The surface of the gastrointestinal tract is lined by a simple columnar epithelium that is folded to form a number of invaginations, or crypts, that are embedded in the connective tissue. Each crypt contains approximately 250 cells, depending on its species and anatomical location. Crypt size and organization are generally uniform within a given region of the gastrointestinal tract (1). Of the major gut epithelial cell types, all but one (the Paneth’s cells of the small intestine) move upward toward the lumen of the gut as they mature. Hence, the differentiated, functional cells are found mainly on the villi (small intestine) or toward the top of the colonic crypt — the intercrypt table — in the large intestine. During the latter stages of the process, these mature epithelial cells become senescent and are shed intact into the lumen. Cells shed from the gut must be replaced by a steady supply of cells generated in the low- to mid-crypt region, where, at least in the mouse, up to 60% of the crypt cells divide twice daily.
Because of this continuous upward migration, the location of a cell within the migratory stream indicates its stage in the process of maturation. Intestinal stem cells reside at the origin of the migration, which is found just above the crypt base in the small intestine and at the crypt base in the colon (2). As with normal homeostatic proliferation, crypt regeneration after cytotoxic damage also appears to originate at this site. Unfortunately, the stem cells responsible for tissue homeostasis and regeneration cannot be identified morphologically or distinguished from other epithelial cells by any recognized set of markers. Hence, most interpretations of stem-cell behavior are based upon monitoring cohorts of cells before and after perturbation of the tissue. This approach offers direct insights into the dynamics of the crypt-cell population, but only limited steady-state behavioral information on the cells themselves. This limitation may be inevitable in the absence of good molecular markers, but it must be kept in mind when interpreting many current experiments.
Estimates of stem-cell number vary widely, from 0.4% to 60% of the crypt cells, with the smallest value implying that a single stem cell occurs in each crypt. This discrepancy arises largely because of differences in the operational definition of the stem cell. It is now generally accepted that “stemness” is not a single property, but a number of properties that can be manifested under different conditions. Thus, a stem cell must be undifferentiated (relative to the other epithelial cell types, but not necessarily relative to embryonic cells) and capable of proliferation and self-maintenance, producing many differentiated progeny, and regenerating the tissue after injury (3). It must also retain the ability to switch between these options when appropriate. Hence, the properties, and probably the number, of stem cells in a crypt may change in response to circumstances, including the choice of experimental manipulations (which often fail to mimic the conditions that these cells normally encounter in vivo). Nevertheless, these manipulations have brought to light some interesting properties of this system, including the possible ability of partially differentiated cells to dedifferentiate and replenish the supply of true stem cells. This degree of plasticity is unexpected based on the traditional understanding of stem-cell function.
Various experiments suggest that the number of stem cells per crypt is tightly regulated, implying that stem cells can somehow detect each other’s presence and respond appropriately. The number of functioning stem cells per crypt greatly influences numerous aspects of crypt organization and control. Thus, as discussed below, the changes in stem-cell number can alter stem-cell cycle time, the number of divisions before differentiation, the number of lineages each stem cell normally generates or is capable of generating, and the number of cells capable of tissue regeneration after damage. Finally, because these cells are maintained throughout the lifetime of an animal, the number of stem cells may correspond to the number of cells that are capable of generating a carcinoma.
In a crypt with multiple stem cells, a central question is whether each stem cell produces just one cell type among the various differentiated phenotypes, or whether each stem cell is fully pluripotent, capable of producing all the intestinal epithelial cell types under steady-state conditions. A single stem cell is certainly capable of producing more than one lineage, as seen in a regenerative situation where only a single clonogenic cell remains and yet the whole crypt cell repertoire is reestablished. However, this does not prove conclusively that this also occurs in a normal steady-state crypt. However, it is unlikely that each stem cell is unipotent in the steady state, because one would then expect to observe dramatic fluxes in the number of each differentiated cell type as stem cells are deleted or undergo symmetrical division. This has not been reported. Further evidence for steady-state pluripotency comes from following the expression of G6PD polymorphisms or populations of mutant cells with varying lectin-binding properties, as described below. In such studies, crypts contain cells of various phenotypes, and the mutant areas change over time in size and extent.
The mechanism by which a cell commits and proceeds to full differentiation is currently unknown. However, certain homeobox transcription factors are likely to be involved. Homeobox genes determine cell fate and general pattern formation in many tissues, particularly in regard to cephalocaudal patterning, and the homeobox-containing proteins cdx-1 and cdx-2 appear to regulate epithelial differentiation, possibly by transducing signals from the underlying mesenchyme.
Actual and potential stem cells
By current estimates, a normal adult steady-state crypt contains four-to-six functional or actual stem cells, upon which the entire crypt ultimately depends. These cells appear to be located about four cell diameters up from the small intestinal crypt base, spatially distributed amongst their daughters in ring of about 16 cells (4). These cells occur above the underlying Paneth’s cells, and because the position of the highest Paneth’s cell varies circumferentially, the stem cells probably do not lie in a single plane. The daughters of these cells normally then undergo a limited and defined number of divisions, although how this limit is determined remains uncertain. These daughter cells, or dividing transit cells, are found in the mid-crypt region and can mature into one of five different mature cell types. As such a cell matures, it gradually loses its stemness so that the cells of the upper crypt do not have the capacity to regenerate a crypt after radiation injury (i.e., are not clonogenic) (5). The immature first- to third-generation daughter cells of the actual stem cells, however, may retain some stemness and are thus potential stem cells or clonogenic cells that are capable of stem-cell function if necessary (i.e., if called upon after damage). The existence of many clonogenic cells (up to 30–40 per crypt, see below), but few actual stem cells may explain some of the variation among estimates of stem-cell number determined after crypt perturbation.
A series of experiments using different doses of irradiation to reduce crypt stem-cell numbers to different extents have been used to measure the ability of the crypt to survive and regenerate (4, 6). This ability relates directly to the number of clonogenic cells that survive the irradiation, and with certain limited assumptions, can be used to estimate the number of potential stem cells per crypt. Such assays have led to the proposal of a hierarchical stem-cell organization containing three categories of stem cells, with low, medium, and high radiotolerance. The four-to-six actual stem cells appear to be very sensitive to DNA damage, cannot repair such damage, and are killed by 1 Gy of gamma radiation (7), apparently as a result of p53-mediated apoptosis (8). This extreme sensitivity may normally stop the crypts from maintaining a mutation in this long-lived cell, which could otherwise undergo carcinogenic transformation.
Approximately six additional clonogenic cells per crypt that survive 1 Gy can apparently maintain the crypt when the actual stem cells are killed, but these cells are themselves killed by higher doses of radiation. These cells therefore represent a second tier of stem cells that under normal steady-state conditions would not exhibit their stem-cell potential, but would become dividing transit cells and ultimately differentiate. However, at this early stage, they retain some stem-cell function (i.e., are uncommitted) and can be called upon to express their stem-cell potential if required. Additionally, these cells also appear to have acquired the ability to repair their DNA, a process that seems to involve p53-mediated cell-cycle arrest, because they express high levels of p53 protein after exposure to irradiation (8). Ultimately, they may regenerate the first-tier compartment and a normal crypt.
At higher doses of radiation, the estimate of clonogenic cell numbers increases to about 30–40 per crypt, suggesting that a third tier of 16–24 additional radioresistant cells exists, which is the final clonogenic resource. Thus, there is a gradual loss of stemness over the first two divisions, and although a crypt may normally use only four-to-six stem cells, a reserve force of clonogenic cells ensures crypt survival. Later generations possess no clonogenic regenerative capacity. It remains unclear whether the recruitment of these potential stem cells involves dedifferentiation or whether the commitment to differentiation occurs only at the third generation in the lineage.
Stem-cell division
Seeking to understand the control of cell division in the first few generations of the stem-cell daughters is an area of intense research. It has been proposed that a specific type of mitosis occurs in the functioning stem cells that is responsible for the self-maintenance of this cell type. In general, normal stem-cell mitosis must proceed asymmetrically to produce one daughter stem cell and one daughter that continues to divide, mature, and differentiate. However, mathematical modeling suggests that about five percent of the time, a stem cell may divide symmetrically to produce either two stem cells or two maturing cells. In the latter case, a stem cell is lost from the crypt by differentiation, displacement, or even apoptosis. Changes in the tissue environment may require different cellular outputs from a crypt and induce the stem cells to favor one of these forms of division. Stem-cell competition is one consequence of this variable outcome of stem-cell division and is thought to occur normally within crypts (9). Thus, occasional (possibly random) symmetrical divisions by a single stem cell may gradually populate a crypt with its own stem-cell daughters, which then displace existing stem cells and their daughter cells. Even in steady-state conditions, a single stem cell may gradually replace the other stem cells in a crypt with its own progeny, ultimately producing a monoclonal population of epithelial cells. This repopulation of the stem-cell compartment may require a considerable amount of time, perhaps about 100 days, depending on the number of stem cells per crypt, the cell-cycle time, and the frequency of symmetrical divisions. Stem-cell competition provides an alternative explanation for the observation of monoclonal crypts in the adult intestine, a finding that has been taken as evidence that only a single stem cell functions within a crypt. Instead, a single actual stem cell amongst a population of four to six may have gradually replaced the other stem cells, either randomly or through some form of competitive advantage. Again, this process implies a careful stem-cell counting mechanism.
Stem-cell markers
Proving that such forms of specialized division occur and a stem-cell hierarchy actually exists has been extremely difficult because there are no definitive stem-cell markers. Various transgenic approaches and polymorphisms have provided results that are consistent with stem-cell competition, but the definitive experiments on stem-cell characterization have proven elusive. For example, experiments relying upon the loss of expression of a lectin-binding site after mutation have produced crypts containing a mixture of mutated and nonmutated cells that gradually become monophenotypic and produce a ribbon of marked cells moving up the villus (10). The length of time needed to see such monophenotypic crypts (an interval much longer than the cell turnover time) and the changing width of the resultant villus ribbons is consistent with stem-cell competition. The usual caveats about such experiments apply to this finding, because the behavior being examined occurs in response to a perturbation of the crypt, namely, exposure to mutagens that are known to induce stem-cell apoptosis (reviewed in 4). Further circumstantial evidence comes from a natural human polymorphism in which expression of G6PD can be seen in entire or partial crypts (11). Unfortunately, the time course of the competition cannot be followed, so the spread of the polymorphism from a single stem cell to the entire stem-cell population (and hence crypt) cannot be measured. Ideally, a model should be developed in which this process can be observed in real time, possibly (as discussed below) by introducing a reporter gene into a stem cell and tracking the process, either in vitro using confocal microscopy or in vivo using endoscopy.
Asymmetric division suggests that there is a preferential inheritance of instructions in specific daughter cells that directs their fate: each cell receives cytoplasmic or nuclear information (or both) that determines whether it becomes a committed transit cell or a stem cell. In the epidermis and the hair follicle, the conservation of a population of cells with a labeled strand of DNA from birth to adulthood has provided evidence for DNA segregation during asymmetric cell division, such that the labeled template DNA seemingly remains in a stem cell, whereas the newly synthesized daughter strand is segregated into a transit cell (12, 13). However, such experiments in the intestine have been unsuccessful, probably due to the low tolerance of intestinal stem cells to damage from incorporating label. Recent unpublished work within our laboratory, however, appears to suggest that this type of DNA strand segregation also occurs in small intestinal stem cells. After a dose of 8–10 Gy of radiation, stem cells divide symmetrically to repopulate the crypt, so repeated doses of tritiated thymidine during this time will lead to incorporation of the label into these stem cells. After sufficient time, all the labeled proliferating cells will have been shed into the lumen, and even the slower cycling stem cells should have diluted any label below detection limits. However, cells are found that continue to retain label and are themselves cycling (as observed by double labeling with bromodeoxyuridine), suggesting that selective DNA segregation has occurred in which the stem cells preferentially retain the template DNA strand (Figure 1). Similar results are observed if young mice are labeled when undergoing stem-cell expansion (symmetrical division) in the growing intestine and then examined in later in adult life.
Figure 1.
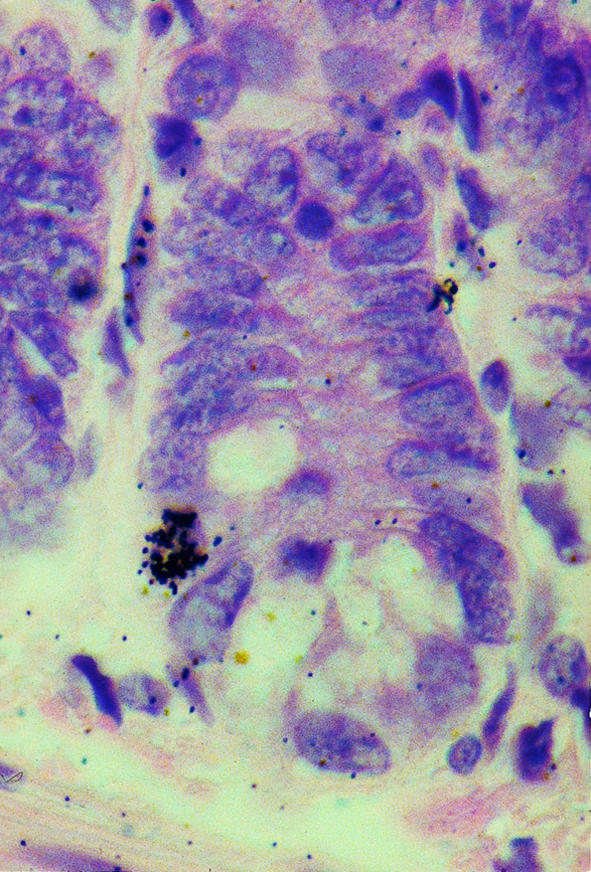
A small intestinal crypt (stained with hematoxylin and eosin) with autoradiographic silver grains overlying a single nucleus in the stem-cell region. This label-retaining cell was obtained by administering tritiated thymidine every 6 hours for 48 hours after irradiation with 8 Gy. The sample was taken 8 days later.
It is possible that no natural marker has been found simply because stem cells must maintain a restricted repertoire of expression in order to retain their undifferentiated state. However, recent preliminary and unpublished work in our laboratory has revealed an intriguing pattern of expression in the small intestine for the RNA binding protein Msi-1 (14), which is thought to be involved in asymmetric divisions in neuroendocrine stem-cell development. This protein appears to be expressed in a few cells in the stem-cell region of steady-state crypts, but is induced over the entire expanded clonogenic region after irradiation, indicating that it may mark functioning stem cells(C. Booth and C.S. Potten, manuscript in preparation). Because this protein also appears to be expressed in the developing crypts, it is possible that Msi-1 is the first natural intestinal stem-cell marker to be identified.
The stem-cell niche
One major unresolved issue in stem-cell biology is whether stem cells are intrinsically different from their dividing cells or whether they are instructed by their environment to behave differently — that is, whether there is a permissive niche. The localization of stem cells within the crypt and the gradual loss of stemness in the upwardly migrating daughter cells may indicate that the environment in which the stem cells are located is ideal for maintaining this phenotype, and that factors that maintain this environment are diluted as cells move away. Niche factors may control the longer stem-cell cycle time and self-maintenance properties but inhibit differentiation and the upward migration of stem cells by increasing their attachment properties. A number of extracellular matrix proteins and growth factors and receptors have been reported to be over- or underexpressed at the stem-cell location; these may have a role in controlling this niche. To date however, no single factor has been identified as having a major influence on such a niche, although the T-cell factor (TCF) family of factors, particularly TCF-4, may be implicated in stem-cell maintenance, because TCF-4 knockout mice appear to lose their crypt stem-cell population and die. Because this factor interacts with β-catenin, it may be involved in regulating either cell proliferation or cell movement within the stem-cell niche.
A number of spatial problems arise when considering growth factor control of a stem-cell niche. Stem cells in the small intestine are thought to be scattered around the crypt annulus at a distance of two to seven (with an average of four) cell diameters above the base of the crypt. It is therefore unlikely that any two stem cells will be in direct contact, and is much more likely that a stem cell may be surrounded by first- to third-generation daughter cells. It is therefore difficult to envision an environment that is so restricted as to exhibit very different levels of control on adjacent cells. Similarly, it is difficult to imagine a mechanism by which separated stem cells detect and compensate for changes in their own number. One possibility is that each stem cell is located adjacent to a Paneth’s cell, and this cell type contributes to stem-cell control. However, because some animals do not contain Paneth’s cells, these are obviously not necessary for stem-cell function. Another possibility is that the stem cells generate a field of stemness and the overall concentration of the relevant factors helps determine cell fate.
Regulation of stem-cell number
As long as a single functional stem cell remains within a crypt, any stem or clonogenic cell deficiency can be compensated for by symmetrical cell division to repopulate the crypt. Conversely, excess but otherwise healthy stem cells may normally be removed by a process of spontaneous apoptosis, a process that also removes stem cells that may have incurred DNA damage. Although more than one percent of crypt cells are apoptotic at any one time, these cells are concentrated in the stem-cell zone, suggesting that up to five to ten percent of stem cells may be undergoing apoptosis. Such stringent control of stem-cell numbers is vital, because one additional stem cell could lead to an extra 60–120 cells per crypt. Apoptosis in the small intestine is a very effective policeman of replication errors: despite the rapid proliferation rate, cancers are very rare.
Apoptosis is thought to be suppressed by bcl-2 in the colon (15), a process that may have evolved to protect the crypt from a constant need to undergo symmetrical division to restore cells lost to apoptosis in the fermenting cytotoxic environment of the colon. However, the bcl-2–induced suppression of spontaneous apoptosis may itself allow crypt stem-cell numbers to gradually drift upward, leading to a hyperplastic crypt. The balance of risk and benefit for each process is no doubt fine-tuned through evolution, but with current medical advances increasing life expectancy, the protective effects of apoptosis suppression may now represent a greater cancer risk than do the colonic contents themselves.
Should stem-cell numbers drift upward without incurring further mutation, it is thought that the natural response is for the crypt to divide, or bifurcate. In the growing animal, extensive symmetrical divisions probably expand stem-cell numbers in order to generate frequent crypt bifurcations for the growing gut. Indeed, many dividing crypts can be seen in the growing animal, and almost all of this type of division appears to originate toward the crypt base, i.e., at the stem-cell position. In geriatric animals, on the other hand, stem-cell number may drift upward, but fewer crypts are found per unit length.
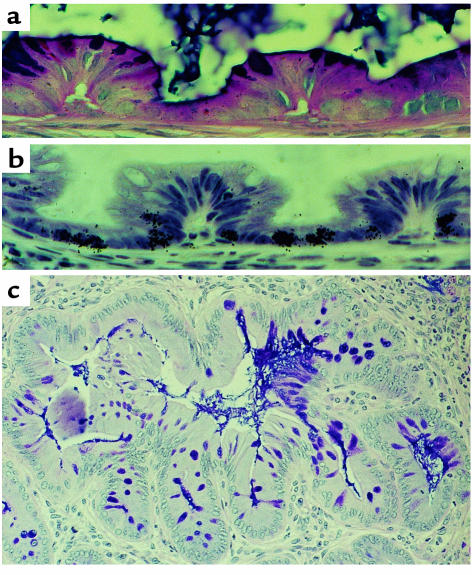
After cytotoxic exposure, such as after radio- or chemotherapy, some crypts will die and a similar situation will occur: remaining potential stem cells will begin to function as stem cells and will divide symmetrically to regenerate the crypt and finally generate more crypts via bifurcation (Figure 2), until eventually an intact mucosa is restored. However, in this situation, the stem cells initiating bifurcation are likely to be located at a slightly higher position up the crypt, being daughter cells of up to the third generation. One would therefore expect the origin of bifurcation to be slightly higher up the crypt. Preliminary analysis indicates that this is indeed the case, with new crypts branching out from the middle of the crypt. In cases of severe damage, in which all the clonogens are recruited to act as stem cells, multiple crypts can be generated from a single surviving crypt. Why one crypt survives when adjacent ones are killed is unclear, but crypts lying close to Peyer’s patch lymphoid tissue appear to be more radiotolerant, implying that these cells produce survival factors. The presence of a few more resistant “master crypts” also has been suggested, which may be responsible for populating areas, or patches, of mucosa during either development or cytotoxic regeneration, although this remains an unproven hypothesis.
Figure 2.
(a and b) Examples of “spontaneous” crypt fission (bifurcation) in normal adult mouse small intestine. (c) Multiple fission after irradiation with 12 Gy. A new crypt can be seen developing from the mid-crypt region as well as from the crypt base. Each neocrypt contains Paneth’s cells. (d) Similar bifurcation from a higher crypt-cell position can be seen in the colon after irradiation (10 Gy).
Comparisons of cell-cycle times and sensitivity to DNA damage (apoptotic frequency) in the actual stem cells have been made in juvenile, middle-aged, and geriatric mice, and to date have revealed an increase in the number of susceptible cells in geriatric mice (16). It also appears that the number of potential stem cells (clonogens) increases with age, although the speed with which a crypt recognizes and repairs DNA damage and initiates crypt regeneration is compromised as the animal ages (17). The reason for this less efficient homeostatic mechanism is unclear.
Interestingly, the cycle times calculated imply that during the three-year lifetime of a laboratory mouse, approximately 1,000 stem-cell divisions occur (up to 5,000 in man). Because every mitosis causes a shortening of chromosomal telomere length, one would expect stem cells to have a relatively high level of telomerase (the enzyme responsible for telomere synthesis), thereby ensuring sustained division potential. Consistent with this assumption, telomerase RNA and protein have been detected at the base of human colonic crypts, or just above the Paneth’s cells in the small intestine, as expected for stem cells (Figure 3). However, telomerase-null mice do not exhibit any intestinal phenotype until the sixth mouse generation, indicating that telomere regeneration is not absolutely required until this point. This could indicate that telomerase is not directly involved in stem-cell maintenance, but because mice themselves naturally have extremely long telomeres, it may simply indicate that in this species the telomeres can ultimately support up to 6,000 rounds of stem-cell division. Humans, with shorter telomeres, might prove more sensitive to transient loss of telomerase activity.
Figure 3.
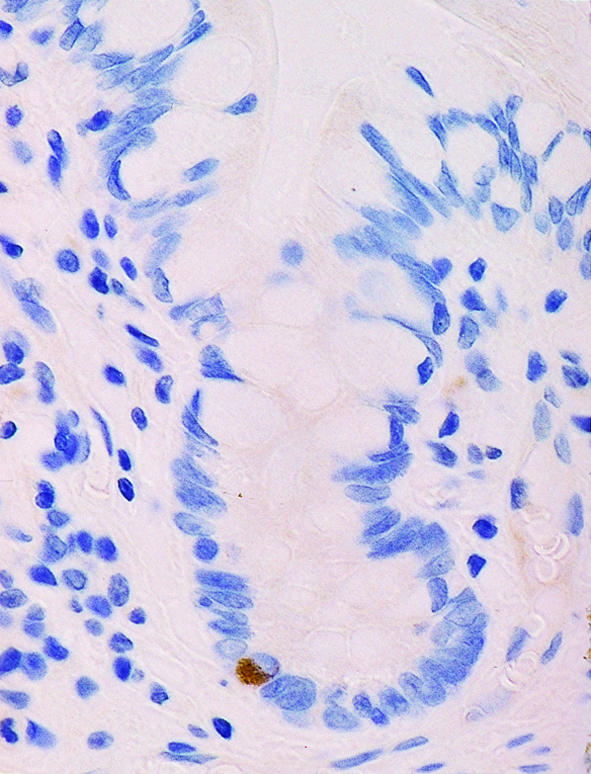
A human small intestinal crypt immunostained for telomerase. Labeling can be seen toward the crypt base, indicating telomere regeneration in the stem cell region.
Future model systems
Our understanding of intestinal stem cells is likely to progress rapidly in the next few years, thanks to two main advances: stem-cell transfection and in vitro stem-cell maintenance. Expression of reporter genes such as GFP in individual stem cells should enable many of the theories presented here to be tested directly, particularly if the marked crypts can also be maintained in vitro and examined by real-time confocal microscopy. For example, one should be able to observe stem-cell competition within a crypt, demonstrate the existence of distinct tiers of stem cells, and follow their reorganization during crypt regeneration or carcinogenesis. Analysis of individual marked cells, either in culture after growth factor administration or in transgenic animals, may help determine which factors influence stem-cell cycling or apoptotic susceptibility.
Ultimately this technology should produce enough knowledge for us to manipulate stem cells for clinical benefit, by altering stem-cell cycle kinetics, stem-cell number, or sensitivity to apoptosis. A number of cancer therapies may be improved as a consequence. With existing technology, we have made initial steps in this direction by pretreating mice experiencing radiation damage (a model for cancer radio- or chemotherapy), for example with the stem-cell inhibitory factor TGF-β3. TGF-β3 increases clonogenic cell survival, thereby reducing the level of normal intestinal tissue damage (side-effect damage) and increasing the speed with which it is repaired. This reduced severity of mucositis should improve the quality of life of the patient and also allow doses of chemotherapeutic drugs to be increased if appropriate (18). The lessons learned from in vivo transfection with reporter genes should also enable effective in vivo gene therapies. The long-term introduction of genes such as APC may reduce cancer risk in certain patients, whereas introduction of CFTR could be used to treat cystic fibrosis. Even short-term expression could be used to improve responses to cancer therapy by introducing p53, bcl-2, or DNA repair enzymes into the relevant tissue sites.
Until recently, in vitro models of normal intestinal stem cells have been nonexistent. Some researchers have argued that certain tumor or fetal cell lines are equivalent to stem cells, but this is generally due to the presence of one or two stem-cell properties, not the complete range necessary to define a stem cell. The cells are certainly not from a normal adult source, are always subjected to the various selective pressures during cloning, and generally contain one or more mutations (particularly p53). Therefore, the most promising methods of addressing stem-cell questions in vitro are primary culture models. These are relatively short-term cultures derived from isolated intact crypts (19) or dispersed crypt cells (20), and are being used to develop colony-forming assays similar to those routinely used for bone marrow cells.
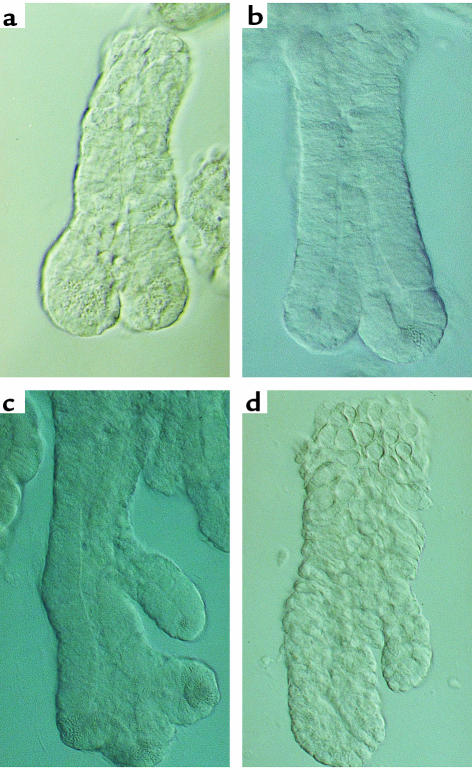
Cultures from isolated crypts maintain functional stem cells for at least the first week in culture, as judged by their ability to mediate crypt regeneration. Because the correct tissue culture conditions required to reproduce stem-cell lineages, crypt regeneration, differentiation, and spatial organization remain to be determined, stem-cell function has been assayed by subcutaneous transplantation in an immunocompromised animal, after various time periods in culture. After 4–12 weeks, a topographically correct intestinal-like structure is regenerated, complete with differentiated cell types (Figure 4). The structures are very similar to those observed during developmental organogenesis, and may involve similar homeobox-dependent signaling pathways. An intriguing question remaining to be addressed in this system concerns whether each structure is derived from one or multiple crypts. If the former (as preliminary data in some cases seems to indicate), then the stem cells, (potential new crypts), appear capable of redistributing themselves around the cyst-like structure and organizing morphological regeneration to create multiple crypts from one original crypt. Whether this situation involves the recruitment of the existing potential stem cells or induces stem-cell amplification via symmetrical division remains to be determined, but subcutaneous grafting of crypts with stem cells expressing a GFP reporter acting as a cell marker may help answer some of these questions.
Figure 4.
(a and b) Four-week-old grafts of colonic epithelium derived from isolated crypts. Invaginations similar to those observed during normal crypt development can be seen, with differentiated goblet cells and secreting mucins at the apical pole, and proliferating cells (labeled with tritiated thymidine) at the basal pole. (c) At later times some of these grafts develop a structure more typical of adult intestinal crypts. Adapted from ref. 19.
Stem-cell amplification is the ultimate aim of most in vitro work because this is necessary for mucosal transplantation to be a feasible clinical option. The culture conditions suitable for this have so far proven elusive, but with the development of colony-forming assays, combined with stem-cell marking, it should not be too long before the factors necessary to achieve this goal are identified. Such assays may also address the central question of whether a stem cell is preprogrammed for a particular region of the intestine or whether the underlying connective tissue has the major influence. Grafting colonic stem cells onto regions of the small intestine that have been denuded of epithelial cells could be used to address this question, because the former model predicts that large intestinal, rather than small intestinal, mucosa would be regenerated.
Conclusions
Despite the lack of marker proteins, a large amount of information has been collected over the years that collectively provides a detailed characterization of the stem cells of the small intestinal epithelium. Their number, cycle times, proliferative potential, and responses to a number of situations have been explored in great detail. The next major advances will involve stem-cell transfection, for both research and clinical purposes. In vitro, this will provide information on the factors controlling proliferation, apoptosis, differentiation, and general proliferative potential. In vivo transfection will support this data and also provide invaluable information on the lifetime behavior of stem cells. Ultimately, both have tremendous clinical potential. It is probably not too optimistic to expect successful in vitro maintenance and transfection of stem cells, before transplanting them back into the gut within the not-too-distant future.
Acknowledgments
Both authors are funded by the United Kingdom Cancer Research Campaign.
References
- 1.Potten, C.S. 1995. Structure, function and proliferative organisation of the mammalian gut. In Radiation and gut. C.S. Potten and J.H. Hendry, editors. Elsevier Science. Amsterdam, The Netherlands. 1–31.
- 2.Kaur P, Potten CS. Cell migration velocities in the crypts of the small intestine after cytotoxic insult are not dependent on mitotic activity. Cell Tissue Kinet. 1986;19:601–610. doi: 10.1111/j.1365-2184.1986.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 3.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 4.Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997;78:219–243. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendry JH, et al. The response of the murine intestinal crypts to short range prometheium-147 radiation. Radiat Res. 1989;118:364–374. [PubMed] [Google Scholar]
- 6.Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci. 1998;353:821–830. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and γ irradiation. Nature. 1977;269:518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 8.Merritt AJ, et al. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994;54:614–617. [PubMed] [Google Scholar]
- 9.Loeffler M, Birke A, Winton D, Potten CS. Somatic mutation monoclonality and stochastic models of stem cell organisation in the intestinal crypt. J Theor Biol. 1993;162:471–491. doi: 10.1006/jtbi.1993.1031. [DOI] [PubMed] [Google Scholar]
- 10.Winton DJ, Blount MA, Ponder BAJ. A clonal marker induced by mutation in mouse intestinal epithelium. Nature. 1988;33:463–466. doi: 10.1038/333463a0. [DOI] [PubMed] [Google Scholar]
- 11.Williams ED, Lowes AP, Williams D, Williams GT. A stem cell niche theory of intestinal crypt maintenance based on a study of somatic mutation in colonic mucosa. Am J Pathol. 1992;141:773–776. [PMC free article] [PubMed] [Google Scholar]
- 12.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;15:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 13.Potten CS, Hume WJ, Reid P, Cairns J. The segregation of DNA in epithelial stem cells. Cell. 1978;15:899–906. doi: 10.1016/0092-8674(78)90274-x. [DOI] [PubMed] [Google Scholar]
- 14.Sakakibara S, Okano H. Expression of neural RNA-binding proteins in the postnatal CNS: implications of their roles in neuronal and glial cell development. J Neurosci. 1997;17:8300–8312. doi: 10.1523/JNEUROSCI.17-21-08300.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merritt AJ, et al. Differential expression of bcl-2 in intestinal epithelia-correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995;108:2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- 16.Martin K, Kirkwood TB, Potten CS. Age changes in stem cells of murine small intestinal crypts. Exp Cell Res. 1998;241:316–323. doi: 10.1006/excr.1998.4001. [DOI] [PubMed] [Google Scholar]
- 17.Martin K, Potten CS, Roberts SA, Kirkwood TB. Altered stem cell regeneration in irradiated intestinal crypts of senescent mice. J Cell Sci. 1998;111:2297–2303. doi: 10.1242/jcs.111.16.2297. [DOI] [PubMed] [Google Scholar]
- 18.Potten CS, Booth D, Haley JD. Pretreatment with transforming growth factor beta-3 protects small intestinal stem cells against radiation damage in vivo. Br J Cancer. 1997;75:1454–1459. doi: 10.1038/bjc.1997.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth C, O’Shea JA, Potten CS. Maintenance of functional stem cells in isolated and cultured intestinal epithelium. Exp Cell Res. 1999;249:359–366. doi: 10.1006/excr.1999.4483. [DOI] [PubMed] [Google Scholar]
- 20.Whitehead RH, Demmler K, Rockman SP, Watson NK. Clonogenic growth of epithelial cells from normal colonic mucosa from both mice and humans. Gastroenterology. 1999;117:858–865. doi: 10.1016/s0016-5085(99)70344-6. [DOI] [PubMed] [Google Scholar]