal lubrication, and expansion and swelling of external genitals. Orgasmic disorders can be categorized with FASD and are described as the persistent or recurrent difficulty, delay in, or absence of, attaining orgasm following sufficient sexual stimulation and arousal that leads to personal distress.
Sexual pain disorders are another form of FSD and are diagnosed as followed: dyspareunia, the recurrent or persistent genital pain associated with sexual intercourse, vaginismus, the recurrent or persistent involuntary spasm of the musculature of the outer third of the vagina that interferes with vaginal penetration, and noncoital sexual pain disorder, the recurrent or persistent genital pain induced by noncoital sexual stimulation.
The incidence of FSD is alarmingly high, where 30 years ago, 76% of women described some symptom of sexual dysfunction [8]. More recent evidence suggests 43% have some form of sexual difficulty [9]. By comparison, the Massachusetts Male Aging Study found that 34.8% of men (40–80 years old) displayed moderate to complete erectile dysfunction [10]. These data, along with data based on the 1999 US National Health and Social Life Survey, where females report more cases of sexual dysfunction than male (43 vs 31%) [9], clearly demonstrate that sexual dysfunction is not gender specific and highlight an elevated prevalence in women. However, FSD remains relatively understudied and therapeutic breakthroughs, such as phosphodiesterase type 5 (PDE 5) inhibitors used for erectile dysfunction, have yet to be discovered.
Throughout society, sexual disorders for women are influenced by both health-related and psychosocial factors. Taken together, this dynamic is associated with impaired quality of life and interpersonal relationships [11]. Significant improvements in overall clinical care have allowed the management of quality of life complications and not just the treatment of life-threatening diseases. Importantly, several studies have linked cardiovascular diseases with sexual dysfunction, in both females [12] and males [13]. Therefore, the treatment of FSD as purely a lifestyle disorder may severely underestimate the seriousness of the situation.
Recently, Schwarz et al. reported a high correlation between women with sexual dysfunction and chronic compensated heart failure. This report showed that 87% of middle-aged women with heart failure reported some degree of sexual dysfunction [14]. More specifically, 80% of these patients reported reduced lubrication that resulted in frequent unsuccessful intercourse (76%) and 63% of the same population had difficulties achieving an orgasm. Indeed, the prevalence of sexual dysfunction in women with chronic compensated heart failure suggests a reduction in quality of life.
Compared to the extensive sexual function studies conducted in diabetic men [15], substantially less is known regarding diabetic women. However, recent studies have demonstrated that diabetic women experience increased incidences of sexual dysfunction [16–18], including reduced sex drive, little to no arousal, vaginal dryness, difficulty in achieving orgasm and overall diminished sexual satisfaction [16]. Despite these observations, correlation between FSD and diabetes is not without controversy. A report on the frequency of psychosexual difficulties from diabetic women found secondary sexual dysfunction was reported in 73.3% of diabetic women, however no direct association between sexual disorders and diabetic complications were found [19]. These authors concluded that, in diabetic women, sexual dysfunction was prominently a psychogenic complication.
Broadly interpreting these data, sexual dysfunction very well may be considered an early marker/risk factor for cardiovascular diseases and consequently a life-threatening condition. Therefore, a more comprehensive understanding of the etiology and treatment options of FSD is crucial for improving existing conditions seen in women, as well as preventative measures of future, more fatal, pathologies.
Basic Science/Experimental Challenges
Animal models have been used to investigate female sexual function and dysfunction over the past 20 years and several experimental approaches have been developed. Particular aspects of female sexual function, more specifically desire and peripheral arousal, are currently under investigation in basic science laboratory settings. Accurately modeling FSD is an experimental challenge. However, investigating comorbid diseases, such as diabetes, cardiovascular disease and depression models, allows end-point measurements involved in FSD to be examined. This section will describe techniques currently in use and the challenges that investigators face studying FSD.
Sexual desire in humans can be described as the presence of desire for sexual activity. Desire in animal models can be assessed by monitoring particular appetitive behaviors that occur during copulation as well as from certain unconditioned copulatory determinants [20]. In female rats, increased dopamine release in the striatum and nucleus accumbens leads to repetitive voluntary return (by depressing an access lever) to attain access to male rats [21, 22]. These authors also showed that cage pacing (an animalistic sexual desire response) was increased when the same central pathways were stimulated [23, 24]. Translating findings similar to these to the clinical setting has revealed that dopamine agonists (discussed further in the following section) indeed increase sexual desire in women and is a viable treatment option for women that suffer from HSDD.
Sexual arousal encompasses a variety of outputs, including vaginal blood flow, clitoral, labial and vestibular bulb engorgement [20]. These physiological responses are neuronally controlled, which affects the contractility of vascular smooth muscle cells throughout the genitals. Pelvic nerve stimulation (PNS) is a common technique used to induce tumescence of erectile tissue. Intracavernous pressure and blood flow can be measured by inserting a probe into vaginal tissue and the corpus cavernosum, and signals to a laser Doppler blood flow monitor and thus, following PNS, changes in vaginal and clitoral blood flow can be measured. Recently, Angulo and colleagues demonstrated that treatment with vardenafil, a PDE5 inhibitor, increased vaginal and clitoral blood flow following PNS, which was assessed by laser Doppler, in a FSD-rabbit model where the animals were treated with anti-depressants [25]. The use of the female New Zealand white rabbit has given tremendous insight into genital hemodynamic measurements. A study by Park and colleagues demonstrated that vaginal engorgement and clitoral erection induced by PNS in rabbits was diminished in an atherosclerotic/high cholesterol rabbit model [26]. Additionally, Giuliano and coworkers demonstrated using a scanning laser Doppler system that rat vaginal blood flow, as well as contractions and temperature were increased following PNS [27].
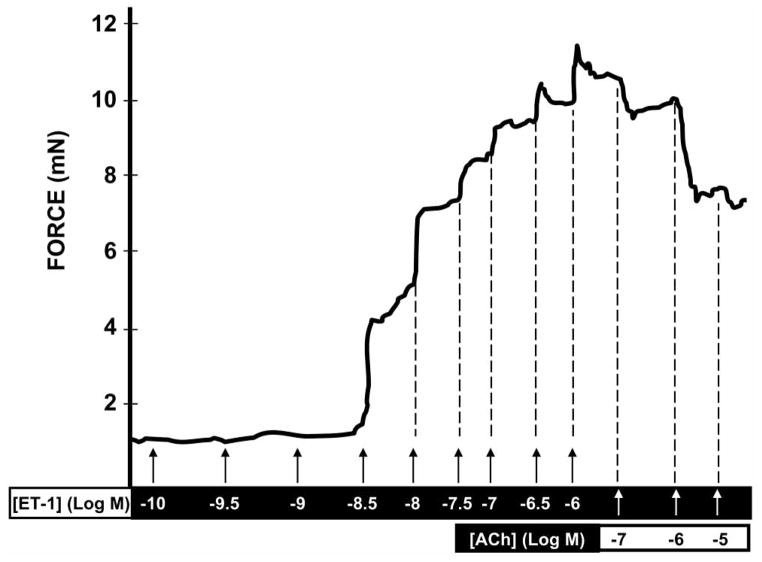
Ex vivo investigation of clitoral and vaginal strips, as well as the vasculature that delivers blood to these end-organs, have revealed contractility and relaxation states of these tissues. By using wire myographs, we have begun to characterize the contractile properties of internal pudendal arteries as well as the clitoral arteries, the vasculature that feed blood to the clitoris and labia minora, in female rats [28]. Using this technique, we have measured alterations in contraction, relaxation, signaling, and drug effectiveness in physiological and pathological conditions (Fig. 2). Other researchers have demonstrated that experimentally-induced diabetic rats have diminished adrenergic-, cholinergic- and NANC-neurotransmitter mechanisms in the smooth muscle of the vagina compared to control [29]. As well, Myung and colleagues demonstrated that an overactive bladder model in female rabbits deteriorated clitoral engorgement, which was associated with greater force generation through increased calcium sensitization and subsequently decreased relaxation, via activation of endothelin-1 (ET-1) and Rho-kinase system [30], which support our previous findings [28].
Fig. (2).
Representative trace showing changes in force (contraction and relaxation) of a female internal pudendal artery stimulated with increased concentrations of endothelin-1 (ET-1) and acetylcholine (ACh), respectively. The internal pudendal artery supplies blood to the clitoris and labia minora of the vagina. Relaxation of the internal pudendal artery is essential to achieve tumescence during sexual stimulation. A compromised state of relaxation in this artery may play a role in female sexual dysfunction.
These basic science techniques have and continue to advance our understanding of FSD, however several experimental challenges still remain. Due to undefined anatomical characteristics and limitations in structure, physiological and pharmacological aspects of the rat clitoris have not been thoroughly investigated. In contrast, clitoral function and characteristics have been studied in larger animal studies (rabbits and dogs), however these species are limited in experimental design when compared to rat.
The investigation of FSD is complicated by many factors. Experimentally, modeling FSD is challenging due to the multifaceted and varied inputs that define this disorder. Clinically, treatment success is variable in women with sexual dysfunction due to the rationale that not every woman responds to sexual stimulus/treatment the same. Therefore endpoint measurements such as clitoral and vaginal blood flow, internal pudendal artery compliance and nerve-stimulated increases in pressure assist in the quantification of animal responses. Direct study of FSD in animal models has proven difficult and therefore the disorder has been investigated in the study of other comorbid conditions (diabetes, hypertension, ect.) where more established models have been created. This approach could complicate the study of FSD in that the researcher must interpret data in conjunction with an additional disease condition. However, this may be a more realistic approach due to the commonalities between FSD and some cardiovascular diseases.
Therapeutic Options
Currently there are few pharmacological options available in the treatment of FSD. Historically, FSD patients were treated through psychological therapy; however as we have come to understand the extensiveness of the disorder, more basic science research and clinical recognition have been developed to address the problem. Several pharmacological initiatives are in development aimed at increasing blood flow to the genitals, improving androgen deficiencies and enhancing central nervous system stimulation. Table 1 summarizes the potential and current treatment options available for FSD.
Table 1.
Potential and Current Therapeutic Options Available for the Treatment of Female Sexual Dysfunction
| General Target | Product | Brand, Company | Mechanism of Action |
|---|---|---|---|
| Peripheral Vaginal/Clitoral Blood flow | |||
| PDE5 inhibitors | Sildenafil Tadalafil Vardenafil |
(Viagra®, Pfizer) (Cialis®, Lilly) (Levitra®, Bayer) |
 cGMP availability; mediates vascular smooth muscle (VSM) relaxation cGMP availability; mediates vascular smooth muscle (VSM) relaxation |
| Prostaglandin | Alprostadil | (Femprox®, NexMed) (Alista®, Vivus) |
Binds to EP2 receptor; cAMP and mediates VSM relaxation cAMP and mediates VSM relaxation |
| Nitric oxide | L-arginine- yohimbine L-arginine |
(NMI-870®, NitroMed) (ArginMax®, The Daily Wellness Co.) |
 NO production; augments NO production; augmentscGMP availability; mediates VSM relaxation |
| VIP | Candoxatril | (Candoxatrilat®, Pfizer) | Inhibits degradation of VIP; VSM relaxation VSM relaxation |
| Hormonal | |||
| Estrogen | Estradiol | (Vagifem®, Upjohn) (Premarin®, Wyeth) |
Improves vaginal dryness and irritation |
| Testosterone | Testosterone Testosterone Testosterone |
(Intrensa®, Watson) (Tostrelle®, Cellegy) (Androsorb®, Novavax) |
 sexual activity, libido and pleasure sexual activity, libido and pleasure |
| Synthetic | Tibolone | (Livial®, Organon) | Improves vaginal dryness and overall sexual function |
| CNS | |||
| Dopaminergic agonist | Apomorphine Bupropion |
(Uprima®, Tap) (Wellbutrin XL®, GlaxoSmithKline) |
Binds to D receptors; increases sexual responsiveness |
| Synthetic α-melanocortin- stimulating hormone | Bremelanotide | (PT-141®, Palatin) | Binds to MC4 receptors; contributes to VSM relaxation |
Increasing Blood Flow
PDE5 Inhibitors
Increasing blood delivery to the genitals with the development of the first marked PDE5 inhibitor, sildenafil revolutionized the treatment of erectile dysfunction in men. The physiological mechanism responsible for relaxation of smooth muscle of cavernous tissue (both male and female) is initiated with the release of nitric oxide (NO) from adjacent nerve endings and/or endothelial cells upon mental and sensory stimuli via spinal reflex [31]. (See Fig. 3). NO produced by the sexual stimuli passes through the plasma membrane of the smooth muscle cell and binds to guanylyl cyclase (GS) and soluble GS (sGS). This binding results in an enzyme conformational change, resulting in the production of 3′-5′-cyclic guanosine monophosphate (cGMP) from guanosine 5′-trisphsophate (GTP). cGMP activates cGMP-dependent protein kinase (PKG), which upon activation, phosphorylates several intracellular proteins, resulting in decreased free cytosolic calcium and ultimately dilation of the vascular smooth muscle cell. Phosphodiesterase type 5 converts cGMP to 5′-GMP, thus reducing cGMP availability. Phosphodiesterase type 5 inhibitors reduce the catalysis of cGMP by PDE5 and thereby increase cGMP availability. Other recent reviews articles regarding NO signaling may be useful for understanding additional aspects of this mechanism [32, 33].
Fig. (3).
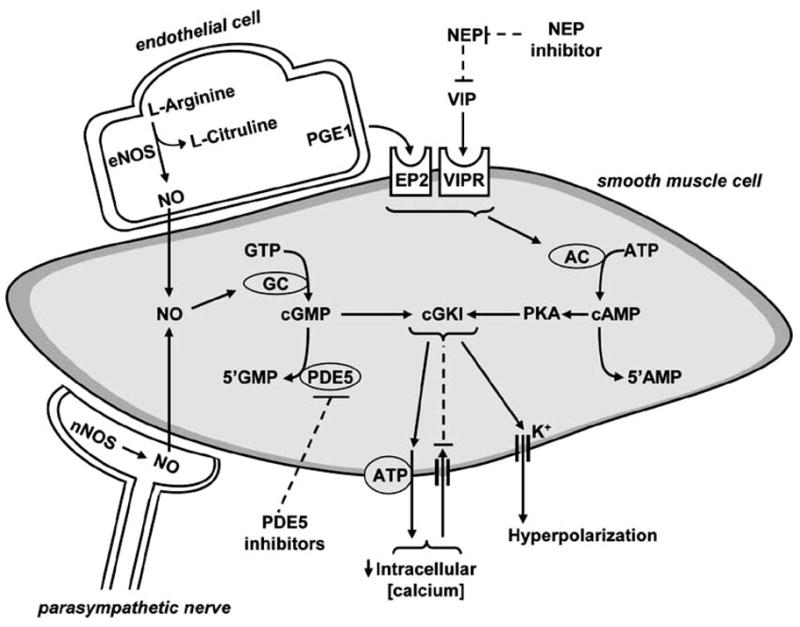
Mechanism of smooth muscle relaxation and peripheral inhibition sites. Abbreviations: nitric oxide (NO), neuronal NO synthase (nNOS), endothelial NOS (eNOS), prostaglandin (PGE1), PGE1 receptor (EP2), guanosine triphosphate (GTP), guanylyl cyclase (GC), cyclic guanosine monophosphate (cGMP), phosphodiesterase type 5 (PDE5), adenosine triphosphate (ATP), cGMP-dependent protein kinase type I (cGKI), protein kinase A (PKA), cyclic adenosine monophosphate (cAMP), vasoactive intestinal peptide (VIP), VIP receptor (VIPR) and neutral endopeptidase (NEP).
Several PDE5 inhibitors (sildenafil, vardenafil and tadalafil) are available for the treatment of erectile dysfunction. Though success of PDE5 inhibitors in males with sexual dysfunction did stimulate interest in treating FSD, the same effectiveness of this drug class has not been found across genders. Early clinical trials conducted by questionnaires in women with HSDD and FSAD showed some promise, revealing that sildenafil improved the ability to achieve orgasm and state of arousal [34, 35]. In addition, Cavalcanti et al showed that sildenafil significantly improved clitoral blood flow in postmenopausal women with orgasmic dysfunction, measured by color and pulse Doppler [36]. Conversely however, an additional study investigating a broad spectrum of sexual dysfunction in women did not report beneficial effects of the PDE5 inhibitor [37]. Inconsistent reports on the effects of sildenafil between male and females may be explained by the different definitions or states of arousal between the genders. PDE5 inhibitors increase genital engorgement [35] and blood flow [36] and recently, expression of numerous PDE isoforms were found in the human clitoris, vagina, and labia minora [38]. This further indicates that an increase in cGMP or cAMP could mediate vaginal and clitoral blood flow, and continued investigation into PDE inhibition is needed.
Prostaglandins
Prostaglandins (PG) are found in virtually all tissues and organs. They are autocrine and paracrine lipid molecules, which are quickly metabolized, and participate in a variety of physiological events, including blood flow regulation. Specifically, the PG isoform PGE1 (signaling through its EP2 receptor) causes smooth muscle relaxation in the vaginal, uterine, as well as penile smooth muscle [39]. PGE1/EP2 activation leads to increases in cAMP resulting in activation of protein kinase A, which causes smooth muscle relaxation. Prostaglandins have been used in male sexual dysfunction, especially erectile dysfunction (administered through penile injection), for some time and have displayed positive outcomes for certain women with genital sexual arousal disorder, most likely through increasing vaginal secretion and arterial smooth muscle relaxation [40].
A synthetic version of PGE1, alprostadil (Femprox® from NexMed and Alista® from Vivus; still in clinical trials), has displayed positive results for the treatment of FSAD. Administered topically, thereby not resulting in systemic side effects, application of alprostadil demonstrated positive responses in genital vasocongestion, vaginal erythema, and transudate volume; however these effects were not consistently superior to placebo effects [41]. In a recent randomized, double blind, placebo-controlled study, 400 female patients (pre- and post-menopausal) with FSAD displayed an improved sexual arousal rate when topical alprostadil was applied prior to vaginal intercourse [42]. Although topical alprostadil is a potential new therapy for the treatment of FSAD, more conclusive results from ongoing clinical studies are needed to further validate the use of topical alprostadil in the treatment of FSAD.
Nitric Oxide Donor and Combination Therapy
It is well established that the production of NO is essential in vascular relaxation to numerous stimuli. PDE5 inhibitors augment NO-initiated dilation by propagating the downstream mediator, cGMP, through the activation of guanylate cyclase. Thus, activation of the NO-NO synthase (NOS) system is a potential site for pharmacological intervention. Pacher et al., demonstrated topical application of a NO donor, DS1, a linear polyethylenimine-nitric oxide/nucleophile adduct, increased vaginal blood flow in anesthetized rats [43]. Some NO-donor creams are available (Sensua!®), however these topical therapies have yet to become FDA approved. NO-independent stimulators and activators of sGS, including YC-1, BAY 41-2272 and A-350619, have shown the ability to improve erectile function in rodents and could be targeted towards the treatment of FSD as well [44]. Combination therapy of L-arginine, the substrate in NO production, and yohimbine, a competitive α2-adrenergic receptor antagonist, (NMI-870; NitroMed) has completed Phase I and IIa clinical trials; however trials have not continued and the current stage of this product is unknown [45]. Two additional α-adrenergic receptor antagonists, phentolamine (non-selective α-adrenergic receptor antagonist) as well as REC2615 (α1-adrenergic receptor antagonist) have begun clinical trials but their current status is uncertain [46].
Another L-arginine product, ArginMax®, has demonstrated positive results in the treatment of FSD. In a 4-week, placebo-controlled study, women (in the pre, peri or post menopausal state) who reported lack of sexual desire exhibited statistically significant increases in clitoral sensitivity, sexual satisfaction, increased frequency of sexual intercourse and decreased vaginal dryness, compared to women in the control group [47, 48]. The authors reported that the changes in sexual satisfaction and elevated state of desire were stronger in pre-menopausal and less in the post-menopausal group, potentially due to alterations in hormonal levels. ArginMax® is a nutritional supplement containing extracts from ginseng, ginkgo, damamiana, arginine, and includes various vitamins and minerals and further scientific investigation of this natural enhancement compound is needed.
Vasoactive Intestinal Peptide
Vasoactive intestinal peptide (VIP) is a polypeptide hormone containing 28 amino acid residues and is produced in many areas of the human body. VIP has potent vasorelexant effects and has been suggested to contribute to vaginal blood flow control [49, 50]. Like many peptidic therapies, oral administration of VIP is complicated by low bioavailability and high rate of clearance. Therefore, an alternative approach using an inhibitor of neutral endopeptidase (NEP), the primary enzyme responsible for the degradation of VIP, has been in development under the assumption that inhibition of NEP will lead to more VIP in the circulation, which can increase clitoral and vaginal blood flow when sexually stimulated [2]. Pfizer has developed a cardiovascular agent, Candoxatrilat®, a diacid NEP inhibitor, which requires delivery as the prodrug form, Candoxatril, to assure systemic exposure [51]. This inhibition, as well as dual inhibitors of NEP and soluble endopeptidase (SEP), has been demonstrated to inhibit NEP/SEP-mediated degradation of VIP [52] and could potentially aid in the treatment of FSD. However, there are no current data demonstrating the effectiveness of these agents in improving clitoral or vaginal blood circulation.
Hormones
Key endocrine hormones, estrogen, progesterone and testosterone are involved in the sexual response [53] and deficiencies in the levels of these androgens potentially are involved in FSD [54]. Low levels of testosterone are associated with a decline in libido, arousal, genital sensation and orgasm [55].
Testosterone
The use of testosterone to treat FSD has delivered mixed results. A primary concern in testosterone therapy is the long-term side effects including: hirsutism, acne and masculinization [2]. Given the results following the Woman’s Health Initiative, replacement therapy with estrogen and progestin revealed elevation in coronary heart disease, stroke and thrombosis formation [56], a certain amount of caution must be taken in the treatment of FSD with hormones.
Nevertheless, several products have been developed to administer testosterone in a variety of ways. Intrensa® is a transdermal testosterone (300μg/day) patch currently in use (Watson Pharmaceuticals/Theratech and Proctor & Gamble). The patch, placed just below the navel, has been shown to produce an increase in sexual activity and pleasure in a 6-month study trial. Several doses of testosterone were examined during clinical trials (150, 300 and 450 μg/day), however the most effective dose was 300 μg/day [57]. Other studies have reported similar results, however the observed changes, though clinically meaningful are considered modest at best [58, 59].
Application of testosterone by topical cream or gel has been developed as well. Tostrelle® (Cellegy), a transdermal testosterone metered gel that has completed phase I/II trials, has reportedly restored testosterone levels in menopausal women [60]. No further information is available on the progress of this product. Additionally, Androsorb® (Novavax), a testosterone cream is in early stages of clinical trials and preliminarily has shown heightened libido in postmenopausal women [60].
Estrogen
Estrogen plays a vital role in the regulation of female sexual function. Alterations in estradiol levels can result in vaginal wall smooth muscle atrophy and increased vaginal canal acidity, ultimately leading to discomfort and stress [60]. The findings from the Woman’s Health Initiative raised concerns on estrogen replacement therapy, however the benefits of estrogen in normal function are well accepted. In the treatment of FSD, Vagifem® (Pharmacia Upjohn) and Premarin® (Wyeth Pharmaceuticals) have been shown to improve vaginal dryness and irritation. Interestingly, it does not appear that these products specifically deliver estrogen solely to the genitals. Labrie and colleagues recently demonstrated that, following a 1-week treatment with Vagifem® (25 μg/pill) and Premarin® (0.625 mg; topical cream), these compounds caused an approximately fivefold increase in serum estradiol in postmenopausal women [61]. The lack of specific delivery or localization of estradiol to the vagina indicates that systemic effects could be expected after application of these intravaginal estrogen preparations and further evaluation of these systemic effects must be studied.
Tibolone is an additional hormonal modulator option. It is a synthetic steroid commonly used for the treatment of menopausal symptoms, including diminished vaginal lubrication. Recently, Nijland and colleagues demonstrated that tibolone (2.5 mg) treatment improved overall sexual function, increased frequency of sexual events and reduced sexually-related personal distress in naturally postmenopausal women with sexual dysfunction [62]. The effectiveness of this compound may be because of tibolone’s combined estrogenic and androgenic properties and could prove to be a viable treatment option for FSD.
Centrally Mediated Stimulation
The sexual response for men and women is distinct. Regarding treatment of male ED, PDE5 inhibitors have proven to be very successful, whereas in FSD similar achievements have not been made. Treating FSD through central acting mediators has recently received more attention. This area of investigation has gained momentum by recent publication demonstrating that several hypothalamic nuclei are activated in rodent sexual response [20]. Therefore, central regulation/activation of the female sexual response could mark an alternative approach for treating FSD.
A central player in behavioral states, including sexual function, dopamine signals through its receptors (D1-like: D1 and D5; D2-like: D2, D3 and D4) and mediates a variety of responses. Dopamine agonists, apomorphine and bupropion, also used in the treatment of Parkinson’s disease, have displayed mixed results in increasing sexual function in males [63–65]. Dosing limitations in apomorphine and bupropion administration has reduced effectiveness of this therapy as high exposure leads to adverse side effects, such as nausea, vomiting and headaches. The use of apomorphine in women showed an increase in sexual responsiveness [66, 67], though patients involved still reported unwanted side effects. New administration therapies, especially intranasal and sublingual distribution have reported equal effectiveness in the increase of sexual desire while minimizing side effects. Additionally, intranasally delivered apomorphine in male rats significantly increased brain distribution and accumulation of the dopamine agonist [68], which lead to a faster and more efficacious treatment option.
Another centrally acting agent that has been in development in recent years is α-melanocortin-stimulating hormone, a 13 amino acid peptide that signals through its receptors (MC1-MC5, most preferentially through MC4) located in the brain and more specifically in the paraventricular hypothalamic nucleus. Recently Aughton and colleagues demonstrated that α-melanocortin-stimulating hormone (1 μM) relaxed both vaginal strips and vaginal arteries from rats [69]. Bremelanotide (PT-141, Palatin®) is a synthetic analogue of α-melanocortin-stimulating hormone that shows highest binding affinity for the MC4 receptor (similar to its endogenous peptidic counterpart). However, as reported by Aughton and colleagues, the endogenous form, α-melanocortin-stimulating hormone was much more effective in relaxing rabbit vaginal wall and vaginal artery smooth muscle than bremelanotide [69]. In pre-clinical trials, use of bremelanotide in health women showed increased vaginal blood flow in response to visual sexual stimulation as well as an increase in sexual desire and arousal in menopausal women [2]. Administration of these central acting agonists varies between oral, intranasal and sublingual. Intranasal appears to be the most effective delivery method where oral administration results in unwanted side effects such as vomiting, nausea and headache.
CONCLUSION
The study of FSD is as complex as any disorder or disease. Our understanding of female sexuality was only first formally addressed roughly 50 years ago. During this period, and even now, the treatment of FSD has primarily focused on psychosocial/cultural therapy, and highlights that our limited knowledge is reflective of the inadequate treatment options available. Due to the complexity of FSD, a multifaceted approach, addressing neurobiological, vasoactive, hormonal as well as psychosocial/cultural aspects [70] would be more comprehensive and would address the needs and concerns of the women that suffer from this disorder. Directly modeling FSD in animals may be difficult. However, the comorbidity observed between FSD and many other diseases, as well as well characterized animal models, potentially will be the future of basic science research of this disorder.
ABBREVIATIONS
- ATP
Adenosine 5′-trisphsophate
- cAMP
3′-5′-cyclic adenosine monophosphate
- cGMP
3′-5′-cyclic guanosine monophosphate
- FASD
Female sexual arousal disorders
- FSD
Female sexual dysfunction
- GTP
Guanosine 5′-trisphsophate
- HSDD
Hypoactive sexual desire disorder
- NANC
Non-adrenergic- non-cholinergic
- NEP
Neutral endopeptidase
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- PDE5
Phosphodiesterase type 5
- PG
Prostaglandins
- PKG
cGMP-dependent protein kinase
- PNS
Pelvic nerve stimulation
- SEP
Soluble endopeptidase
- VIP
Vasoactive intestinal peptide
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest to disclose.
References
- 1.Basson R. Women’s sexual function and dysfunction: current uncertainties, future directions. Int J Impot Res. 2008;20:466. doi: 10.1038/ijir.2008.23. [DOI] [PubMed] [Google Scholar]
- 2.Brown AD, Blagg J, Reynolds DS. Designing drugs for the treatment of female sexual dysfunction. Drug Discov Today. 2007;12:757. doi: 10.1016/j.drudis.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein I. Sexual dysfunction in women: what can urologists contribute? Curr Urol Rep. 2008;9:475. doi: 10.1007/s11934-008-0081-5. [DOI] [PubMed] [Google Scholar]
- 4.Sommer F, Caspers HP, Esders K, Klotz T, Engelmann U. Measurement of vaginal and minor labial oxygen tension for the evaluation of female sexual function. J Urol. 2001;165:1181. [PubMed] [Google Scholar]
- 5.Maravilla KR, Yang CC. Magnetic resonance imaging and the female sexual response: overview of techniques, results, and future directions. J Sex Med. 2008;5:1559. doi: 10.1111/j.1743-6109.2008.00839.x. [DOI] [PubMed] [Google Scholar]
- 6.NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83. [PubMed] [Google Scholar]
- 7.Salonia A, Munarriz RM, Naspro R, Nappi RE, Briganti A, Chionna R, Federghini F, Mirone V, Rigatti P, Goldstein I, Montorsi F. Women’s sexual dysfunction: a pathophysiological review. BJU Int. 2004;93:1156. doi: 10.1111/j.1464-410X.2004.04796.x. [DOI] [PubMed] [Google Scholar]
- 8.Frank E, Anderson C, Rubinstein D. Frequency of sexual dysfunction in “normal” couples. N Engl J Med. 1978;299:111. doi: 10.1056/NEJM197807202990302. [DOI] [PubMed] [Google Scholar]
- 9.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 10.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol. 1994;151:54. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 11.Hatzimouratidis K, Hatzichristou D. Sexual dysfunctions: classifications and definitions. J Sex Med. 2007;4:241. doi: 10.1111/j.1743-6109.2007.00409.x. [DOI] [PubMed] [Google Scholar]
- 12.Jackson G. Sexual dysfunction and the cardiac patient--no room for gender bias. Int J Clin Pract. 1999;53:411. [PubMed] [Google Scholar]
- 13.Kostis JB, Jackson G, Rosen R, Barrett-Connor E, Billups K, Burnett AL, Carson C, 3rd, Cheitlin M, Debusk R, Fonseca V, Ganz P, Goldstein I, Guay A, Hatzichristou D, Hollander JE, Hutter A, Katz S, Kloner RA, Mittleman M, Montorsi F, Montorsi P, Nehra A, Sadovsky R, Shabsigh R. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference) Am J Cardiol. 2005;96:85M. doi: 10.1016/j.amjcard.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz ER, Kapur V, Bionat S, Rastogi S, Gupta R, Rosanio S. The prevalence and clinical relevance of sexual dysfunction in women and men with chronic heart failure. Int J Impot Res. 2008;20:85. doi: 10.1038/sj.ijir.3901613. [DOI] [PubMed] [Google Scholar]
- 15.Rosen RC, Wing RR, Schneider S, Wadden TA, Foster GD, West DS, Kitabchi AE, Brancati FL, Maschak-Carey BJ, Bahnson JL, Lewis CE, Gendrano IN., 3rd Erectile Dysfunction in type 2 diabetic men: relationship to exercise fitness and cardiovascular risk factors in the look AHEAD trial. J Sex Med. 2009;6(5):1414. doi: 10.1111/j.1743-6109.2008.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fatemi SS, Taghavi SM. Evaluation of sexual function in women with type 2 diabetes mellitus. Diab Vasc Dis Res. 2009;6:38. doi: 10.3132/dvdr.2009.07. [DOI] [PubMed] [Google Scholar]
- 17.Olarinoye J, Olarinoye A. Determinants of sexual function among women with type 2 diabetes in a Nigerian population. J Sex Med. 2008;5:878. doi: 10.1111/j.1743-6109.2007.00649.x. [DOI] [PubMed] [Google Scholar]
- 18.Rutherford D, Collier A. Sexual dysfunction in women with diabetes mellitus. Gynecol Endocrinol. 2005;21:189. doi: 10.1080/09513590400021110. [DOI] [PubMed] [Google Scholar]
- 19.Newman AS, Bertelson AD. Sexual dysfunction in diabetic women. J Behav Med. 1986;9:261. doi: 10.1007/BF00844773. [DOI] [PubMed] [Google Scholar]
- 20.Giraldi A, Marson L, Nappi R, Pfaus J, Traish AM, Vardi Y, Goldstein I. Physiology of female sexual function: animal models. J Sex Med. 2004;1:237. doi: 10.1111/j.1743-6109.04037.x. [DOI] [PubMed] [Google Scholar]
- 21.Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Horm Behav. 2003;43:503. doi: 10.1016/s0018-506x(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins WJ, Becker JB. Role of the striatum and nucleus accumbens in paced copulatory behavior in the female rat. Behav Brain Res. 2001;121:119. doi: 10.1016/s0166-4328(00)00394-6. [DOI] [PubMed] [Google Scholar]
- 24.Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav Neurosci. 1995;109:354. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- 25.Angulo J, Cuevas P, Cuevas B, Bischoff E, de Tejada IS. Antidepressant-induced inhibition of genital vascular responses is reversed by vardenafil in female rabbits. J Sex Med. 2006;3:988. doi: 10.1111/j.1743-6109.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 26.Park K, Goldstein I, Andry C, Siroky MB, Krane RJ, Azadzoi KM. Vasculogenic female sexual dysfunction: the hemodynamic basis for vaginal engorgement insufficiency and clitoral erectile insufficiency. Int J Impot Res. 1997;9:27. doi: 10.1038/sj.ijir.3900258. [DOI] [PubMed] [Google Scholar]
- 27.Giuliano F, Allard J, Compagnie S, Alexandre L, Droupy S, Bernabe J. Vaginal physiological changes in a model of sexual arousal in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R140. doi: 10.1152/ajpregu.2001.281.1.R140. [DOI] [PubMed] [Google Scholar]
- 28.Allahdadi KJ, Tostes R, Webb RC. Contribution of Endothelin-1 in Constriction of Pudendal Artery in Female Rats: Potential Role in Female Sexual Dysfunction. Joint Meeting of the European (ESSM) and International (ISSM) Societies for Sexual Medicine, Brussels, Belgium, Journal of Sexual Medicine; Brussels, Belgium: Wiley-Blackwell; 2008. p. 60. [Google Scholar]
- 29.Giraldi A, Persson K, Werkstrom V, Alm P, Wagner G, Andersson KE. Effects of diabetes on neurotransmission in rat vaginal smooth muscle. Int J Impot Res. 2001;13:58. doi: 10.1038/sj.ijir.3900648. [DOI] [PubMed] [Google Scholar]
- 30.Myung SC, Lee MY, Lee SY, Yum SH, Park SH, Kim SC. Contractile changes of the clitoral cavernous smooth muscle in female rabbits with experimentally induced overactive bladder. J Sex Med. 2008;5:1088. doi: 10.1111/j.1743-6109.2008.00777.x. [DOI] [PubMed] [Google Scholar]
- 31.Corbin JD. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res. 2004;16(Suppl 1):S4. doi: 10.1038/sj.ijir.3901205. [DOI] [PubMed] [Google Scholar]
- 32.Bryan NS, Bian K, Murad F. Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci. 2009;14:1. doi: 10.2741/3228. [DOI] [PubMed] [Google Scholar]
- 33.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berman JR, Berman LA, Lin H, Flaherty E, Lahey N, Goldstein I, Cantey-Kiser J. Effect of sildenafil on subjective and physiologic parameters of the female sexual response in women with sexual arousal disorder. J Sex Marital Ther. 2001;27:411. doi: 10.1080/713846815. [DOI] [PubMed] [Google Scholar]
- 35.Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG. 2001;108:623. doi: 10.1111/j.1471-0528.2001.00143.x. [DOI] [PubMed] [Google Scholar]
- 36.Cavalcanti AL, Bagnoli VR, Fonseca AM, Pastore RA, Cardoso EB, Paixao JS, Soares JM, Jr, Saad F, Baracat EC. Effect of sildenafil on clitoral blood flow and sexual response in postmenopausal women with orgasmic dysfunction. Int J Gynaecol Obstet. 2008;102:115. doi: 10.1016/j.ijgo.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Basson R, McInnes R, Smith MD, Hodgson G, Koppiker N. Efficacy and safety of sildenafil citrate in women with sexual dysfunction associated with female sexual arousal disorder. J Womens Health Gend Based Med. 2002;11:367. doi: 10.1089/152460902317586001. [DOI] [PubMed] [Google Scholar]
- 38.Uckert S, Oelke M, Albrecht K, Stief C, Jonas U, Hedlund P. Immunohistochemical description of cyclic nucleotide phosphodiesterase (PDE) isoenzymes in the human labia minora. J Sex Med. 2007;4:602. doi: 10.1111/j.1743-6109.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 39.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205. [PubMed] [Google Scholar]
- 40.Fourcroy JL. Female sexual dysfunction: potential for pharmacotherapy. Drugs. 2003;63:1445. doi: 10.2165/00003495-200363140-00002. [DOI] [PubMed] [Google Scholar]
- 41.Kielbasa LA, Daniel KL. Topical alprostadil treatment of female sexual arousal disorder. Ann Pharmacother. 2006;40:1369. doi: 10.1345/aph.1G472. [DOI] [PubMed] [Google Scholar]
- 42.Liao Q, Zhang M, Geng L, Wang X, Song X, Xia P, Lu T, Lu M, Liu V. Efficacy and safety of alprostadil cream for the treatment of female sexual arousal disorder: a double-blind, placebo-controlled study in chinese population. J Sex Med. 2008;5:1923. doi: 10.1111/j.1743-6109.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- 43.Pacher P, Mabley JG, Liaudet L, Evgenov OV, Southan GJ, Abdelkarim GE, Szabo C, Salzman AL. Topical administration of a novel nitric oxide donor, linear polyethylenimine-nitric oxide/nucleophile adduct (DS1), selectively increases vaginal blood flow in anesthetized rats. Int J Impot Res. 2003;15:461. doi: 10.1038/sj.ijir.3901045. [DOI] [PubMed] [Google Scholar]
- 44.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meston CM, Worcel M. The effects of yohimbine plus L-arginine glutamate on sexual arousal in postmenopausal women with sexual arousal disorder. Arch Sex Behav. 2002;31:323. doi: 10.1023/a:1016220225392. [DOI] [PubMed] [Google Scholar]
- 46.Pfaus JG, Kippin TE, Coria-Avila G. What can animal models tell us about human sexual response? Annu Rev Sex Res. 2003;14:1. [PubMed] [Google Scholar]
- 47.Ito TY, Polan ML, Whipple B, Trant AS. The enhancement of female sexual function with ArginMax, a nutritional supplement, among women differing in menopausal status. J Sex Marital Ther. 2006;32:369. doi: 10.1080/00926230600834901. [DOI] [PubMed] [Google Scholar]
- 48.Ito TY, Trant AS, Polan ML. A double-blind placebo-controlled study of ArginMax, a nutritional supplement for enhancement of female sexual function. J Sex Marital Ther. 2001;27:541. doi: 10.1080/713846828. [DOI] [PubMed] [Google Scholar]
- 49.Giraldi A, Alm P, Werkstrom V, Myllymaki L, Wagner G, Andersson KE. Morphological and functional characterization of a rat vaginal smooth muscle sphincter. Int J Impot Res. 2002;14:271. doi: 10.1038/sj.ijir.3900886. [DOI] [PubMed] [Google Scholar]
- 50.Ottesen B, Gerstenberg T, Ulrichsen H, Manthorpe T, Fahrenkrug J, Wagner G. Vasoactive intestinal polypeptide (VIP) increases vaginal blood flow and inhibits uterine smooth muscle activity in women. Eur J Clin Invest. 1983;13:321. doi: 10.1111/j.1365-2362.1983.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 51.Kaye B, Brearley CJ, Cussans NJ, Herron M, Humphrey MJ, Mollatt AR. Formation and pharmacokinetics of the active drug candoxatrilat in mouse, rat, rabbit, dog and man following administration of the prodrug candoxatril. Xenobiotica. 1997;27:1091. doi: 10.1080/004982597240046. [DOI] [PubMed] [Google Scholar]
- 52.Ziegler D, Witte K, Straub M, Weske M. Medicaments containing dually acting inhibitors of neutral endopeptidase and of human soluble endopeptidase for the treatment of sexual dysfunction. (WO/2005/112939) 2005 Available from http://www.wipo.int/pctdb/en/wo.jsp?wo=2005112939.
- 53.Bancroft J. The endocrinology of sexual arousal. J Endocrinol. 2005;186:411. doi: 10.1677/joe.1.06233. [DOI] [PubMed] [Google Scholar]
- 54.Bancroft J. Sexual effects of androgens in women: some theoretical considerations. Fertil Steril. 2002;77(Suppl 4):S55. doi: 10.1016/s0015-0282(02)02961-8. [DOI] [PubMed] [Google Scholar]
- 55.Davis SR. Androgens and female sexuality. J Gend Specif Med. 2000;3:36. [PubMed] [Google Scholar]
- 56.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 57.Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, Bachman G, Aguirre OA, Lucas JD, Rodenberg C, Buch A, Watts NB. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med. 2005;165:1582. doi: 10.1001/archinte.165.14.1582. [DOI] [PubMed] [Google Scholar]
- 58.Davis SR, van der Mooren MJ, van Lunsen RH, Lopes P, Ribot C, Rees M, Moufarege A, Rodenberg C, Buch A, Purdie DW. Efficacy and safety of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Menopause. 2006;13:387. doi: 10.1097/01.gme.0000179049.08371.c7. [DOI] [PubMed] [Google Scholar]
- 59.Vigersky RA. Goldilocks and menopause. Arch Intern Med. 2005;165:1571. doi: 10.1001/archinte.165.14.1571. [DOI] [PubMed] [Google Scholar]
- 60.Berman JR. Physiology of female sexual function and dysfunction. Int J Impot Res. 2005;17(Suppl 1):S44. doi: 10.1038/sj.ijir.3901428. [DOI] [PubMed] [Google Scholar]
- 61.Labrie F, Cusan L, Gomez JL, Cote I, Berube R, Belanger P, Martel C, Labrie C. Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause. 2009;16:30. doi: 10.1097/gme.0b013e31817b6132. [DOI] [PubMed] [Google Scholar]
- 62.Nijland EA, Weijmar Schultz WC, Nathorst-Boos J, Helmond FA, Van Lunsen RH, Palacios S, Norman RJ, Mulder RJ, Davis SR. Tibolone and transdermal E2/NETA for the treatment of female sexual dysfunction in naturally menopausal women: results of a randomized active-controlled trial. J Sex Med. 2008;5:646. doi: 10.1111/j.1743-6109.2007.00726.x. [DOI] [PubMed] [Google Scholar]
- 63.Dula E, Bukofzer S, Perdok R, George M. Double-blind, crossover comparison of 3 mg apomorphine SL with placebo and with 4 mg apomorphine SL in male erectile dysfunction. Eur Urol. 2001;39:558. doi: 10.1159/000052503. [DOI] [PubMed] [Google Scholar]
- 64.Giammusso B, Colpi GM, Cormio L, Ludovico G, Soli M, Ponchietti R, Montorsi F, Panzironi C, Guastella B. An open-label, randomized, flexible-dose, crossover study to assess the comparative efficacy and safety of sildenafil citrate and apo-morphine hydrochloride in men with erectile dysfunction. Urol Int. 2008;81:409. doi: 10.1159/000167838. [DOI] [PubMed] [Google Scholar]
- 65.Modell JG, May RS, Katholi CR. Effect of bupropion-SR on orgasmic dysfunction in nondepressed subjects: a pilot study. J Sex Marital Ther. 2000;26:231. doi: 10.1080/00926230050084623. [DOI] [PubMed] [Google Scholar]
- 66.Bechara A, Bertolino MV, Casabe A, Fredotovich N. A double-blind randomized placebo control study comparing the objective and subjective changes in female sexual response using sublingual apomorphine. J Sex Med. 2004;1:209. doi: 10.1111/j.1743-6109.2004.04030.x. [DOI] [PubMed] [Google Scholar]
- 67.Caruso S, Agnello C, Intelisano G, Farina M, Di Mari L, Cianci A. Placebo-controlled study on efficacy and safety of daily apomorphine SL intake in premenopausal women affected by hypoactive sexual desire disorder and sexual arousal disorder. Urology. 2004;63:955. doi: 10.1016/j.urology.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 68.Lu W, Jiang W, Chen J, Yin M, Wang Z, Jiang X. Modulation of brain delivery and copulation by intranasal apomorphine hydrochloride. Int J Pharm. 2008;349:196. doi: 10.1016/j.ijpharm.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 69.Aughton KL, Hamilton-Smith K, Gupta J, Morton JS, Wayman CP, Jackson VM. Pharmacological profiling of neuropeptides on rabbit vaginal wall and vaginal artery smooth muscle in vitro. Br J Pharmacol. 2008;155:236. doi: 10.1038/bjp.2008.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perelman MA. Clinical application of CNS-acting agents in FSD. J Sex Med. 2007;4(Suppl 4):280. doi: 10.1111/j.1743-6109.2007.00611.x. [DOI] [PubMed] [Google Scholar]