Abstract
Recent research suggests that pulse pressure (PP), a putative marker of vascular integrity, may be associated with brain microvascular damage and age-related cognitive decline. Thus, the present study examined the relationship between PP and cognition in a sample of healthy nondemented older adults. One hundred nine participants were administered neurological and neuropsychological evaluations and determined to be nondemented. Regression analyses were used to examine the relationships among pulse pressure (PP) [systolic blood pressure (SBP) – diastolic blood pressure (DBP)], age, and cognition. PP and related measures were inversely correlated with global cognitive functioning and scores on a composite measure of language function, even after adjusting for age, education, and relevant vascular risk factors. Results indicate that increases in the pulsatile component of blood pressure may convey added risk of global cognitive decline and specific impairment in language abilities.
Keywords: Blood pressure, Hypertension, Aging, Cognition, Stroke, Arterial stiffness
INTRODUCTION
Blood pressure is typically measured at two points within the cardiac cycle, systole and diastole, which are used to calculate the steady component of blood pressure, mean arterial pressure (MAP), or its pulsatile component, pulse pressure (PP) (Nichols & O’Rourke, 1998). The steady component, MAP, is conceptualized as the product of cardiac output and total peripheral resistance. The pulsatile component, PP, is determined by a more complex combination of factors, including stroke volume, arterial compliance, and wave reflection (Safar, Levy, & Struijker-Boudier, 2003). Extensive research has investigated the effects of systolic blood pressure (SBP) and diastolic blood pressure (DBP) on cognitive functioning (Qiu, Winblad, & Fratiglioni, 2005). However, when examined in isolation, these two points on the pressure curve do not fully capture important information about the pulsatile component of blood pressure. Indeed, comparatively few studies have examined the relationship between the pulsatile aspect of blood pressure and cognitive functioning, despite substantial evidence that it is related to vascular disease (Jankowski, Bilo, & Kawecka-Jaszcz, 2007; Nakayama, Hayashi, Yoshimaru, Tsumura, & Ueda, 2002).
In healthy individuals, the elastic properties of the aorta, carotid, and other large arteries reduce pressure fluctuations that occur during the cardiac cycle. Arterial elasticity allows stretching to reduce pressure increases during systole and recoil to buffer decreased pressure during diastole (Safar et al., 2003). These factors assist in the maintenance of relatively constant pressure at the level of distal arterioles and capillary beds (Mitchell, 2008). Changes in elastin and collagen fibers within the elastic lamina of larger vessels may be responsible for stiffening of large arteries with age (Mitchell, 2008). As large arteries stiffen, they lose compliance and no longer buffer fluctuations in pressure throughout the cardiac cycle. The resulting increase in pulsatile stress on the vessel wall may contribute to the development of atherosclerosis, which can further exacerbate arterial stiffening (Zuckerman, Weisman, & Yin, 1989). These changes could expose smaller vessels to harmful pressure fluctuations during each cardiac cycle, potentially leading to microvascular damage (Mitchell, 2008).
This problem may be particularly salient in highly perfused organs with a rich, autoregulated microvasculature, such as the brain. This hypothesis is supported by studies showing that measures of pressure fluctuation and arterial stiffness, such as PP and pulse wave velocity (PWV), have been associated with microvascular damage [white matter lesions (WML)] in older adults with hypertension (Kearney-Schwartz et al., 2009) and those with Alzheimer’s disease (AD) (Lee, Jeong, Choi, Sohn, & Chui, 2006). Additionally, elevated PP has been associated with declines in global cognitive functioning and memory in healthy populations (Waldstein, Rice, Thayer, Najjar, Scuteri, & Zonderman, 2008) and those with various vascular risk factors (Abbatacola et al., 2008; Kearney-Schwartz et al., 2009). Findings have been less consistent with regard to the relationship between PP and measures of working memory, visuospatial abilities, executive functioning, and psychomotor speed (Dahle, Jacobs, & Raz, 2009; Kearney-Schwartz et al., 2009; Muller, Grobbee, Aleman, Bots, & van der Schouw, 2007; Raz, Dahle, Rodrigue, Kennedy, & Land, 2009; Waldstein et al., 2008 ). Inconsistencies across studies may be related to methodological differences as well as variations in sample characteristics (e.g., age, degree of cognitive impairment, and prevalence of vascular risk factors).
Studies have found that the relationship between standard blood pressure measures and cognitive functioning is complex, curvilinear, and age-dependent (for review, Qiu, Winblad, & Fratiglioni, 2005). Longitudinal studies have repeatedly found that elevated blood pressure in midlife is predictive of cognitive decline in late life, but cross-sectional studies indicate that both high and low blood pressure are associated with cognitive decline in older adults. Specifically, low DBP has repeatedly been associated with cognitive decline in older adults (Kennelly, Lawlor, & Kenny, 2009). Although SBP increases with age, MAP remains constant and DBP typically decreases, leading to elevated PP (Mitchell, 2008). Thus, increased PP may be more important in age-related cognitive decline than standard blood pressure measures. Beyond the pulsatile strain produced by increased PP, elevated PP also represents an indirect measure of the increased arterial stiffness and atherosclerosis that occurs with age. Thus, even in the context of blood pressure that is well controlled by medications, PP may remain elevated and continues to be associated with cardiovascular events (Van Bortel, Struiker-Boudier, & Safar, 2001). Taken together these considerations point toward PP as a particularly important hemodynamic measure in studies examining age-related cognitive decline.
Given the potential differential contribution of SBP and DBP to cognitive performance in older adults, measures of pulsatile pressure that correct for these values may be more sensitive to age-related changes in cognition. These measures, referred to as fractional systolic pressure (FSP) and diastolic pressure (FDP), have been associated with vascular disease in animal and human models (Jankowski et al., 2007 ; Nakayama et al., 2002), but their relationship with cognition has not been extensively investigated. Thus, the aim of the present study was to examine nondemented healthy older adults to (1) further examine the relationship between measures of the pulsatile component of blood pressure and cognitive functioning and (2) compare these findings with those of other more commonly used hemodynamic measures (e.g., systolic and diastolic blood pressure).
METHODS
Participants
One hundred nine nondemented individuals were recruited from ongoing community-based studies of normal aging and AD, as well as through the community via word-of-mouth and flyers. All participants underwent medical, neurological, and laboratory evaluations. Individuals with a history of alcoholism, drug abuse, learning disability, neurological, or psychiatric disease were excluded. Participants also underwent a comprehensive neuropsychological battery, including measures of global cognitive functioning, attention, language, executive functioning, visuospatial abilities, memory, and functioning in daily living. To screen for possible dementia, participants with a Dementia Rating Scale (DRS) score of ≤ 126 were excluded from analyses (see also Jak et al., 2009). All data were obtained in compliance with institutional guidelines.
Stroke Risk Factors and Hemodynamic Measures
Hemodynamic measures and vascular risk factors were obtained through clinical interview and physical examination. Blood pressure measures were obtained from single recordings. Participants were seated comfortably for a few minutes before taking blood pressure measures. Self-reported blood pressure was used for participants that frequently measure their own blood pressure. PP and MAP were calculated from SBP and DBP using the following equations: PP = SBP − DBP; MAP = ((2 × DBP) + SBP)/3. FSP and FDP were calculated using the following equations, respectively: FSP = PP/SBP; FDP = PP/DBP (Jankowski et al., 2007; Nakayama et al., 2002).
Neuropsychological Assessment
Cognitive testing was performed using a comprehensive neuropsychological battery described previously (Salmon & Butters, 1992). The battery included an assessment of global cognitive functioning as well as the following six neurocognitive domains: Global cognitive functioning was assessed by the DRS total score. Attention was measured by the attention subscale of the DRS and the Digit Span sub-test of the Wechsler Adult Intelligence Scale – Revised. Language was measured with the Boston Naming Test, category (animals) fluency, and the supermarket items fluency subtest of the DRS. Visuospatial functioning was determined by performance on the Block Design subtest of the Wechsler Intelligence Scale for Children (WISC) and the construction subscale of the DRS. The use of the WISC block design is based on extensive use of this test in research at the UCSD Alzheimer’s Disease Research Center (see Salmon & Butters, 1992) on cognitive changes with aging and dementia. Executive functioning was assessed by the modified Wisconsin Card Sorting Test, 48 card version (perseverative responses), Trail Making Test Part B, and the letter (FAS) fluency test. Verbal Memory was assessed by the Logical Memory subtest of the Wechsler Memory Scale – Revised (immediate and delayed free recall) and the California Verbal Learning Test (Trials 1–5 total recall and long delay free recall). Processing Speed was measured by the Trail Making Test Part A. Raw scores were converted into Z-scores based on the mean and standard deviation of the sample of participants identified as normally aging (see criteria set forth by Jak et al., 2009 ). Cognitive domain scores represented the average Z-score for the tests within each domain.
Statistical Analyses
Non-normally distributed cognitive measures were log-transformed before z-transformation. Independent samples t tests were used to investigate differences in hemodynamic measures and cognitive domain scores between men and women, individuals on or off antihypertensive medications, and those with or without various stroke risk factors, including history of atrial fibrillation (AF), cardiovascular disease (CVD), and transient ischemic attack (TIA) or minor stroke. Pearson product-moment correlations and t tests were used to examine the relationships between hemodynamic measures and neuropsychological test scores, as well as identification of appropriate covariates from demographic and stroke risk data. Bivariate correlations between hemodynamic and neuropsychological measures were treated as omnibus tests by domain, with Bonferroni correction for multiple comparisons being applied for each cognitive domain (i.e., p value of .05/7 = .007). Only those hemodynamic variables found to be significantly related to cognitive domain scores after correction for multiple comparisons were retained in regression models. Multiple hierarchical regression was used to test relationships between hemodynamic parameters and cognitive functioning after controlling for relevant covariates. All significance tests were 2-tailed with a cutoff of p < .05, after correction for multiple comparisons.
RESULTS
Participant Characteristics
Forty-eight participants were men (44.0%). The average age at examination was 74.2 years (SD = 10.0), ranging from 48 to 93 years, average DRS total score was 139.9 (SD = 3.7), ranging from 127 to 144, and average education was 16.2 years (SD = 2.3). Average SBP was 125.8 (SD = 12.8), average DBP was 73.2 (SD = 9.4), 48.0% were on anti-hypertensive medications, 21.2% had evidence of CVD, 11.5% had a history of TIA or minor stroke, and 16.2% had AF. Results of independent samples t tests indicated no significant differences in any hemodynamic measures between men and women, individuals on or off antihypertensive medications, or those with or without a history of AF (p > .10). Age showed a significant positive correlation with SBP (r = 0.25; p < .01) and a nonsignificant trend toward a negative correlation with DBP (r = −0.18; p = .06). Age was strongly correlated with PP measures, including PP (r = 0.38; p < .0001), FSP (r = 0.36; p = .0001), and FDP (r = 0.36; p = .0001). The steady component of blood pressure, MAP, was not correlated with age (r = −0.01; p > .90).
Hemodynamic Measures and Cognition
Commonly used hemodynamic measures, including SBP, DBP, and MAP, showed no significant correlations with scores on composite measures of cognitive functioning, after controlling for multiple comparisons. PP was inversely correlated with language ability (p = .0001), but showed no significant relationship with other cognitive domains after correction for multiple comparisons. Fractionated measures of PP, including FSP and FDP, were also significantly correlated with language ability (p < .001). Additionally, FDP showed a significant negative correlation with global cognitive functioning (p = .003) (Table 1).
Table 1.
Correlations between hemodynamic and cognitive measures
| Cognitive Domain | PP | FSP | FDP | SBP | DBP | MAP |
|---|---|---|---|---|---|---|
| Global | −0.21 | −0.23 | −0.29** | −0.06 | 0.22 | 0.13 |
| Attention | −0.04 | 0.01 | −0.004 | −0.08 | −0.05 | −0.08 |
| Language | −0.36*** | −0.34*** | −0.36*** | −0.23 | 0.19 | 0.02 |
| Visuospatial | −0.02 | −0.04 | −0.08 | 0.01 | 0.03 | 0.03 |
| Executive | −0.24 | −0.20 | −0.24 | −0.17 | 0.09 | −0.02 |
| Memory | 0.02 | 0.03 | −0.03 | 0.02 | 0 | 0.01 |
| Processing Speed | −0.21 | −0.15 | −0.18 | −0.24 | −0.03 | −0.13 |
Note. p values are displayed only for relationships significant after correction for multiple comparisons.
p<.01.
p<.001.
Regression Analyses
Analysis of potential covariates for multiple regression analyses indicated a negative correlation between age and language (r = −0.32; p < .01). Education was positively correlated with language (r = 0.29; p < .01) and global cognitive functioning (r = 0.40; p < .0001). Additionally, t tests indicated that individuals on antihypertensive medications exhibited lower global cognitive functioning (p < .01) and those with AF displayed lower language ability (p < .05). Consequently, these variables were retained in regression models.
The relationship between PP and language ability remained significant after controlling for age, education, and history of AF, β = −0.26; p < .01; ΔR2 = 0.06 (Figure 1). The inverse correlation between age and language ability was no longer significant after controlling for PP and these covariates, β = −0.13, p > .10, ΔR2 = .01. The relationship between FSP and language ability remained significant using the same model, β = −0.24; p = .01; ΔR2 = 0.05, with similar results holding for FDP, β = −0.24; p < .05; ΔR2 = 0.05.
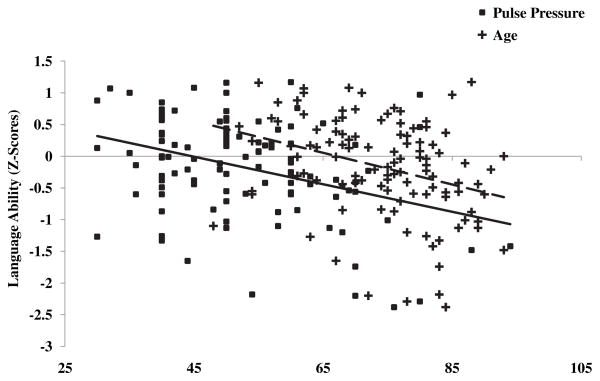
Fig. 1.
Scatterplots of age and pulse pressure (PP) by language ability are displayed. Age (+, dashed line) and PP (■, solid line) exhibited significant inverse correlations with language ability: r = −0.32, p < .01 and r = −0.36, p < .001, respectively.
The relationship between FDP and global cognitive functioning remained significant after controlling for education and antihypertensive medication, β = −0.22; p < .05; ΔR2 = 0.05 (Figure 1).
DISCUSSION
Our results demonstrated that nondemented older adults with elevated measures of the pulsatile component of blood pressure performed more poorly on tests of language, as well as global cognitive functioning, but not on tasks assessing attention, executive, visuospatial, memory, and processing speed abilities. The observed relationships between PP measures and cognition were of medium effect size and were not fully accounted for by relevant covariates, including age, education, and vascular risk factors. Importantly, other more commonly used hemodynamic measures, including SBP, DBP, and MAP, showed no significant relationships with measures of cognitive function after controlling for multiple comparisons. All measures of the pulsatile component of blood pressure, including fractionated measures of PP that adjust for absolute values of SBP and DBP, showed similar negative associations with cognition, suggesting that the results were not greatly influenced by either of these blood pressure points in isolation. In fact, FDP appeared to show the most consistent relationship with cognition, displaying negative relationships with language and global cognitive functioning.
All three measures of PP showed stronger relationships with language functioning than any other cognitive ability, suggesting that this cognitive domain may be particularly impacted by elevations in the pulsatile component of blood pressure. In older adults, insidious decline in language abilities and executive processes have both been associated with functional alterations within frontal-subcortical networks, suggesting that the ability to access and retrieve semantic information may underlie these word-finding problems (Wierenga et al., 2008). Increased pulsatile pressure could be impacting these functional networks through disruption of frontal-subcortical white matter tracts, as neuroimaging studies have associated increased PP with subcortical leukoaraiosis (Kearney-Schwartz et al., 2009; Lee et al., 2006).
However, the current study did not observe a significant relationship between PP and executive functioning, suggesting that other mechanisms of injury may be involved. Beyond evidence of subcortical ischemic lesions, there may be more insidious neuropathologic changes associated with alterations in hemodynamic parameters. For example, both animal and human studies have found that blood pressure elevation can cause reductions in cerebral blood flow and increased cerebral atrophy, even in the absence of frank vascular lesions (Fujishima, Ibayashi, Fujii, & Mori, 1995; Gesztelyi, Finnegan, DeMaro, Wang, Chen, & Fenstermacher, 1993). These subtle neuropathological changes have been associated with cognitive dysfunction (Dai, Lopez, Carmichael, Becker, Kuller, & Gach, 2008; Vlek et al., 2009), and it is possible that increased pulse pressure may also convey vulnerability through more subtle changes in neurovascular function. Another possibility is that more richly perfused areas, such as cortical gray matter, could be susceptible to damage or dysfunction from increased PP. It has been proposed that increased microvascular perfusion in the context of elevated PP may be associated with particularly high risk of damage to these brain areas (Mitchell, 2008). While it is possible that increased PP is causing impairment through cerebrovascular damage, it is also possible that PP is simply serving as a marker of large artery stiffness and cerebrovascular disease. Thus, PP may represent a measure of systemic vascular disease, a causative factor in cerebrovascular dysfunction, or some combination of both. Future studies examining the relationship between PP and cerebral structure, function, and perfusion may provide more information regarding the mechanism underlying the association between PP and language function.
Although the present study did not find a significant association between executive function and measures of PP after correction for multiple comparisons, performance of category fluency tasks involves some executive processes and has been periodically included as a measure of executive function in prior studies. Given the high prevalence of age-related decline in language ability, it may be more favorable to combine category fluency measures into a language composite in studies involving older adults. These semantic abilities frequently decline with age and are among the earliest cognitive abilities to decline in the course of AD (see Bondi, Jak, Delano-Wood, Jacobson, Delis, & Salamon, 2008 for review). Studies have found that patients with AD exhibit elevated PP relative to controls and that elevated PP is associated with increased risk of developing AD (Lee et al., 2006; Qiu et al., 2003). The current study did not examine patients with AD. Thus, the significance of the current findings remains unclear in terms of risk of AD, although we have demonstrated significant declines in semantic memory in the years preceding mild cognitive impairment (MCI) or AD diagnoses (Mickes et al., 2007). Future longitudinal studies examining whether PP predicts declines in language function, or progression to MCI or dementia, will further establish the significance of this finding for dementia research.
The findings of the current study are limited by the nature of our sample, which was relatively well educated and medically healthy. The relationship between PP and cognitive functioning may be different in individuals with a greater number of risk factors for cognitive impairment.
Acknowledgments
This work was supported by grant IIRG 07-59343 from the Alzheimer’s Association (M.W.B.); National Institute on Aging grants R01 AG012674 (M.W.B.), K24 AG026431 (M.W.B.), and P50 AG05131 (D.P.S.); National Institutes of Health Ruth L. Kirschstein National Research Service Award MH18399 (D.A.N.); and by the Department of Veterans Affairs (D.C.D., L.D.W., A.J.J., C.E.W.).
References
- Abbatacola AM, Barbieri M, Rizzo MR, Grella R, Laieta MT, Quaranta E, et al. Arterial stiffness and cognition in elderly persons with impaired glucose tolerance and microalbuminuria. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2008;63:991–996. doi: 10.1093/gerona/63.9.991. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Jak AJ, Delano-Wood L, Jacobson MW, Delis DC, Salmon DP. Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychology Review. 2008;18:73–90. doi: 10.1007/s11065-008-9054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognition: Blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychology and Aging. 2009;24:154–162. doi: 10.1037/a0014283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller H, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke. 2008;39:349–354. doi: 10.1161/STROKEAHA.107.495457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima M, Ibayashi S, Fujii K, Mori S. Cerebral blood flow and brain function in hypertension. Hypertension Research. 1995;18:111–117. doi: 10.1291/hypres.18.111. [DOI] [PubMed] [Google Scholar]
- Gesztelyi G, Finnegan W, DeMaro JA, Wang JY, Chen JL, Fenstermacher J. Parenchymal microvascular systems and cerebral atrophy in spontaneously hypertensive rats. Brain Research. 1993;611:249–257. doi: 10.1016/0006-8993(93)90510-t. [DOI] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski P, Bilo G, Kawecka-Jaszcz K. The pulsatile component of blood pressure: Its role in the pathogenesis of atherosclerosis. Blood Pressure. 2007;16:238–245. doi: 10.1080/08037050701428166. [DOI] [PubMed] [Google Scholar]
- Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, et al. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke. 2009;40:1229–1236. doi: 10.1161/STROKEAHA.108.532853. [DOI] [PubMed] [Google Scholar]
- Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk of dementia: A double edged sword. Aging Research Reviews. 2009;8:61–70. doi: 10.1016/j.arr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Lee AY, Jeong SH, Choi BH, Sohn EH, Chui H. Pulse pressure correlates with leukoaraiosis in Alzheimer disease. Archives of Gerontology and Geriatrics. 2006;42:157–166. doi: 10.1016/j.archger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Mickes L, Wixted JT, Fennema-Notestine C, Galasko D, Bondi MW, Thal LJ, et al. Progressive impairment on neuropsychological tasks in a longitudinal study of preclinical Alzheimer’s disease. Neuropsychology. 2007;21:696–705. doi: 10.1037/0894-4105.21.6.696. [DOI] [PubMed] [Google Scholar]
- Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. Journal of Applied Physiology. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Grobbee DE, Aleman A, Bots M, van der Schouw YT. Cardiovascular disease and cognitive performance in middle-aged and elderly men. Atherosclerosis. 2007;190:143–149. doi: 10.1016/j.atherosclerosis.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Hayashi T, Yoshimaru K, Tsumura K, Ueda H. Low fractional diastolic pressure in the ascending aorta increased the risk of coronary heart disease. Journal of Human Hypertension. 2002;16:837–841. doi: 10.1038/sj.jhh.1001489. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O’Rourke M. McDonald’s blood flow in arteries: Theoretical, experimental and clinical principles. 4. London: Arnold; 1998. [Google Scholar]
- Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of Alzheimer’s disease in persons aged 75 years and older: A community-based, longitudinal study. Stroke. 2003;34:594–599. doi: 10.1161/01.STR.0000060127.96986.F4. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurology. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land S. Effects of age, genes, and pulse pressure on executive functions in healthy adults. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Butters N. Neuropsychological assessment of dementia in the elderly. In: Katzman R, Rowe JW, editors. Principles of geriatric neurology. F.A. Davis Company; London distributors: Williams & Wilkins, Ltd; Philadelphia, PA: 1992. [Google Scholar]
- Van Bortel LM, Struijker-Boudier HA, Safar ME. Pulse pressure, arterial stiffness, and drug treatment of hypertension. Hypertension. 2001;38:914–921. doi: 10.1161/hy1001.095773. [DOI] [PubMed] [Google Scholar]
- Vlek AL, Visseren FL, Kappelle LJ, Geerlings MI, Vincken KL, Mali WP, et al. Blood pressure and progression of cerebral atrophy in patients with vascular disease. American Journal of Hypertension. 2009;22:1183–1189. doi: 10.1038/ajh.2009.166. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJ, et al. Age-related changes in word retrieval: Role of bilateral frontal and subcortical networks. Neurobiology of Aging. 2008;29:436–451. doi: 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Zuckerman BD, Weisman HF, Yin FC. Arterial hemodynamics in a rabbit model of atherosclerosis. American Journal of Physiology. 1989;257:H891–H897. doi: 10.1152/ajpheart.1989.257.3.H891. [DOI] [PubMed] [Google Scholar]