Abstract
Children with traumatic brain injury (TBI) often experience memory deficits, although the nature, functional implication, and recovery trajectory of such difficulties are poorly understood. The present fMRI study examined the neural activation patterns in a group of young children who sustained moderate TBI in early childhood (n = 7), and a group of healthy control children (n = 13) during a verbal paired associate learning (PAL) task that promoted the use of two mnemonic strategies differing in efficacy. The children with TBI demonstrated intact memory performance and were able to successfully utilize the mnemonic strategies. However, the TBI group also demonstrated altered brain activation patterns during the task compared to the control children. These findings suggest early childhood TBI may alter activation within the network of brain regions supporting associative memory even in children who show good behavioral performance.
Keywords: Traumatic brain injury, children, functional magnetic resonance imaging (fMRI), associative memory
1. Introduction
It is estimated that 250 in every 100,000 children will sustain a traumatic brain injury (TBI) each year [3]. Although individuals with TBI often sustain physical impairments from the injury, other impairments such as problems with emotional, behavioral, and cognitive functioning are the most debilitating consequences of TBI. Pediatric TBI is one of the most frequent causes of interruption to the course of normal cognitive development [3]. Research suggests children who sustain TBI commonly experience deficits in linguistic abilities, psychomotor skills, attention, and executive function [4,75]. In addition, these children can experience memory and new learning deficits, although the nature and outcome of such deficits are not well understood [12]. Memory deficit is not only one of the most common residual effects of TBI, it could have the most functionally significant impact due to its disruption of daily activities and school performance [44].
1.1. Memory deficits in pediatric TBI
Memory deficits are common sequelae of moderate to severe TBI as these injuries often damage regions of the brain supporting memory function [67]. In individuals with TBI, the most common brain lesions are frontal and anterior temporal contusions due to their location near the anterior and middle fossae of the skull [74]. Widespread diffuse axonal injury (DAI) due to shear-strain injuries occurring at the boundaries between white and grey matter is also common in patients with moderate to severe TBI, and can potentially disrupt the interconnections between the prefrontal, subcortical, and posterior cortical regions comprising the memory network [7].
Moderate to severe TBI in children frequently results in impairments in learning and rememberingnew information [12,31,73–75]. Studies suggest that although memory significantly improves during the first year following pediatric TBI, persisting deficits in memory have been documented up to 5 years post-injury [12,31,75]. Studies using a list-learning test designed to assess memory performance and encoding strategy use, the California Verbal Learning Test for Children (CVLT-C), reported learning and memory impairments in children with moderate to severe injury at 1 to 5 years post-injury compared to both age-matched healthy controls and to orthopedic or non-TBI trauma controls [30,31,55,73,75]. A consistent relationship has been observed between verbal memory deficits and injury severity, with greater injury severity resulting in poorer performance on the CVLT-C [6,28,30,55] and on a story recall task [6].
The literature on memory functioning in children with TBI thus far has focused largely on verbal list learning, while another important type of memory, associative memory, has yet to be examined in depth in this group. Through associative learning, previously unassociated ideas and experiences are linked and bound together. Adults with TBI have been shown to demonstrate impaired associative memory performance on verbal paired associate learning (PAL) tasks (for review see [67]), but this has not been investigated in the pediatric TBI literature.
1.2. Mnemonic strategy use in typically-developing children and children with TBI
A systematic strategy for strengthening long-term retention and retrieval of information is referred to as a mnemonic strategy. Mnemonic strategies leading to effective encoding of verbal materials often demand attention [62] and entail semantic, organizational or elaborative processing of the to-be-remembered events [32]. Developmental research suggests children can benefit from memory strategies, although there is a developmental progression in competent memory strategy use from preschool through adolescence, which parallels the development of attention and working memory capacity [61]. By age four, children have the competence to generate elaborative imagery, and by the age of 11 children can use imagery mnemonics in the same way as adults [53]. As children mature, they not only progress from being non-strategic to strategic, they also gradually increase the frequency and number of mnemonic strategies used [59]. The cognitive development literature distinguishes different types of deficiencies that occur as children develop mature strategy use [59]. A production deficiency occurs when a child cannot produce a strategy spontaneously but when prompted can utilize and benefit from the strategy. A utilization deficiency occurs when a child applies an appropriate strategy, either spontaneously or with prompting, but does not benefit in recall or benefits less than does an equally strategic older child [59].
Although memory strategy use has not been examined in depth in the pediatric TBI population, the existing literature suggests that children with severe TBI may have deficiencies in memory strategy use. In studies examining performance on list learning tasks, children with severe TBI were less likely to use an efficient recall strategy than children with mild or moderate TBI [37] and were more likely to use an inefficient strategy such as serial clustering [6] or a passive rehearsal strategy [27]. These studies suggest that children with TBI are less likely to spontaneously employ effective memory strategies. Training in the use of mental imagery strategies has been found to improve memory performance in adults with brain damage [54], but this has not been studied in a pediatric TBI population.
1.3. Neural correlates of associative encoding
Neuroimaging research has begun to elucidate the neural substrates of associative memory encoding (i.e., successful binding or association of information together in long-term memory). Neuropsychological studies [16,21] confirm the role of the medial temporal lobe (MTL) in binding together multiple features or components of an experience to form coherent memory traces. Although controversy remains regarding the precise division of labor between the structures of the MTL and within the hippocampus itself [19], the role of MTL in associative encoding is well-established. Neuroimaging studies in adults have reliably demonstrated activation in the hippocampus during a number of associative encoding tasks, including encoding word pairs [29,42], word triplets [2,20], object pairs [1], and face-name pairs [64].
The MTL functions as part of a complex neural network in adults to support associative encoding. Frontal lobe based cognitive control abilities including selection mechanisms, organization, and strategic processes (such as generating associations between items) are required for successful memory encoding [8–10,25]. Indeed, activation in prefrontal regions including the ventrolateral [8],dorsolateral prefrontal cortex [42],and inferior frontal gyrus [2] has been documented during associative encoding tasks. A number of additional brain regions have also been implicated in successful associative memory encoding, including the inferior parietal lobule, anterior cingulate, and fusiform gyrus [2,29,36,42]. While recent brain imaging studies have examined the neural substrates of associative learning in adults, only a handful of studies to date have examined the neural correlates of memory encoding and memory retrieval in children [41,49,50], and no studies have yet examined the brain structures supporting associative learning in either healthy children or children with acquired brain injuries.
1.4. Neuroimaging studies of TBI
Neuroimaging studies have only recently begun to examine neural activation patterns in individuals who sustained TBI. Several studies of TBI in adults have successfully demonstrated alterations in brain activation patterns during performance of working memory tasks compared to controls using fMRI. These studies found adults with mild TBI [38,39] and moderate to severe TBI [57] demonstrated hyperactivation in the neural regions mediating working memory shortly after the injury compared to healthy controls or orthopedically injured controls. Similar studies of adults with moderate to severe TBI observed a more distributed representation of working memory in individuals with TBI compared to non-injured controls [15,51,58]. Similarly, two recent fMRI studies comparing children with moderate to severe TBI to orthopedically injured controls have observed hyperactivation of relevant brain networks during a verb-generation task [33] and a continuous performance task [34]. Increased activation of relevant brain regions during working memory tasks was also observed in groups of children [45] and adolescents [46] with moderate to severe TBI compared to typically-developing age-matched controls. These imaging studies suggest activation of neural circuitries may be altered after a brain injury, but it is unclear if this finding holds true when considering associative memory processing in children with less severe TBI.
This study sought to characterize the neural activation pattern engaged by effective associative learning in young children with moderate TBI compared to a control group of healthy, typically-developingchildren. The study was novel in its use of fMRI to examine the long-term (i.e., two to four years post-injury) consequences of early pediatric TBI on associative memory ability. In this PAL task, participants were trained to use two different mnemonic strategies to learn pairs of words (e.g., cat-tree) so they could later recall the 2nd word (i.e., tree) given the 1st word (i.e., cat). The mnemonic strategies of interest were an imagery strategy (imagining the items in the pair interacting in some way) and a rote repetition strategy (repeating the word pairs covertly). The imagery strategy promoted relational encoding of the word pairs, while the repetition strategy instead promoted non-associative encoding of the word pairs. In verbal PAL paradigms, typically developing children demonstrate a memory advantage when imagining the two words of the pair interacting in some way (associative encoding) over simply repeating the word pairs [23,52].
It was hypothesized that on behavioral measures of memory performance, children with TBI would have poorer recall performance than the healthy children, although both groups were expected to demonstrate an equal amount of memory advantage (i.e., remember more word pairs) when using the imagery mnemonic compared to using the repetition strategy. Both children with TBI and healthy control children were expected to activate similar networks of brain regions supporting associative encoding during the task although the children with TBI were hypothesized to demonstrate greater activation of brain regions supporting associative memory encoding compared to the healthy control children.
2. Methods
2.1. Participants
Participants in the TBI group were recruited from a larger ongoing study of recovery from TBI in young children and their families conducted at four hospitals with Level 1 Trauma Centers. Eligibility requirements for the participants with TBI in the larger study included: age between 36 to 84 months at the time of injury, English as the primary spoken language in the home, and a TBI requiring overnight admission to the hospital and either evidence of altered neurological status on the Glasgow Coma Scale (GCS) (i.e., total score < 15) or abnormalities on imaging (MRI or CT scan). Exclusionary criteria included: previous history of brain injury, pre-existing neurological disorder or medical problem affecting the CNS, diagnosis of mental retardation or developmental disability, documentation in the medical chart or in the parent interview of child abuse as a cause of the injury, or history of severe pre-morbid psychiatric disorder requiring hospitalization. Children who sustained non-blunt head trauma (e.g., projectile wounds, strokes, drowning) were also excluded. Written informed consent was obtained from a parent or guardian, and written assent was obtained from the child at the time of the study. This study was approved by the medical institutional review boards at Cincinnati Children's Hospital Medical Center and the University of Cincinnati.
We contacted all children in the larger study who had sustained a mild or moderate TBI (see below for definition) and who were at least six years of age and at least 12 months post injury regarding participation in this study. We also recruited healthy, normal control children (NC) from postings at local universities, elementary schools, and hospitals to match the TBI children as closely as possible for age, sex, and handedness. Exclusion criteria for NC participants included an estimated Full Scale IQ of less than 70 or parent report of previous TBI or pre-existing physical, neurological, psychiatric, or developmental disorder. All children in this study had normal hearing per parent report and passed the standard checklist of MRI exclusion criteria used by the Cincinnati Children's Hospital Radiology department in routine clinical scanning.
Nineteen children with TBI were identified from the parent project based on these criteria, and 17 consented to participate in the imaging study. This subset was representative of the larger parent sample on demographic and neuropsychological variables (described below). Nineteen NC children were recruited from the community.
Twenty children (7 TBI and 13 NC) were retained for analysis. While the intent of this project was to study mild to moderate TBI severity, only three children with mild TBI were recruited and only one child with a mild TBI successfully completed the scanning protocol. As such, the decision was made to exclude the child with mild TBI in order to focus on children with injuries of moderate severity. In addition, one NC was excluded due to poor in-scanner task performance (0% recall across conditions) despite intact neuropsychological performance, suggesting lack of task compliance during the scan. The remainder of those excluded had unusable data due to excessive motion, defined as exceeding 0.75 voxel size for more than 25% of the functional imaging data.
Table 1 provides the demographic information for participants included in the current report. Clinical brain scans of the TBI participants performed at the time of injury were used to determine TBI severity, together with other clinical information. Consistent with previous studies [24], mild TBI was defined by a lowest GCS of 13–15 with no documented evidence of brain insult or deterioration of consciousness after the injury; moderate severity was defined as a lowest GCS score of 9–12 or a score of 13–15 accompanied by a skull fracture, intracranial mass, lesion, or contusion, diffuse cerebral swelling, posttraumatic neurologic abnormality, or loss of consciousness for more than 15 minutes; and severe TBI was defined by a lowest GCS score of 8 or less. Using these criteria, all seven children with TBI had moderate injuries.
Table 1.
Demographic and injury-related characteristics of participants
| ID | Age (yrs) | Sex | Race | Maternal Education | Injury Mechanism | Age at Injury (yrs) | Time Since Injury (yrs) | GCS | Imaging at injury | TBI Severity |
|---|---|---|---|---|---|---|---|---|---|---|
| TBI_1 | 8.08 | M | 0 | 4 | Fall | 4.30 | 3.67 | 14 | Abnormal | Moderate |
| TBI_2 | 8.00 | M | 0 | 1 | Car Crash | 3.58 | 4.33 | 9 | Abnormal | Moderate |
| TBI_3 | 7.17 | F | 0 | 3 | Fall | 5.17 | 2.17 | 15 | Abnormal | Moderate |
| TBI_4 | 10.25 | M | 0 | 2 | Fall | 6.33 | 3.92 | 15 | Abnormal | Moderate |
| TBI_5 | 10.17 | F | 0 | 2 | Fall | 5.67 | 4.50 | 15 | Abnormal | Moderate |
| TBI_6 | 9.58 | F | 0 | 5 | Fall | 6.58 | 2.92 | 9 | Abnormal | Moderate |
| TBI_7 | 8.08 | F | 0 | 3 | Car Crash | 6.00 | 2.08 | 15 | Abnormal | Moderate |
| NC_1 | 10.50 | M | 0 | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_2 | 10.08 | F | 0 | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_3 | 10.25 | F | 0 | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_4 | 13.42 | F | 1 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_5 | 9.25 | F | 0 | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_6 | 10.67 | F | 0 | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_7 | 7.33 | M | 0 | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_8 | 7.25 | F | 0 | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_9 | 11.58 | F | 0 | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_10 | 8.58 | M | 0 | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_11 | 8.00 | M | 0 | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_12 | 9.42 | M | 0 | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
| NC_13 | 9.58 | M | 0 | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Note 1. Race: 0 = Caucasian; 1 = Non-Caucasian.
Note 2. Maternal education level: 1 = 2 yrs high school; 2 = high school degree; 3 = 2 years college; 4 = 4 years college; 5 = graduate degree.
2.2. Neuropsychological battery
Several behavioral measures from standardized neuropsychological tests were administered to all participants in order to characterize the samples’ overall intellectual abilities, word reading abilities (as an estimate of academic achievement), and memory abilities. The testing was completed on the same day as the fMRI scan. These measures included: the Vocabulary and Matrix Reasoning subtests from the Wechsler Abbreviated Scale of Intelligence (WASI [68]), the Word Identification subtest from the Woodcock Johnson Test of Achievement – Third Edition (WJ-III [40]), the Digit Span subtest from the Wechsler Intelligence Scale for Children – Third Edition (WISC-III [69]), and the Stories, Word Pairs, Dot Location, and Faces subtests of the Children's Memory Scale (CMS [17]).
2.3. Stimuli and behavioral task used for fMRI
Task procedures, stimuli, and mnemonic strategies were developed and piloted in our laboratory with both children and adults [35]. After consent/assent, participants received instruction and practice, and the child was then placed in the scanner. After the initial scanner calibration and anatomical scan, the participant performed the encoding task, which lasted approximately seven minutes. The encoding task had three conditions: Imagery Condition, Repetition Condition, and Rest (a control condition in which no word pairs are presented). Pairs of words were presented auditorily and were preceded by an auditory cue denoting whether the participant should imagine or repeat the word pair. For half of the word pairs, the participant was asked to imagine the two items in the pair interacting in some way he or she devises (Imagery Condition), and for the other half of the pairs, they were asked to repeat the word pair until the next pair is presented (Repetition Condition). An auditory presentation condition was chosen to minimize the possible interference of the mental imagery process from visually presented stimuli. Each trial was 11 seconds long. Fourteen trials of each of the three conditions were intermixed (42 trials total), with the constraint that each successive triplet included one trial from each of the three conditions.
After completing the encoding task, the participant was removed from the scanner. Approximately five minutes later, after the child had relaxed and reoriented, a cued recall task was administered. In this recall task, the examiner read aloud the first word of each pair heard during the task (and, in addition, 14 distractor words not used in the task) and asked the participant if he or she heard that word during scanning. If the word was recognized, the participant was then asked what word completed the pair. In addition to the cued recall measure of interest, this procedure also provided a measure of single-item recognition memory, which was used to verify encoding task performance.
The stimuli for the encoding task were pairs of words presented auditorily by the same female speaker on a pre-recorded CD. Inside the scanner, the stimuli were presented through pneumatic headphones. The nouns chosen to be stimuli for this study were taken from Cycowicz and colleagues [18] and selected based on the following criteria in order to be appropriate for all child participants: rated highly familiar by 5–7 year olds, rated low in visual complexity by 5–7 years olds, and having a high mean frequency in the English language as reported in the American Heritage Word Frequency book [11]. The words were then paired randomly to avoid producing pairs of words having any obvious preexisting associative relationship.
2.4. fMRI data acquisition and analyses
The fMRI methods used in this experiment are the same as those used previously in our laboratory [13, 14]. The scans were performed on a Bruker Biospec 30/60 MRI scanner based on a 60-cm, 3.0 Tesla magnet (Bruker Medizintechnik, Karlsruhe, Germany). A T2* weighted, gradient-echo, echo-planar imaging (EPI) sequence was used for fMRI scans (TR = 2000 ms, TE = 38 ms, FOV = 25.6 × 25.6 cm, matrix = 64 × 64, 25 slices, slice thickness = 5 mm). There were 42 trials in the run, and each trial began with a 5 second scanner-silent window followed by 6 seconds of fMRI acquisition (three volumes collected over three TRs), for a total imaging time of 7 minutes and 42 seconds. A T1-weighted, 3-D Modified Driven Equilibrium Fourier Transform whole brain scan was performed for anatomical co-registration. Stimuli were presented using an MRI-compatible audiovisual system (Resonance Technologies Inc.).
FMRI image post-processing was completed using Cincinnati Children's Hospital Image Processing Software, in-house software written in Interactive Data Language (Research Systems, Inc., Boulder, CO). The EPI images were corrected for geometrical distortion and Nyquist ghost artifacts using the multiecho reference method [60]. Because of the nonequilibrium T1 effects as a function of acquisition frame (first, second, and third frame for each trial), each frame set was individually co-registered to correct for motion artifacts [66] and then recombined. The data were then normalized into stereotactic space [65] using a linear affine transformation shown previously to be valid for individuals 5 to 18 years of age [43,71] spatially filtered in three dimensions with a 4mm Gaussian filter and detrended. Magnetic resonance volumes acquired during the same temporal frame (1, 2, or 3) from different trial types (imagery, repeat, or rest) were directly contrasted with paired t-tests, pooling data across frames and trials, according to the General Linear Model [72]. A paired t-test map was generated for each participant for each contrast of interest (i.e., Imagery condition compared to Rest, Repetition condition compared to Rest, and Imagery condition compared to Repetition condition), and the individual t-maps were entered into a random effects analysis to form composite maps for the two groups of children, which were compared using the General Linear Model to examine group differences. The Rest condition allowed us to verify that auditory activation was observed in the main experimental conditions, but due to the uncertainty associated with what mental processes might have occurred during this period for each group of participants, we focused on analyses concerning the contrast between Imagery and Repetition conditions. The comparison between Imagery and Repetition conditions controls for task- and stimuli – specific activation and identifies brain activation related to associate encoding. Significant activation was defined by a nominal z = 6.0, cluster = 25, corrected p ≤ 0.05 for multiple comparisons.
3. Results
3.1. Demographic and neuropsychological variables
Descriptive statistics were compared between the two groups using the Fisher exact test or Mann-Whitney U test for categorical variables (gender,race, and maternal education) and t test for continuous variables (age, neuropsychological variables, and task performance).
Children with TBI who successfully completed the scan were significantly more likely to be Caucasian (Fisher's Exact, 2-tailed, p = 0.01; TBI unusable group 30% Caucasian) and have a higher estimated IQ (t(15) = −3.78, p < 0.01; TBI unusable group M = 94.3, SD = 12.2) than children with TBI who had unusable scanning data. NC children who successfully completed the scan were also more likely to be Caucasian than NC children who had unusable scanning data, a difference which approached significance (Fisher's Exact, 2-tailed, p = 0.07; NC unusable group 50% Caucasian). For both groups, children who completed the scan were more likely to have a better task performance than those with unusable data (TBI Imagery Advantage: t(15) = −2.43, p = 0.03, TBI unusable group M = 7.14, SD = 11.17; NC Imagery Advantage: t(17) = −2.46, p = 0.03, NC unusable group M = 15.47, SD = 15.26). See Section 3.2 for explanation of Imagery Advantage task performance parameter. As performance on standardized memory testing did not differ between children with usable data compared to those with unusable data, poor performance on the in-scanner memory task is likely related to task non-compliance (TBI General Memory: t(15) = −1.23, p = 0.24, TBI unusable group M = 103.00, SD = 14.28; NC General Memory: t(17) = −0.09, p = 0.92, NC unusable group M = 116.00, SD = 20.00). Children were not significantly different from their group members with unusable data on any other demographic or neuropsychological variables as confirmed by the appropriate parametric or nonparametric test.
Demographic data for participants included in the analyses are reported in Table 1. Mean age of the TBI group was 8.8 years while mean age of the NC group was 9.7 years. The groups did not differ in age (t(18) = 1.26, p = 0.23), sex (Fisher's Exact, 2-tailed, p = 1.0), or race (Fisher's Exact, 2-tailed, p = 1.0). However, the TBI group had a significantly lower maternal education level than the NC group (Mann-Whitney U = 17.00, p = 0.02). Children in the TBI group on average had mothers with a high school education, and children in the NC group on average had mothers with a four-year college education. Maternal education level is frequently used as an index of socioeconomic status (SES) [22].
Neuropsychological test performance data is reported in Table 2. T-tests revealed no significant group differences on measures of WASI Full Scale IQ (t(18) = −0.71, p = 0.49), WISC-III Digit Span (t(18) = 0.91, p = 0.38), WJ-III Word Identification (t(18) = 0.35, p = 0.73), and CMS General Memory Ability (t(18) = 1.02, p = 0.32).
Table 2.
Group differences on cued recall and neuropsychological measures
| Mean (SD) |
||
|---|---|---|
| TBI group N = 7 | NC group N = 13 | |
| Cued Recall Task Performance | ||
| Imagery Condition (Percentage pairs recalled) | 35.71 (27.97) | 43.59 (20.17) |
| Repeat Condition (Percentage pairs recalled) | 5.10 (5.40) | 4.95 (6.11) |
| Percentage Advantage (Imagery-Repeat) | 30.61 (27.88) | 38.64 (20.44) |
| Neuropsychological Measures | ||
| WASI: FSIQa | 118.29 (13.90) | 114.00 (12.40) |
| CMS: General Memory Abilitya | 111.14 (11.92) | 116.69 (11.38) |
| WISC-III: Digit Span subtestb | 9.43 (2.44) | 10.38 (2.14) |
| WJ-III: Word Identification subtesta | 104.57 (12.29) | 106.31 (9.50) |
Note: WASI = Weschler Abbreviated Scale of Intelligence; CMS = Children's Memory Scale; WISC-III = Weschler Intelligence Scale for Children, Third Edition; WJ-III = Woodcock Johnson Test of Achievement, Third Edition.
Standard Score
Scaled Score.
3.2. Behavioral performance on memory task
Cued recall task performance data were collected from all participants (see Table 2). Although the data passed tests for skew and kurtosis, the range of word pairs recalled in the Repetition condition was severely restricted due to floor effects on recall. There was no evidence of floor or ceiling effects in the number of word pairs recalled in the Imagery condition, and data passed tests for skew and kurtosis. Mean proportion of word pairs recalled in the Imagery condition (t(18) = 0.73, p = 0.48) and Repetition condition (Mann-Whitney U = 43.5, p = 0.88) did not differ significantly between the TBI and NC groups. Although there were considerable individual differences, children within each group remembered significantly more imagined word pairs than repeated word pairs (TBI group: Wilcoxon Signed Rank Z = −3.19, p < 0.01; NC group: Wilcoxon Signed Rank Z = −1.99, p = 0.05) with an average recall advantage of 31% more word pairs recalled in the Imagery condition over the Repetition condition for the TBI group and a 39% recall advantage for the NC group (a non-significant group difference, t(18) = 0.74, p = 0.47). This performance parameter, determined by subtracting mean proportion of word pairs in the Repetition condition recalled in the post-scan Cued Recall task from the mean proportion of word pairs in the Imagery condition recalled, will be referred to as “Imagery Advantage.” Imagery Advantage was used in subsequent analyses as a measure of task performance with acceptable range and variability.
Both groups of children demonstrated well above chance level performance on the single-item recognition memory task embedded in the cued recall task as measured by hit rate minus false alarm rate (termed “corrected hit rate”). In the NC group, mean corrected hit rate was 60.1% for the Imagery condition and 40.4% for the Repetition condition (Imagery hit rate M = 60.1, SD = 27.5; Repetition hit rate M = 40.4, SD = 25.2; false alarm rate M = 0.0, SD = 0.0). In the TBI group, mean corrected hit rate was 48.4% for Imagery and 37.0% for Repetition (Imagery hit rate M = 50.5, SD = 17.7; Repetition hit rate M = 39.0, SD = 14.5; false alarm rate M = 2.0, SD = 3.5). These results suggest that despite the variability in cued recall performance, the participants were attending to the stimuli during the task, and were likely attempting to encode the word pairs.
3.3. Relationship between task performance and neuropsychological variables
The relationship of Imagery Advantage to age and neuropsychological measures was investigated with partial correlations, controlling for group status (TBI vs. NC), uncorrected for multiple comparisons. Higher Imagery Advantage was significantly correlated with older age (r = 0.54, p = 0.02) and higher General Memory Ability score from the CMS (r = 0.46, p = 0.05). However, recall advantage was not significantly correlated other neuropsychological variables including estimated FSIQ from the WASI (r = 0.05, p = 0.83), Digit Span subtest (r = 0.26, p = 0.29), or WJ-III (r = 0.16, p = 0.54). Recall advantage was not significantly correlated with maternal education for the TBI group (dichotomized as high school diploma and below versus some college and above, point biserial correlation r = −0.09, p = 0.85).
3.4. fMRI analysis: Condition comparisons by group
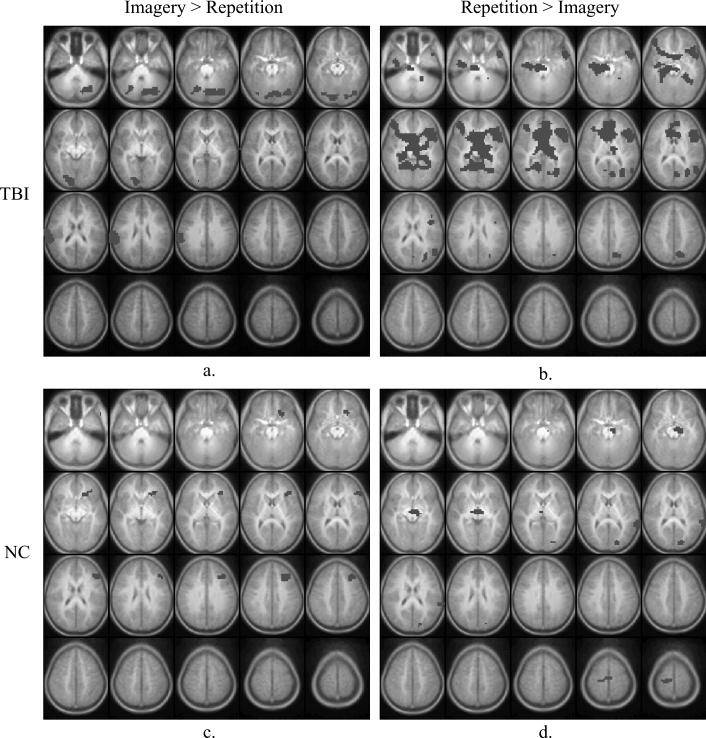
Figure 1 presents the statistical parametric maps (composite Z-score maps) of brain regions demonstrating significantly greater activation during the Imagery condition compared to Repetition condition (“Imagery – Repetition”), and vice versa (“Repetition – Imagery”), for each group.
Fig. 1.
Brain activation maps of task contrasts, by group. Images are horizontal slices 5 mm apart and start at z = −30 mm from the top left to z = +65 mm on the bottom right. Images are in radiological convention: left side of the images corresponds to the right hemisphere. Image parameters are as follows: nominal z = 6.0, cluster = 25, corrected p < 0.05 for multiple comparisons. Positive activation foci only are shown. Panel 1a: TBI group, Imagery – Repetition. 1b: TBI group, Repetition – Imagery. 1c: NC group, Imagery – Repetition. 1d: NC group, Repetition – Imagery. Please see Tables 3 and 4 for listings of activation foci.
Figure 1a and Table 3 present brain regions with significantly greater activation during the Imagery condition compared to Repetition condition for the TBI group. Significantly greater activation during the Imagery condition compared to Repetition condition was observed in the bilateral cerebellum, bilateral visual cortices (including inferior occipital lobes, lingual gyrus, and fusiform gyrus, Brodmann's Area [BA] 17, 18, 19), right inferior parietal lobe (BA 40), and right postcentral gyrus (BA 3). In contrast, greater activation was seen in the Repetition condition compared to Imagery condition in the left superior temporal gyrus (BA 22), left inferior and middle frontal lobes (BA 45, 47), right lingual gyrus (BA 19), bilateral fusiform gyrus (BA 37), bilateral parahippocampal gyrus (BA 35), right hippocampus, posterior cingulate, and left precuneus (BA 7, 31). See Fig. 1b and Table 3.
Table 3.
Regions of interest showing significant differences in brain activation between conditions for the TBI group (N = 7)
| Brodmann's Areas | Talairach Coordinates |
|||
|---|---|---|---|---|
| x | y | z | ||
| Imagery > Repetition | ||||
| Postcentral gyrus (R) | 3 | 58 | –17 | 25 |
| Fusiform gyrus (B) | 19 | 34 | –73 | –10 |
| Lingual gyrus (R) | 17/18 | 10 | –85 | –5 |
| Inferior occipital lobe (B) | 18 | 22 | –85 | –5 |
| Inferior parietal lobe (R) | 40 | 58 | –29 | 20 |
| Cerebellum (B) | – | 10 | –77 | –15 |
| Repetition > Imagery | ||||
| Fusiform gyrus (R) | 20 | 38 | –9 | –25 |
| Superior temporal gyrus (L) | 22 | –50 | 11 | –5 |
| Inferior frontal gyrus (L) | 45/47 | –38 | 23 | –10 |
| Inferior frontal gyrus (L) | 9 | –46 | 15 | 20 |
| Parahippocampal gyrus (B) | 35 | –18 | –33 | –5 |
| Hippocampus (R) | – | 30 | –13 | –15 |
| Fusiform gyrus (B) | 37 | –30 | –37 | –10 |
| Middle frontal gyrus (R) | 10 | 38 | 39 | 0 |
| Anterior cingulate (B) | 32 | –2 | 39 | 15 |
| Posterior cingulate gyrus (L) | 30 | –30 | –69 | 15 |
| Middle temporal gyrus (L) | 19 | –50 | –61 | 15 |
| Cuneus (L) | 18 | –6 | –73 | 15 |
| Lingual gyrus (L) | 19 | –30 | –61 | 5 |
| Precuneus (L) | 7/31 | –26 | –69 | 20 |
Note: L=left, R=right, B=bilateral.
In the direct comparison between Imagery and Repetition conditions within the NC group, significantly greater activation during the Imagery condition was seen in the left inferior frontal and dorsolateral pre-frontal cortex (BA 46, 47), left middle frontal cortex (BA 8, 9), and left insula (BA 13). In contrast, greater activation was seen in the Repetition condition in the left occipital gyrus and lingual gyrus (BA 18), left cuneus (BA 17), left parahippocampal gyrus (PHG), and left superior temporal gyrus (BA 22, 42). See Figs 1c and 1d and Table 4 for complete listing of activation foci.
Table 4.
Regions of interest showing significant differences in brain activation between conditions for the NC group (N = 13)
| Brodmann's Areas | Talairach Coordinates |
|||
|---|---|---|---|---|
| x | y | z | ||
| Imagery > Repetition | ||||
| Inferior frontal gyrus (L) | 46 | –50 | 27 | 15 |
| Inferior frontal gyrus (L) | 47 | –18 | 23 | –10 |
| Middle frontal gyrus (L) | 8 | –30 | 27 | 40 |
| Middle frontal gyrus (L) | 9 | –38 | 27 | 30 |
| Insula (L) | 13 | –26 | 27 | 0 |
| Repetition > Imagery | ||||
| Superior temporal gyrus (L) | 22/42 | –66 | –37 | 15 |
| Parahippocampal gyrus (L) | 34 | –14 | –13 | –15 |
| Lingual gyrus (L) | 18 | –22 | –81 | 5 |
| Cuneus (L) | 18 | –18 | –85 | 15 |
| Precentral gyrus (R) | 6 | 2 | –13 | 60 |
Note: L=left, R=right, B=bilateral.
3.5. fMRI analysis: Maternal education
Maternal education level differed significantly between the TBI and NC groups. Consequently, maternal education level was examined in greater depth to determine if this variable had a significant impact on brain activation. Although maternal education level is a variable of interest in the TBI literature, it was not hypothesized to affect memory performance. Indeed behaviorally, the TBI and NC groups demonstrated equivalent cued recall performance in both the Imagery and Repetition conditions, and the groups were not significantly different on any of the neuropsychological variables that could be hypothesized to affect memory performance (i.e., overall IQ, word reading, Digit Span). An analysis examining the correlation between maternal education and the brain activation pattern in the Imagery – Repetition contrast in the TBI group revealed small areas of positive correlation in the cerebellum, bilateral medial temporal gyrus (BA 21 and 22), and inferior frontal gyrus (BA 46), and postcentral gyrus (BA 2). However, when level of maternal education was entered as a covariate in the analysis of Imagery – Repetition in the TBI group, the pattern of brain activation was identical to the activation pattern produced without controlling for maternal education level, suggesting maternal education does not appear to directly impact regions of activation in which there is greater activation in the Imagery – Repetition contrast of interest. Due to a lack of variability, the relationship between maternal education and brain activation was not able to be similarly examined in the NC group. Because it remains unclear to what extent maternal education affects neural activation patterns in either a healthy or injured brain, we proceeded with group comparisons given the groups were equally matched on all other demographic, neuropsychological, and task-related variables.
3.6. fMRI analysis: Group comparisons
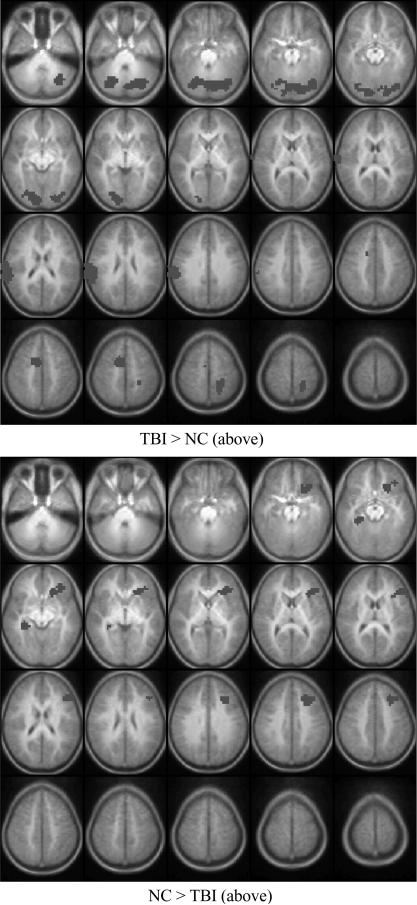
With respect to group-related differences, participants in the TBI and NC groups demonstrated differential patterns of brain activation in the Imagery – Repetition contrast, as shown in Fig. 2 and Table 5. In the direct contrast between the Imagery and Repetition conditions, the TBI group demonstrated greater activation than the NC group in primary and association visual cortices (BA 17, 18, 19), superior temporal lobe (BA 22, 42), and motor cortices (BA 3, 6). The NC group showed greater activation than the TBI group in medial frontal regions (BA 8, 9), dorsolateral prefrontal cortex (BA 46), inferior prefrontal cortex (BA 47), right parahippocampal gyrus and right hippocampus.
Fig. 2.
Group differences in activation in Imagery – Repetition comparison. Top: The TBI group had significantly higher levels of activation in a variety of brain regions relative to the NC group. Bottom: Brain regions showing greater activation for the NC group. Please see Table 5 for listing of activation foci. Image conventions and parameters are as in Fig. 1.
Table 5.
Between group differences in brain regions with greater activation in Imagery compared to Repetition condition
| Brodmann's Areas | Talairach Coordinates |
|||
|---|---|---|---|---|
| x | y | z | ||
| Children with TBI > NC (N = 7) | ||||
| Fusiform gyrus (B) | 19 | 38 | –73 | –10 |
| Inferior occipital lobe (B) | 17/18 | 22 | –89 | –10 |
| Medial occipital lobe (B) | 19 | 38 | –73 | –5 |
| Lingual gyrus (B) | 17/18 | 2 | –85 | –10 |
| Cuneus (R) | 17 | 6 | –93 | 0 |
| Superior temporal lobe (R) | 22/42 | 62 | –17 | 20 |
| Medial frontal lobe (R) | 6 | 6 | 3 | 50 |
| Postcentral gyrus (L) | 3 | –18 | –33 | 55 |
| Cerebellum (B) | – | 22 | –65 | –25 |
| NC > Children with TBI (N = 13) | ||||
| Middle frontal gyrus (L) | 8 | –30 | 27 | 40 |
| Middle frontal gyrus (L) | 9 | –30 | 27 | 35 |
| Dorsolateral prefrontal cortex (L) | 46 | –46 | 27 | 25 |
| Insula (L) | 13 | –26 | –27 | 5 |
| Parahippocampal gyrus (R) | 36 | 30 | –33 | –10 |
| Hippocampus (R) | – | 26 | –29 | –5 |
| Inferior frontal gyrus (L) | 47 | –22 | 27 | –5 |
Note: L=left, R=right, B=bilateral.
3.7. fMRI analysis: Relationship with cued recall performance
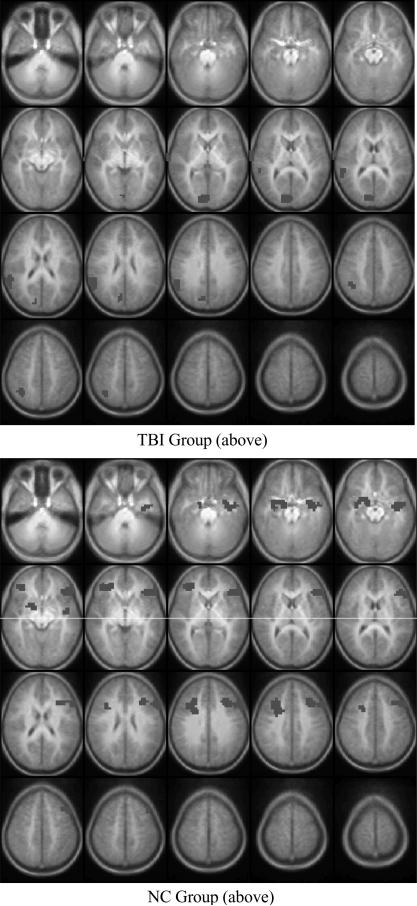
This analysis identified brain regions, by group, in which activation in the Imagery-Repetition contrast significantly correlated with magnitude of Imagery Advantage. In the TBI group, greater Imagery Advantage was positively correlated with activation in the right superior temporal gyrus (BA 22), right insula (BA 13), right inferior parietal lobe (BA 40), and right cuneus and precuneus (BA 7). In the NC group, magnitude of Imagery Advantage was positively correlated with activation in the left inferior and medial temporal lobe (BA 20, 21), bilateral parahippocampal gyrus (BA 36), bilateral hippocampus, bilateral inferior frontal gyrus (BA 47), left dorsolateral prefrontal cortex (BA 46), bilateral medial frontal regions (BA 8,9), and left insula (BA 13). Figure 3 and Table 6 present the Talairach coordinates for these regions.
Fig. 3.
Statistical parametric map showing brain regions, by group, in which activation level in the Imagery – Repetition contrast was significantly correlated with memory performance. Top: TBI group, and Bottom: NC group. Only positively correlated activation foci are shown. Please see Table 6 for listing of activation foci. Image conventions and parameters are as in Fig. 1.
Table 6.
Brain regions showing significant correlation between task performance and activation by group
| Brodmann's Areas | Talairach Coordinates |
|||
|---|---|---|---|---|
| x | y | z | ||
| Children with TBI (N = 7) | ||||
| Superior temporal gyrus (R) | 22 | 66 | –9 | 10 |
| Insula (R) | 13 | 58 | –37 | 20 |
| Inferior parietal lobe (R) | 40 | 54 | –45 | 25 |
| Cuneus (R) | 7 | 2 | –69 | 30 |
| Precuneus (R) | 7 | 10 | –73 | 35 |
| NC (N = 13) | ||||
| Inferior temporal lobe (L) | 20/21 | –42 | –5 | –25 |
| Parahippocampal gyrus (B) | 34 | 18 | 3 | –15 |
| Hippocampus (B) | – | 30 | –9 | –15 |
| Inferior frontal gyrus (B) | 47 | –46 | 35 | –5 |
| Inferior frontal gyrus (L) | 46 | –50 | 36 | 0 |
| Inferior frontal gyrus (L) | 45 | –50 | 27 | 10 |
| Insula (L) | 13 | –42 | –5 | –5 |
| Putamen (R) | – | 18 | 7 | –5 |
| Inferior frontal gyrus (L) | 46 | –46 | 19 | 25 |
| Medial frontal gyrus (B) | 9 | 34 | 19 | 30 |
| Middle frontal gyrus (B) | 8 | 26 | 19 | 35 |
| Superior frontal gyrus (L) | 8 | –42 | 19 | 45 |
Note: L=left, R=right, B=bilateral.
To assess specifically whether brain regions identified in the correlational analysis above are overlapping across groups, we conducted the following analysis. For each group, a mask was first created from brain regions in which activation in the Imagery-Repetition contrast significantly correlated with Imagery Advantage, and then the analyses were re-run with the opposite group's mask as a filter. The results of these analyses demonstrated that the patterns of performance-correlated activation are non-overlapping; neither group demonstrated significant activation within the set of brain regions identified in the other group. This confirmed that the two groups utilized different brain regions to support successful task performance.
4. Discussion
The findings from this study provide preliminary evidence of altered neural activation during an associative encoding task in children following TBI. Despite equivalent behavioral performance between the two groups, the children with TBI demonstrated several areas of increased activation in the contrast between Imagery and Repetition conditions relative to NC children. These findings lend partial support to our hypothesis that children with TBI would activate similar networks of brain regions during the memory task as typically-developing children but would demonstrate hyperactivation of brain regions purported to support associative memory. Overall, these findings are generally consistent with the existing literature on associative memory tasks and neural activation changes after TBI and suggest moderate TBI may alter activation within the network of brain regions supporting associative memory even in children who demonstrate intact behavioral performance.
This study demonstrates the feasibility and utility of studying associative memory in young children following TBI. Our group of children with TBI showed good behavioral performance at an average of three years since injury; their performances on standardized tests of memory abilities were not impaired compared to the NC children or to the normative sample. The structured nature of the task allowed us to examine whether the children with TBI demonstrated utilization deficiencies in mnemonic strategy use, but because participants were not allowed spontaneous strategy use, we were unable to examine production deficiencies. The children with TBI did not demonstrate mnemonic strategy utilization deficiencies. They were able to learn and successfully utilize the mnemonic strategies taught in this paradigm, and although their cued recall advantage for the imagery strategy over the repetition strategy was smaller compared to NC children, this difference was not statistically significant. However, these results suggest despite successful strategy use and equivalent behavioral performance, children with TBI demonstrated altered neural activation patterns during the encoding phase of strategy use.
Our findings of group differences in the Imagery – Repetition comparison are broadly consistent with other fMRI studies observing altered, more extensive, neural activation patterns in adult patients with TBI [15,38, 39,51,57,58]. These findings are also generally consistent with fMRI studies of pediatric TBI in which greater activation was observed in relevant brain networks for children with TBI compared to orthopedically-injured controls [33,34,45,46]. However, our findings do not support a generalized pattern of over-activation in the group of children with TBI, but instead suggest that the two groups of children demonstrated differential patterns of activation within a network of brain regions thought to support associative encoding. In the analysis that identified group differences in brain regions with significantly higher activation in the Imagery condition compared to the Repetition condition, children with TBI activated several brain regions to a greater extent than the NC children did, including regions related to auditory processing and visual processing. In contrast, the NC group showed greater activation compared to the TBI group in the medial frontal cortex, the dorsolateral and inferior prefrontal cortex, and MTL.
As expected, successful associative encoding in the group of typically-developing children was positively correlated with activation in dorsolateral prefrontal cortex, inferior frontal regions, and medial temporal regions, all brain regions that have been implicated in successful associative encoding in adults [20,36]. The positive correlation found in the MTL confirms and extends previous findings from two recent neuroimaging studies of memory encoding in children, although both studies used indoor/outdoor scenes that did not necessarily entail associative encoding [41,49]. In contrast, successful associative encoding in the TBI group was positively correlated with activation in the superior temporal gyrus, inferior parietal lobe, cuneus, and precuneus. Not only did children with TBI over-recruit brain regions supporting visual and multi-modal processing compared to NC children, but higher activation in these regions was also correlated with task performance. It is possible that the task demands for auditory processing (listening for cue words and stimuli) and visual processing (generating images) were higher for the children with TBI compared to NC children, causing the task to be more challenging and more effortful. Children with TBI may have allocated more neural resources to auditory and visual processing as a compensatory mechanism. In the TBI fMRI literature, neural mechanisms such as differences in capacity or allocation of neural resources have been suggested as possible explanations for the altered activation pattern of neural networks following TBI [39]. It is thus possible that children with TBI may allocate limited resources to processing the auditory stimuli and generating images at the expense of PFC and MTL brain regions known to support successful episodic memory encoding.
It is also possible that the processing resources of the TBI group are unimpaired, but the children may nevertheless be unable to efficiently match those available resources to the task demands due to injury-related impairments in executive functioning. Diffuse axonal injury, which potentially disconnects brain circuits mediating memory following TBI, has been correlated with memory performance on a verbal list learning task one year post-injury in a group of children with moderate to severe TBI [56]. As a result of executive dysfunction, the children with TBI may over-commit processing resources to the task without enhancing performance. Studies using in-scanner cognitive tasks with pediatric or adult TBI cohorts have observed a pattern of fewer and smaller regions of performance-related task activation in individuals with TBI group compared to orthopedically-injured controls [34,57], which may represent an inefficient utilization of neural resources. Executive functioning abilities were not assessed as part of this study, but it will important to include this variable in future studies of mnemomic strategy use in this population.
Another possible explanation for the altered activation pattern observed in the TBI group is interruption of neural network maturation. From preschool through adolescence, there is a developmental progression in competent memory strategy use which parallels the development of attention and working memory capacity [59]. One would expect as later-maturing brain regions such as the prefrontal cortex come online during development [26,63], a parallel developmental trajectory of memory functioning would also be apparent in which the distributed network of brain regions supporting associative memory becomes solidified and functions as a cohesive, coordinated unit. Indeed, this is consistent with data from our laboratory in which healthy adults underwent the same fMRI task described here, and demonstrated greater activation compared to the same group of NC children from the present study in a number of brain regions supporting associative memory, including MTL, posterior cingulate, and inferior parietal lobe [35]. Consistent with the existing literature on associative encoding in adults, task performance in the group of healthy adults was positively correlated with brain activation in the MTL, PFC, anterior cingulate, and inferior parietal lobe [35]. Unfortunately we were not able to examine the relationship between age and brain activation in the TBI group due to the confounding effects of age at injury and time since injury. Nevertheless, children who sustain a TBI at a very young age may be especially vulnerable to the disruption of the maturation of this network, resulting in altered brain activation patterns for years after the injury.
The present results must be considered preliminary in light of several methodological limitations. A high percentage of children with TBI recruited for this project had unusable fMRI data due to excessive motion, and those who successfully completed the scan were significantly more likely to be Caucasian and have a higher IQ than those with unusable scanning data. Motion artifact is a methodologicalproblem inherent in pediatric neuroimaging due to the effects of anxiety, fatigue, and restlessness on children's compliance. However, our high failure rate suggests the feasibility of fMRI scanning in children with TBI may be impacted by additional challenges unique to this population. Future neuroimaging protocols and task paradigms need to be designed to maximize the comfort and cooperation of children with TBI. Finally, the event-related fMRI procedure employed in this study was originally inspired by our desire to use an auditory paradigm to minimize interference for visual imagery. However, one potential weakness of this procedure is that MR volumes were acquired during the portion of the canonical hemodynamic response function that corresponded to neural activation occurring shortly after the presentation of auditory stimuli, when participants were presumably engaging the mnemonic strategy. Consequently, we are only able to identify group differences in brain activation during this time window, and due to the covert nature of task, it is unknown if individual or groups differences in task behavior occurred at this time. Future studies might include greater inter-trial intervals to probe a longer time window for encoding processes.
Our sample was small and only included children with moderate TBI; thus, findings may not generalize to the larger TBI population. Although children with moderate to severe TBI have been shown to demonstrate verbal learning deficits [12,75], the sample in this study did not demonstrate behavioral deficits in verbal associative memory. Future studies examining children with a wider range of TBI severity will be needed to more clearly examine associative memory deficits. Regardless, in the present study, the lack of behavioral differences between groups allowed for examination of compensatory mechanisms supporting normal behavioral function, which may have important implications for treatment.
In addition, larger studies are needed to allow for better statistical control of demographic and injury-related confounding variables unable to be addressed here. Studies in the TBI field, including the existing fMRI studies of pediatric and adult TBI, tended to use an orthopedically-injured (OI) control group instead of healthy non-injured individuals in order to assess the consequences of TBI relative to the consequences of trauma not involving the head, as well as to control for demographics and other factors associated with the risk of traumatic injury. However, the assumption that TBI and OI groups are likely to be similar in pre-injury behavior and family characteristics does not always hold. In order to improve control for premorbid factors in pediatric TBI studies, better comparisons may instead be large groups of children with varying severity of TBI. In this study, NC children were used as a control group because no prior behavioral or imaging studies of verbal associative memory exist in the pediatric TBI literature. The two groups were matched on all neuropsychological, task-related, and demographic variables, except for maternal education level. In the TBI literature, SES has been implicated as a moderator of long-term effects of pediatric TBI on family functioning and stress and children's behavioral outcomes [70], and some studies have suggested that lower SES may be associated with an increased risk of accident [70]. However, it remains unclear how SES directly affects cognitive outcomes post-injury. One study observed unexpectedly high rates of poorer outcome for children with a combination of both severe TBI and low SES on selected measures of cognitive functioning, but this pattern was not observed on measures of memory,or for children with mild or moderate TBI [5]. In the present study, SES was not hypothesized to affect associative memory functioning, and indeed maternal education level was not significantly correlated with behavioral performance on the memory task. There is some evidence to suggest a relationship exists between language abilities and SES as evident in behavioral testing [48] and brain activation patterns on an fMRI task of phono-logical language skills [47]. However, it is unknown if verbal associative memory is similarly affected. Very few imaging studies have addressed SES issues or even reported SES status of participants, so clearly this is an area of needed research.
These findings have promising clinical implications for understanding and remediating long-term memory deficits in pediatric TBI. Persistent changes in neural mechanisms years following early childhood TBI suggest that associative memory should be assessed in the chronic phase of TBI. Children with TBI can benefit from learning mnemonic strategies to improve memory, whether or not they demonstrate clinical memory deficits. Imaging studies like ours have the potential to greatly inform the development of cognitive rehabilitation programs aiming to remediate both behavioral and neural changes following pediatric TBI.
Acknowledgements
The first author was supported by a University Dean's Distinguished Dissertation Award sponsored by the University of Cincinnati, and she would like to thank M. Douglas Ris for serving on her dissertation committee. The authors would like to acknowledge Lori Bernard and Karen Oberjohn for recruiting assistance.
This work was supported in part by 1) NIH grant RO1-HD044279 from the National Center for Medical Rehabilitation Research in the National Institute of Child Health and Human Development; 2) UPHS GCRC Grant #M01 RR 08084 from the National Center for Research Resources, NIH; and 3) NIH grant RO1-HD38578 from the U.S. National Institute of Child Health and Human Development. Funding for the fMRI scans was provided by the Association of Volunteers of the Convalescent Hospital for Children, Cincinnati Children's Hospital Medical Center.
References
- 1.Achim AM, Lepage M. Neural correlates of memory for items and associations: an event-related functional magnetic resonance imaging study. Journal of Neuroscience. 2005;17:652–667. doi: 10.1162/0898929053467578. [DOI] [PubMed] [Google Scholar]
- 2.Addis DR, McAndrews MP. Prefrontal and hippocampal contributions to the generation and binding of semantic associations during successful encoding. NeuroImage. 2006;33:1194–1206. doi: 10.1016/j.neuroimage.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 3.Anderson VA, Catroppa C, Rosenfeld J, Haritou F, Morse SA. Recovery of memory function following traumatic brain injury in pre-school children. Brain Injury. 2000;14:679–692. doi: 10.1080/026990500413704. [DOI] [PubMed] [Google Scholar]
- 4.Anderson VA, Catroppa C, Morse SA, Haritou F, Rosenfeld J. Attentional and processing skills following traumatic brain injury in early childhood. Brain Injury. 2005;20:699–710. doi: 10.1080/02699050400025281. [DOI] [PubMed] [Google Scholar]
- 5.Anderson VA, Catroppa C, Dudgeon P, Morse SA, Haritou F, Rosenfeld J. Understanding predictors of functional recovery and outcome 30 months following early childhood head injury. Neuropsychology. 2006;20:42–57. doi: 10.1037/0894-4105.20.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Arroyos-Jurado E, Paulsen JS, Ehly S, Max JE. Traumatic brain injury in children and adolescents: academic and intellectual outcomes following injury. Exceptionality. 2006;14:125–140. [Google Scholar]
- 7.Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean A. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;354:1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- 8.Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- 9.Bor D, Cumming N, Scott CE, Owen AM. Prefrontal cortical involvement in verbal encoding strategies. European Journal of Neuroscience. 2004;19:3365–3370. doi: 10.1111/j.1460-9568.2004.03438.x. [DOI] [PubMed] [Google Scholar]
- 10.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 11.Carroll JB, Davies P, Richman B. The American Heritage Word Frequency Book. Houghton Mifflin; Boston: 1971. [Google Scholar]
- 12.Catroppa C, Anderson V. Recovery in memory function in the first year following TBI in children. Brain Injury. 2002;16:369–384. doi: 10.1080/02699050110104444. [DOI] [PubMed] [Google Scholar]
- 13.Chiu C-YP, Coen-Cummings M, Schmithorst VJ, Holland SK, Keith R, Nabors L, Kramer M, Rozier H. Sound blending in the brain: a functional magnetic resonance imaging investigation. Neuroreport. 2005;16:883–886. doi: 10.1097/00001756-200506210-00002. [DOI] [PubMed] [Google Scholar]
- 14.Chiu C-YP, Schmithorst V, Brown RD, Holland SK, Dunn S. Making memories: an investigation of episodic memory encoding in children using fMRI. Developmental Neuropsychology. 2006;29:321–350. doi: 10.1207/s15326942dn2902_3. [DOI] [PubMed] [Google Scholar]
- 15.Christodoulou C, Deluca J, Ricker JH, Madigan NK, Bly BM, Lange G, Kalnin AJ, Liu W-C, Steffner J, Diamond BJ, Ni AC. Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. Journal of Neurology, Neurosurgery and Psychiatry. 2001;71:161–168. doi: 10.1136/jnnp.71.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory network. Memory. 1997;5:131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MJ. CMS: Children's Memory Scale Manual. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 18.Cycowicz YM, Friedman D, Snodgrass JG, Duff M. Recognition and source memory for pictures in children and adults. Neuropsychologia. 2001;39:255–267. doi: 10.1016/s0028-3932(00)00108-1. [DOI] [PubMed] [Google Scholar]
- 19.Davachi L. Item, context, and relational episodic encoding in humans. Current Opinions in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. Journal of Neurophysiology. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- 21.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature Reviews: Neuroscience. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 22.Ensminger ME, Fothergill KE. A decade of measuring SES: what it tells us and where to go from here. In: Born-stein MH, Bradley RH, editors. Socioeconomic Status, Parenting, and Child Development. Lawrence Erlbaum Associates; Mahwah, NJ: 2003. pp. 13–27. [Google Scholar]
- 23.Ferro SC, Pressley M. Imagery generation by learning disabled and average-achieving 11- to 13-year-olds. Learning Disability Quarterly. 1991;14:231–239. [Google Scholar]
- 24.Fletcher JM, Ewing-Cobbs L, Miner ME, Levin HS, Eisenberg HM. Behavioral changes after closed head injury in children. Journal of Consulting and Clinical Psychology. 1990;58:93–98. doi: 10.1037//0022-006x.58.1.93. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher P, Shallice T, Dolan RJ. “Sculpting the response space” - An account of left prefrontal activation at encoding. NeuroImage. 2000;12:404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- 26.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris JR. Verbal rehearsal and memory in children with closed head injury: a quantitative and qualitative analysis. Journal of Communication Disorders. 1996;29:79–93. doi: 10.1016/0021-9924(94)00035-2. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman N, Donders J, Thompson EH. Novel learning abilities after traumatic head injury in children. Archives of Clinical Neuropsychology. 2000;1:47–58. [PubMed] [Google Scholar]
- 29.Jackson O, III, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. NeuroImage. 2004;21:456–462. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 30.Jaffe K, Fay G, Polissar NL, Martin K, Shurtleff H, Rivara JB, Winn HR. Severity of pediatric traumatic brain injury and neurobehavioral recovery at one year - A cohort study. Archives of Physical Medicine and Rehabilitation. 1993;74:587–595. doi: 10.1016/0003-9993(93)90156-5. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe KM, Polissar NL, Fay GC. Recovery trends over three years following pediatric traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 1995;76:17–26. doi: 10.1016/s0003-9993(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 32.Kapur S, Craik FIM, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: Levels of processing effect. Proceedings of the National Academy of Sciences USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karunanayaka P, Holland S, Yuan W, Altaye M, Egelhoff J, Michaud L, Walz NC, Wade SL. Neural substrate differences in language networks and associated language-related behavioral impairments in children with TBI: A preliminary fMRI investigation. Neurorehabilitation. 2007;22:355–369. [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer ME, Chiu C-YP, Walz NC, Holland SK, Yuan W, Karunanayaka P, Wade SL. Long-term neural processing of attention following early childhood traumatic brain injury: fMRI and neurobehavioral outcomes. Journal of the International Neuropsychological Society. 2008;14:424–435. doi: 10.1017/S1355617708080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer ME, Chiu C-YP. Neural and behavioral correlates of verbal associative memory and mnemonic strategy use in children. doi: 10.3233/PRM-2009-0091. Manuscript in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lepage M, Habib R, Cormier H, Houle S, McIntosh AR. Neural correlates of semantic associative encoding in episodic memory. Brain Research - Cognitive Brain Research. 2000;9:271–280. doi: 10.1016/s0926-6410(00)00005-7. [DOI] [PubMed] [Google Scholar]
- 37.Levin HS, Culhane KA, Mendelsohn D, Lilly MA, Bruce D, Fletcher JM, Chapman SB, Harward H, Eisenberg HM. Cognition in relation to magnetic resonance imaging in head-injured children and adolescents. Archives of Neurology. 1993;50:897–905. doi: 10.1001/archneur.1993.00540090008004. [DOI] [PubMed] [Google Scholar]
- 38.McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ. Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- 39.McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- 40.McGrew KS, Woodcock RW. Woodcock-Johnson III: Technical Manual. Riverside Publishing; Itasca, IL: 2001. [Google Scholar]
- 41.Menon V, Boyett-Anderson JM, Reiss AL. Maturation of medial temporal lobe response and connectivity during memory encoding. Cognitive Brain Research. 2005;25:379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Murray JL, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. The Journal of Neuroscience. 2007;27:5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muzik O, Chugani DC. Statistical parametric mapping: assessment of application in children. NeuroImage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- 44.National Institute of Neurological Disorders and Stroke. Traumatic Brain Injury: Hope Through Research. 2002 NIH Publication No. 02-2478. [Google Scholar]
- 45.Newsome MR, Scheibel RS, Hunter JV, Wang ZJ, Chu Z, Li X, Levin HS. Brain activation during working memory after traumatic brain injury in children. Neurocase. 2007;13:16–24. doi: 10.1080/13554790601186629. [DOI] [PubMed] [Google Scholar]
- 46.Newsome MR, Steinberg JL, Scheibel RS, Toyanskaya M, Chu Z, Hanten G, Lu H, Lane S, Lin X, Hunter JV, Vasquez C, Zientz J, Li X, Wilde EA, Levin HS. Effects of traumatic brain injury on working memory-related brain activation in adolescents. Neuropsychology. 2008;22:419–425. doi: 10.1037/0894-4105.22.4.419. [DOI] [PubMed] [Google Scholar]
- 47.Noble KG, Wolmetz ME, Ochs LG, Farah MJ, McCandliss BD. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factor. Developmental Science. 2006;9:642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- 48.Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 49.Ofen N, Kao Y-C, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JDE. Development of the declarative memory system in the human brain. Nature Neuroscience. 2007;10:1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- 50.Paz-Alonso PM, Ghetti S, Donohue SE, Goodman GS, Bunge SA. Neurodevelopmental correlates of true and false recognition. Cerebral Cortex. 2008;18:2208–2216. doi: 10.1093/cercor/bhm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perlstein WM, Cole MA, Demery JA, Seignourel PJ, Dixit NK, Larson MJ, Briggs RW. Parametric manipulation of working memory load in traumatic brain injury: behavioral and neural correlates. Journal of the International Neuropsychological Society. 2004;10:724–741. doi: 10.1017/S1355617704105110. [DOI] [PubMed] [Google Scholar]
- 52.Pressley M, Levin JR. Task parameters affecting the efficacy of a visual imagery learning strategy in younger and older children. Journal of Experimental Child Psychology. 1977;24:53–59. [Google Scholar]
- 53.Pressley M. Elaboration and memory development. Child Development. 1982;53:296–309. [Google Scholar]
- 54.Richardson JTE. The efficacy of imagery mnemonics in memory remediation. Neuropsychologia. 1995;33:1345–1357. doi: 10.1016/0028-3932(95)00068-e. [DOI] [PubMed] [Google Scholar]
- 55.Roman MJ, Delis DC, Willerman L, Magulac M, Demadura TL, de la Pena JL, Loftis C, Walsh J, Kracun M. Impact of pediatric traumatic brain injury on components of verbal memory. Journal of Clinical and Experimental Neuropsychology. 1998;20:245–258. doi: 10.1076/jcen.20.2.245.1168. [DOI] [PubMed] [Google Scholar]
- 56.Salorio CF, Slomine BS, Grados MA, Vasa RA, Christensen JR, Gerring JP. Neuroanatomic correlates of CVLT-C performance following pediatric brain injury. Journal of the International Neuropsychological Society. 2005;11:686–696. doi: 10.1017/S1355617705050885. [DOI] [PubMed] [Google Scholar]
- 57.Scheibel RS, Newsome MR, Steinberg JL, Pearson DA, Rauch RA, Mao H, Troyanskaya M, Sharma RG, Levin HS. Altered brain activation during cognitive control in patients with moderate to severe traumatic brain injury. Neurorehabilitation and Neural Repair. 2007;21:36–45. doi: 10.1177/1545968306294730. [DOI] [PubMed] [Google Scholar]
- 58.Scheibel RS, Pearson DA, Faria LP, Kotrla KJ, Ayl-ward E, Bachevalier J, Levin HS. An fMRI study of executive functioning after severe diffuse TBI. Brain Injury. 2003;17:919–930. doi: 10.1080/0269905031000110472. [DOI] [PubMed] [Google Scholar]
- 59.Schlagmuller M, Schneider W. Development of organizational strategies in children: evidence from a microgenetic longitudinal study. Journal of Experimental Child Psychology. 2002;81:298–319. doi: 10.1006/jecp.2002.2655. [DOI] [PubMed] [Google Scholar]
- 60.Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20:535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider W, Kron V, Hunnerkopf M, Krajewski K. The development of young children's memory strategies: First findings from the Wurzburg Longitudinal Memory Study. Journal of Experimental Child Psychology. 2004;88:193–209. doi: 10.1016/j.jecp.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RSJ, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- 63.Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. Journal of the International Neuropsychological Society. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- 64.Sperling RA, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. NeuroImage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Talairach J, Tournoux P. Coplanar Stereotactic Atlas of the Human Brain. Thieme; New York, NY: 1988. [Google Scholar]
- 66.Thevenaz P, Unser M. A pyramid approach to sub-pixel registration based on intensity. IEEE Trans Image process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- 67.Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. Journal of Clinical and Experimental Neuropsychology. 2005;27:977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- 68.Wechsler D. WASI Manual. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 69.Wechsler D. WISC-III: Administration and Scoring Manual. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 70.Wade SL, Taylor HG, Yeates KO, Drotar D, Stancin T, Minich NM, Schluchter M. Long-term parental and family adaptation following pediatric brain injury. Journal of Pediatric Psychology. 2006;31:1072–1083. doi: 10.1093/jpepsy/jsj077. [DOI] [PubMed] [Google Scholar]
- 71.Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Human Brain Mapping. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited - Again. NeuroImage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 73.Yeates KO, Blumenstein E, Patterson CM, Delis DC. Verbal learning and memory following pediatric closed head injury. Journal of the International Neuropsychological Society. 1995;1:78–87. doi: 10.1017/s1355617700000138. [DOI] [PubMed] [Google Scholar]
- 74.Yeates KO. Closed-head injury. In: Yeates KO, Ris MD, Taylor HG, editors. Pediatric Neuropsychology: Research, Theory and Practice. Guilford; New York: 2000. pp. 92–116. [Google Scholar]
- 75.Yeates KO, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term neuropsychological outcomes after traumatic brain injury in children. Neuropsychology. 2002;16:514–523. doi: 10.1037//0894-4105.16.4.514. [DOI] [PubMed] [Google Scholar]