Abstract
Risk for Alzheimer's disease escalates dramatically with increasing age in the later decades of life. It is widely recognized that a preclinical condition in which memory loss is greater than would be expected for a person's age, referred to as amnestic mild cognitive impairment, may offer the best opportunity for intervention to treat symptoms and modify disease progression. Here we discuss a basis for age-related memory impairment, first discovered in animal models and recently isolated in the medial temporal lobe system of man, that offers a novel entry point for restoring memory function with the possible benefit in slowing progression to Alzheimer's disease.
Keywords: Memory, mild cognitive impairment, hippocampus, dentate gyrus/CA3, pattern separation, pattern completion, animal models, neuroimaging
INTRODUCTION
As John Bayley wrote about his wife's illness in “Elegy for Iris” (Murdoch), “Alzheimer's, in fact, like an insidious fog, is barely noticeable until everything around has disappeared [1].”
Memory loss is a common complaint at older ages and is often worrisome as memory problems can be the leading edge of an insidious fog, forecasting Alzheimer's disease (AD). Although by no means a certain diagnosis for impending AD, for the elderly with amnestic mild cognitive impairment (aMCI), who have memory impairment greater than would be expected for their age, the conversion to AD is reported to occur at a rate of 8–15% per year [2,3]. The field is fervently looking for markers that give a definitive diagnosis of incipient AD. With the discovery of such markers it is hoped that promising therapies, now under development, could be used to prevent the devastating damage to the brain and profound clinical decline of patients who would otherwise succumb to dementia. Still the common thread of memory loss, which runs through aging, MCI, and AD points to a specific system in the brain. Today we understand how changes in the function of this system can serve as a basis for memory loss in aged laboratory animals. Here we discuss the relevance of those findings for age-related memory loss in man, with the possible implication that this condition could be permissive for the progression of AD.
The structures of the medial temporal lobe in the mammalian brain are critical for memory functions that give us a record of our experience, in acquiring new facts and preserving information about the events in our lives, the latter commonly referred to as episodic memory [4]. This system provides communication between high order cortical circuits and the hippocampal formation. It is appreciated that hippocampal damage at any age causes a clear amnestic syndrome in brains that otherwise function well. Features of memory loss at older ages, in humans and lab animals, resemble key features of this amnestic syndrome. However, as distinct from amnesic patients with frank destruction of the hippocampus, neurons and circuits remain largely intact in aging when deficits in memory occur [5,6]. Indeed, the basis for impaired memory in healthy aging is not neurodegeneration, as once thought, but instead arises from more subtle functional alterations. Recent research has shown how the ability to rapidly encode new information in the dentate gyrus (DG) and CA3 subregions that receive input from the layer II entorhinal cortex is altered in the aged hippocampus [7,8] (and see [9,10] for details on the model and Figure 1 for a schematic of the circuits described further below).
Fig. (1). Schematic of the circuits in the hippocampal formation.
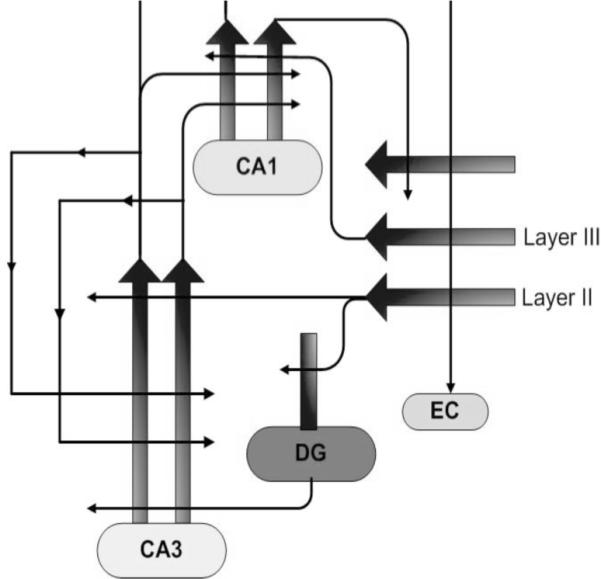
The entorhinal cortex (EC) provides cortically processed information, via layer II neurons, to the dentate gyrus (DG) and distal dendrites of CA3 principal pyramidal neurons. CA3 afferents, in addition to innervation of CA1, form a massive autoassociative input to CA3. Recurrent CA3 input produces generalization/pattern completion. EC input, which is weakened in aging and comprises a significant early lesion in AD, is essential for pattern separation/specific new encoding.
Elegant studies recording from ensembles of single neurons in the brains of young laboratory rats have confirmed what computational models have long predicted about the network properties of cortical input into the DG/CA3. When young rats first explore a familiar arena and then are placed in a second environment, CA3 cells with location-specific encoding (e.g., place fields) rapidly form different patterns of spatial encoding for the two environments [11,12]. In aged rats, on the other hand, hippocampal CA3 neurons can have very similar representations across those two environments [13,7]. Hence, rather than creating distinctive representations, aged rats tend to retrieve the same representation, a process known as `pattern completion', distinguishing it from `pattern separation'. In every day experience the complementary functions of pattern separation and pattern completion are important for episodic memory. Encounters with a colleague or close friend can be represented as distinct episodes while linked to a history of experience in the past with the same person. In computational terms, the aged brain performs more pattern completion and less pattern separation, diminishing the distinctive encoding of experiences.
A second feature of CA3 neurons in aged laboratory animals is tied to the computational shift just described. Namely the CA3 neurons in aged animals with poor memory exhibit excess activity, e.g. higher firing rates [7]. Perhaps this is not surprising because pattern completion is mediated by excitatory autoassociative connections whereby collaterals of the CA3 neurons synapse on many other CA3 neurons, forming the majority of CA3 inputs. A shift in the balance between extrinsic cortical inputs that drive pattern separation and autoassociative control that mediates pattern completion could both elevate CA3 activity and prevent the encoding of new information.
Evidence is now accumulating that the basis of age-related memory impairment in laboratory rats just described may also apply to the aged human brain. In memory tests specifically designed to probe pattern separation abilities in humans, aged subjects exhibit an increased tendency to engage in pattern completion at the expense of pattern separation [14]. Here items were presented in a recognition memory task. Instead of the customary judgments of `old' or `new', indicating whether an item presented was already viewed in the set of presented items, subjects were given a third option of `similar'. The similar judgment was an accurate response to items that resembled, e.g., shared some common elements, with a previous item but was not an exact repeat. Compared to the accuracy of young adults on those similar items, aged participants were more likely to judge them as repetitions, calling them `old', providing behavioral evidence of diminished pattern separation in aging.
A similar computational basis for pattern separation in the DG/CA3 component of the hippocampal network has also been observed in the human brain. Recent research using high-resolution functional magnetic resonance neuroimaging (fMRI) has demonstrated activation in the DG/CA3 consistent with this function in young adults [15]. When subjects viewed an item that was similar but not identical to an item seen before, activation in the DG/CA3 region, but not other components of the hippocampal system, was consistent with encoding of new information in contrast to the activation observed on exposure to a repeated item.
If hippocampal processing is shifted in the aged human to pattern completion, is there also an emergence of greater activity driven by the autoassociative network? Until recently high-resolution fMRI has not been used to address this question. Some earlier reports, however, highlighted greater hippocampal activation observed during memory encoding in MCI [16,17,18,19]. In another recent study of aged individuals (not MCI) increased hippocampal activation was similarly seen in those subjects with poorer memory performance [20]. New evidence has now localized elevated activity in such conditions to the DG/CA3 region. In a three-choice recognition task, as described in the behavioral memory study above — using new, old, similar judgments — increased activation localized to the DG/CA3, but not other subregions of the hippocampal system, was observed in aMCI participants compared to age matched controls (Yassa, M. unpublished data). Those aMCI subjects, compared to their age-matched controls, also had a corresponding deficit in making accurate memory judgments, more often identifying similar items as repeats. Thus, while aging itself, as reported by Toner et al. [14], can impair memory performance when pattern separation is taxed, a further loss is seen in aMCI, along with elevated activation in the DG/CA3 network.
Some have viewed the fMRI activations in MCI as a compensatory phenomenon, suggesting that greater activation is recruited in response to a failing memory system and needed to maintain memory function. The computational account offered here, based on animal studies, suggests otherwise, namely that excess activity is due to a shift in network properties that cause memory failure. If excess activity is not beneficial, moreover, it could have further deleterious consequences in the aged brain contributing to pathophysiology in AD [21]. That suggestion is consistent with evidence, albeit from relatively small-scale studies, of increased hippocampal activation in MCI as a predictor for further cognitive decline and conversion to AD [22]. At the very least it is important to understand what role, if any, the condition associated with memory impairment may have in driving disease progression.
The answer to that important question is not yet forthcoming. It is widely recognized that the earliest pathological markers of AD are found in the hippocampal system, most notably in layer II entorhinal neurons where synaptic failure (at DG/CA3 connections) is thought to precede neuronal degeneration [23,24]. CA1 neurons, which are highly sensitive to excitotoxicity are also lost in large numbers in AD brains, suggesting that excess drive from CA3 could have deleterious effects. If lowering excess activity can shift the DG/CA3 network from autoassociative control to allow improved encoding by extrinsic cortical input, as recent animal studies now suggest, such treatments might also have a benefit in dampening processes that contribute to progressive destruction of this critical brain system.
ACKNOWLEDGEMENT
Dr. Gallagher is the founder and Chairman of AgeneBio, Inc. Dr. Gallagher owns company stock, which is subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
REFERENCES
- [1].Bayley J. Elegy for Iris. St. Martin's Press; New York: 1999. [Google Scholar]
- [2].Luck T, Rieder-Heller SG, Kaduszkiewicz H, Bickel H, Jessen F, Pentzek M, et al. Mild cognitive impairment in general practice: age-specific prevalence and correlate results from the German study on ageing, cognition and dementia in primary care patients (AgeCoDe) Dement Geriatr Cogn Disord. 2007;24:307–316. doi: 10.1159/000108099. [DOI] [PubMed] [Google Scholar]
- [3].Ravaglia GP, Forti P, Montesi F, Lucicesare A, Pisacane N, Rietti E, et al. Mild cognitive impairment: epidemiology and dementia risk in an elderly Italian population. J Am Geriatr Soc. 2008;56:51–8. doi: 10.1111/j.1532-5415.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- [4].Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Ann Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- [5].Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci USA. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Geinisman Y, Ganeshina O, Yoshida R, Berry RW, Disterhoft JF, Gallagher M. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol Aging. 2004;25:407–416. doi: 10.1016/j.neurobiolaging.2003.12.001. [DOI] [PubMed] [Google Scholar]
- [7].Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- [10].Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci. 2008;28:8945–8954. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- [12].Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- [13].Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol Aging. 2003;24:297–305. doi: 10.1016/s0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- [14].Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- [15].Bakker A, Kirwan CB, Miller NI, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- [20].Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008a;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, et al. App processing and synaptic function. Neuron. 2003;37:925–37. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- [22].Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. J Neurol Neurosurg Psychiatry. 2008b;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Scheff SW, Price D, Schmitt F, Mufson E. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27(10):1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]


