Abstract
Skeletal muscle glucose uptake increases dramatically in response to physical exercise. Moreover, skeletal muscle comprises the vast majority of insulin-sensitive tissue and is a site of dysregulation in the insulin-resistant state. The biochemical and histological composition of the muscle is well defined in a variety of species. However, the functional consequences of muscle biochemical and histological adaptations to physiological and pathophysiological conditions are not well understood. The physiological regulation of muscle glucose uptake is complex. Sites involved in the regulation of muscle glucose uptake are defined by a three-step process consisting of: (1) delivery of glucose to muscle, (2) transport of glucose into the muscle by GLUT4 and (3) phosphorylation of glucose within the muscle by a hexokinase (HK). Muscle blood flow, capillary recruitment and extracellular matrix characteristics determine glucose movement from the blood to the interstitium. Plasma membrane GLUT4 content determines glucose transport into the cell. Muscle HK activity, cellular HK compartmentalization and the concentration of the HK inhibitor glucose 6-phosphate determine the capacity to phosphorylate glucose. Phosphorylation of glucose is irreversible in muscle; therefore, with this reaction, glucose is trapped and the uptake process is complete. Emphasis has been placed on the role of the glucose transport step for glucose influx into muscle with the past assertion that membrane transport is rate limiting. More recent research definitively shows that the distributed control paradigm more accurately defines the regulation of muscle glucose uptake as each of the three steps that define this process are important sites of flux control.
Keywords: flux, glucose, in vivo
Introduction
The Journal of Experimental Biology published a series of articles by Weibel, Taylor, Hoppeler and associates in the 1990s that defined the design of pathways for the utilization of oxygen and substrates (Hoppeler and Weibel, 1998; Roberts et al., 1996; Taylor et al., 1996; Vock et al., 1996a; Vock et al., 1996b; Weber et al., 1996a; Weber et al., 1996b; Weibel et al., 1991; Weibel et al., 1996). In these elegant analyses, the relationships of biological structure to functional limitations were defined by a series of transfer steps. These transfer steps conceptualized the delivery of oxygen from the environment, as well as substrates from storage depots, to working muscle. The concept of symmorphosis, whereby “no more structure is built and maintained than is required to meet functional demand”, was central to building the relationship of structure to functional limitations (Weibel et al., 1991).
Here we describe the regulatory factors that operate within the structural framework defined in this classic series of papers, focusing on the functional controllers of glucose influx into skeletal muscle. Blood glucose homeostasis cannot be understood without defining the control of muscle glucose uptake. Indeed, skeletal muscle comprises the bulk of insulin-sensitive tissue, and thus is where glucose uptake is quantitatively the most important. It is also the primary site of glucose uptake during exercise. As exercise and insulin are the primary physiological conditions that stimulate muscle glucose uptake, these conditions act to challenge the systems that control glucose flux into muscle. Here we will highlight studies in which these conditions have been used to perturb the glucoregulatory system. Moreover, defects in flux control that lead to glucose intolerance and insulin resistance resulting from high-fat feeding will be discussed. As in the previous studies summarized by Hoppeler and Weibel (Hoppeler and Weibel, 1998), we will describe the control of glucose flux in terms of the integration of physiological systems, and will emphasize animal models that provide unique insight into metabolic regulation.
Muscle glucose uptake defined
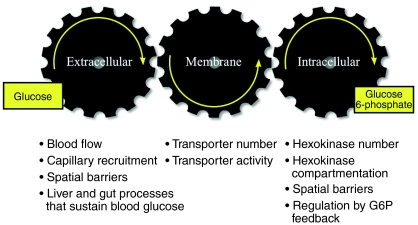
The physiological regulation of muscle glucose uptake requires that glucose travels from the blood to the interstitium to the intracellular space and then is phosphorylated to glucose 6-phosphate (G6P). The coupling of these processes involved in the influx of glucose is illustrated in Fig. 1. Blood glucose concentration, muscle blood flow and recruitment of capillaries to muscle determine glucose movement from the blood to the interstitium. The plasma membrane glucose transporter content determines glucose transport into the cell. Muscle hexokinase (HK) activity, cellular HK compartmentalization and the concentration of the HK inhibitor, G6P, determine the capacity to phosphorylate glucose. Glucose phosphorylation in muscle is irreversible; therefore, with this reaction, glucose is trapped and the uptake process is complete. These three steps – delivery, transport and phosphorylation – comprise muscle glucose uptake. This is not to say that steps downstream of glucose phosphorylation do not affect glucose uptake. It is just that any downstream step must, by definition, act through glucose delivery, transport or phosphorylation. For example, acceleration of glycolysis or glycogen synthesis could reduce G6P, increase HK activity, increase the capacity for glucose phosphorylation and potentially stimulate muscle glucose uptake. Reciprocally, rapid glycogen breakdown such as that which occurs with exercise could increase the G6P pool, inhibit HK, decrease the rate of glucose phosphorylation and, through this mechanism, impede the rate of muscle glucose uptake.
Fig. 1.
Distributed control of muscle glucose uptake. Modified from Wasserman and Halseth (Wasserman and Halseth, 1998) and Wasserman et al. (Wasserman et al., 1967).
The distributed control paradigm (Fig. 1) for muscle glucose uptake has been challenging to study because intracellular glucose, which is the product of membrane glucose transport and the substrate for glucose phosphorylation, cannot be measured directly. It can theoretically be calculated indirectly as the difference between total muscle glucose and interstitial glucose. Numerous theoretical and measurement issues make this calculation untenable. The close coupling of glucose delivery, transport and phosphorylation and the existence of glucose compartmentalization and spatial gradients compelled us to develop new techniques to overcome these difficulties associated with defining muscle glucose uptake.
Studying the whole organism
There have been innumerable studies that have attempted to address the control of glucose uptake in isolated muscle. These studies have provided tremendous insight into the basic cellular mechanisms behind glucose transport. Isolated muscle preparations, as does virtually every experimental model system, have strengths and also limitations. Extramyocellular factors involved in the control of glucose uptake (e.g. glucose delivery to muscle) are necessarily absent. Moreover, glucose uptake by isolated muscle preparations is extremely resistant to insulin (requiring suprapharmacological insulin levels) and contraction (requiring extremely high-intensity contraction). It is likely that in some instances the rates of glucose uptake in vitro do not become high enough to test the glucose phosphorylation capacity of muscle. As isolated muscle preparations are relatively simple to execute and far easier to interpret because they are free of the often complicating variables of the internal environment of the whole organism, most of the literature describes studies conducted in vitro. This body of work has led to the inevitable conclusion that membrane transport is rate limiting for muscle glucose uptake. One difficulty in studying the whole organism is that animal models are often stressed during experiments. This is particularly true of the mouse, whose small size makes the obtainment of blood difficult. The studies from our laboratory that are described below were performed using unique methods that were specifically designed to avoid stress and were validated to be stress-free on the basis of plasma catecholamine concentrations (Ayala et al., 2006; Berglund et al., 2008). By the use of these methods, we show that under physiological conditions the distributed control paradigm, where regulation of flux is distributed between multiple steps (Fig. 1), better defines muscle glucose uptake than the rate-limiting step paradigm, where regulation is dominated by a single step (Wasserman, 2009).
Control of muscle glucose influx during exercise
Regulating the supply of glucose to the working muscle
Blood glucose concentration is a key determinant of the rate at which muscle can consume glucose. If blood glucose concentration falls, the rate of muscle glucose uptake will decline as well. Conversely, an increase in blood glucose concentrations will cause muscle glucose uptake to increase. Liver release of glucose is the primary means by which blood glucose is sustained in the post-absorptive state in the face of constant tissue glucose usage. Thus, the control of liver glucose output is key to the regulation of muscle glucose uptake. Of course, the gut is key in providing glucose after a meal, and the ingestion of glucose can sustain blood glucose concentration under circumstances during which the liver rate of glucose release cannot keep pace with tissue glucose utilization.
Glucagon is the primary controller of hepatic glucose production in the sedentary state (Liljenquist et al., 1977). Exercise is a robust challenge of the processes involved because of the high rates of glucose production necessary to maintain blood glucose (Wasserman, 2009). Glucagon secretion from the pancreatic α cell increases during exercise, whereas insulin secretion from the pancreatic β cell declines. The decline in insulin secretion potentiates the actions of glucagon (Lavoie et al., 1997; Lins et al., 1983; Wasserman et al., 1989c). Studies in animals (Wasserman et al., 1984; Wasserman et al., 1985; Wasserman et al., 1989b) and humans (Hirsch et al., 1991; Lavoie et al., 1997; Wolfe et al., 1986) demonstrate that the increase in glucagon is the primary stimulator of hepatic glucose production during exercise. The powerful effect of glucagon on hepatic glucose production was recently demonstrated by Berglund et al. (Berglund et al., 2009). This study showed that increasing glucagon in sedentary mice to levels similar to those seen during exercise causes a marked discharge of hepatic energy stores so that the adenosine monophosphate (AMP) to adenosine triphosphate (ATP) ratio is increased. This increase in the AMP:ATP ratio, through allosteric mechanisms, facilitates the glucagon-induced breakdown of glycogen and the oxidation of fat in the liver (Wasserman et al., 1989a; Wasserman et al., 1989b).
Some have noted a disassociation between glucagon concentrations and glucose release from the liver and have used this to argue that glucagon does not stimulate hepatic glucose output during exercise (reviewed by Wasserman, 2009). This argument is flawed. The reason for this disassociation is that glucagon is secreted into the hepatic portal venous circulation, which traverses the liver. The liver extracts glucagon, thereby slowing the time course and dampening the rise in the hormone in the peripheral circulation (Coker et al., 1999; Wasserman et al., 1993).
It is unlikely that catecholamines are directly responsible for the increase in glucose production during exercise, as hepatic denervation (Wasserman et al., 1990), selective hepatic β- and α-adrenergic receptor blockade (Coker et al., 1997) and adrenalectomy (Moates et al., 1988) have little or no effect in the exercising dog. These findings are consistent with research in other species, including humans (Wasserman, 1995). Other factors such as interleukin-6 (Febbraio et al., 2004) or an as-yet-undefined regulator may play a role, perhaps by regulating the endocrine pancreas.
Muscle blood flow, the factor besides glucose concentration that determines blood glucose delivery, is markedly increased with exercise. A hallmark of the physiological response to exercise is marked hyperemia and an increase in capillary blood flow. The overall effect of this phenomenon on glucose influx is that more glucose is delivered to the working muscle and there is increased surface area for exchange of glucose. This hemodynamic effect increases the delivery not only of glucose but also of all blood constituents. Simulating the exercise-induced increase in glucose delivery in the absence of an increase in glucose transporter type 4 (GLUT4) protein translocation to the muscle membrane is inadequate by itself, however, in recreating the exercise-induced increase in muscle glucose uptake (Zinker et al., 1993b).
Glucose transport across the plasma membrane of working muscle cells
Membrane transport is almost certainly the primary barrier to muscle glucose uptake in the fasted, sedentary state, as membrane GLUT4 content is low and the membrane is relatively impermeable to glucose. GLUT4 translocation to the muscle membrane is accelerated by muscle contraction (Etgen et al., 1996; Ploug et al., 1992), and the intracellular signaling mechanism(s) resulting in GLUT4 translocation to the muscle membrane (Funai et al., 2009; Kramer et al., 2007; Kramer et al., 2006; Sakamoto and Goodyear, 2002; Witczak et al., 2010) and muscle glucose uptake (Goodyear et al., 1990; Ploug et al., 1984; Richter et al., 1985; Wallberg-Henriksson and Holloszy, 1985; Wasserman et al., 1991; Wasserman et al., 1992) is independent of the actions of insulin. Moreover, the fate of glucose extracted from the blood is different in response to exercise and insulin (Wasserman et al., 1991; Zinker et al., 1993a). The working muscle oxidizes glucose, whereas insulin-stimulated muscle primarily stores glucose.
Glucose phosphorylation within the working muscle cells
The ability of myocytes to phosphorylate glucose is inhibited by G6P. During exercise, the simultaneous increase in glycogen breakdown and glucose uptake can potentially lead an increase in the inhibitory G6P levels. This, combined with exercise hyperemia and increased glucose transport, predicts a shift in the muscle glucose uptake barrier from transport to phosphorylation. The first hint that glucose phosphorylation may become a significant barrier to glucose influx was an observation in muscle from exercised rats. It was shown that HK II mRNA, but not GLUT4 mRNA, was increased following exercise (O'Doherty et al., 1994) because of an increase in gene transcription (O'Doherty et al., 1996). Although increased GLUT4 expression has been reported following exercise (Kraniou et al., 2006; Ren et al., 1994), the HK II gene has since been shown to be considerably more responsive (O'Doherty et al., 1994; Pilegaard et al., 2005). The increase in HK II mRNA in response to a single bout of exercise only makes sense from an adaptive standpoint if glucose phosphorylation is a barrier to muscle glucose uptake.
Determining the functional roles of glucose delivery, transport and phosphorylation
The functional barriers to muscle glucose uptake were subsequently tested during exercise using isotopic glucose analogues in the conscious rat to obtain a surrogate for intracellular glucose by applying the concept of glucose countertransport (Halseth et al., 1998; Halseth et al., 2000; Halseth et al., 2001; O'Doherty et al., 1998; Petersen et al., 2003). In an isotopic steady state, glucose countertransport creates a situation where the distribution of one sugar between intracellular and extracellular water is induced by a transmembrane gradient of a second sugar (Morgan et al., 1964). The distribution of trace 3-O-[3H]methyl-glucose between intracellular and extracellular water can then be used to calculate the glucose concentration at the outer ([G]om) and inner ([G]im) membrane surfaces. The extracellular glucose gradient (arterial glucose minus [G]om), the trans-membrane gradient ([G]om–[G]im) and the intracellular glucose available for phosphorylation ([G]im) can then be calculated from this information. An index of muscle glucose uptake (Rg) can be derived from the accumulation of phosphorylated 2-deoxy[3H]glucose (2[3H]DG). Extracellular, membrane and intracellular resistances to muscle glucose influx can be calculated using a variation of Ohm's Law for electrical circuits where glucose gradients and Rg are analogous to voltage gradient and current, respectively. This model is illustrated in Fig. 2 and is shown with reference to anatomical compartments in Fig. 3.
Fig. 2.
Ohm's Law was applied to determine sites of resistance to muscle glucose uptake. Ga, Ge and Gi are the glucose concentrations in the arterial blood, outer sarcolemmal surface and inner sarcolemmal surface, respectively. RExtracell, RTransport and RPhos are the resistances to glucose influx in the extracellular space, across the membrane and at the phosphorylation step, respectively. Ig is the glucose ‘current’ as estimated using 2[3H]DG. Using the countertransport method, glucose gradients were calculated as described in the text. Transgenic mice were used to alter sites of resistance.
Fig. 3.
The flux of glucose from the blood to the membrane to muscle. Steps 1, 2 and 3 represent glucose delivery, membrane transport and phosphorylation steps, respectively. Hexagons labeled ‘G’ are glucose molecules; those with an associated ‘P’ are glucose 6-phosphate. Green ovals are glucose transporters. The figure illustrates the fasted, sedentary state where few transporters are in the plasma membrane. Glucose 6-phosphate inhibition of glucose phosphorylation is illustrated by a negative feedback loop. The countertransport method estimates glucose gradients across each step using radioactive glucose analogues. Transgenic mice were used to alter sites of resistance at each step.
The countertransport method revealed that exercise decreases the extracellular and muscle membrane glucose gradients (Halseth et al., 1998), reflecting a shift in control of muscle glucose uptake from glucose delivery and transport to glucose phosphorylation. The decrease in resistance to glucose transport is consistent with the translocation of GLUT4 to the plasma membrane. The decrease in resistance to glucose delivery is predictable from the marked exercise hyperemia. The concept that muscle glucose delivery was not a major barrier to muscle glucose uptake during exercise (Halseth et al., 1998) is consistent with results obtained with microdialysis (MacLean et al., 1999). The shift in control of muscle glucose influx to glucose phosphorylation during exercise is consistent with the accumulation of glucose in muscle tissue from exercising humans (Katz et al., 1986; Katz et al., 1991; Richter et al., 1998).
The second approach used to dissect control of glucose flux into working muscle was the application of isotopic techniques to mouse models with genetic increases in muscle GLUT4 and HK II. As was the case with the countertransport model, this approach was also based on Ohm's Law, where 2[3H]DG was used to gain an index of muscle glucose influx. The hypotheses that HK II overexpression (deletion of RPhos in Fig. 2) would increase the capacity of muscle to consume glucose, whereas GLUT4 overexpression (deletion of RTransport in Fig. 2) would have no effect were tested (Fueger et al., 2004a; Halseth et al., 1999). Mice overexpressing GLUT4 (GLUT4Tg) and/or HK II (HKTg) were catheterized and underwent experiments >5 days later. Consistent with predictions of the countertransport approach, HKTg mice had increased exercise-stimulated Rg, whereas GLUT4Tg mice did not. A variation of the ‘control coefficient’ concept was applied to the three steps of muscle glucose uptake and was calculated by the equation derived from control theory (Kacser and Burns, 1995):
| (1) |
where CTg is the control coefficient for the regulatory site of interest and PTg is calculated from the GLUT4 and HK II expression in GLUT4Tg and HKTg mice relative to their wild-type littermates. In a closed system, the control coefficients sum to 1.0. A control coefficient for glucose delivery is calculated as 1.0 minus the sum of control coefficients for transport and phosphorylation steps. It is assumed that the fold overexpression equals the functional increase in the transgene product. The control coefficient in the fasted sedentary state is highest at the transport step. During exercise, the control coefficient for transport falls to zero, suggesting that the muscle membrane is highly permeable to glucose because of recruitment of GLUT4 from intracellular vesicles. The control coefficient calculated for delivery also fell to very low levels, suggesting that exercise-induced hyperemia largely removes the glucose delivery barrier. During exercise, the onus of control very clearly rests with the phosphorylation step. Notably, these results are the same as those obtained using the countertransport approach.
Mice with a heterozygous or homozygous deletion of GLUT4 and mice with a heterozygous HK II deletion (complete knockout is lethal) extend results obtained in GLUT4 and HK II overexpressing mice. The Rg response to exercise was diminished in mice with a partial HK II knockout (Fueger et al., 2003). A heterozygous deletion of GLUT4 did not impair Rg in working muscle (Fueger et al., 2004b); however, a complete deletion of GLUT4 prevented the increase in Rg with exercise and led to marked hyperglycemia (Fueger et al., 2007b). We tested the hypothesis that if the glucose phosphorylation barrier in GLUT4 knockout mice was lowered by HK II overexpression, muscle would then be sensitive to reduced transport capacity. This was in fact the case (Fig. 4). Lowering the phosphorylation barrier resulted in a shift in control so that muscle glucose uptake was sensitive to a 50% reduction in GLUT4 during exercise (Fueger et al., 2004c).
Fig. 4.
Resistance to glucose phosphorylation and the impact of a 50% reduction in GLUT4 on the index of skeletal muscle glucose uptake (Rg) during exercise in mice. The absence of a single GLUT4 allele (GLUT4+/–) in mice does not affect Rg during exercise when resistance to phosphorylation is high. However, it leads to a marked reduction in Rg when the resistance to glucose phosphorylation is reduced by HK II overexpression. ‡P<0.05 compared with Rg in wild-type (WT) mice. *P<0.05 compared with Rg in mice overexpressing HK II (HKTg). Data are from Fueger et al. (Fueger et al., 2004c).
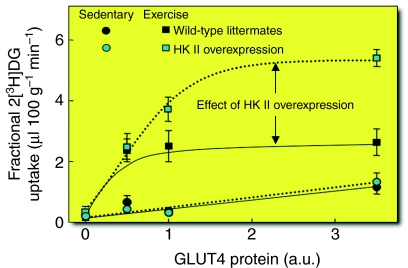
Fig. 5 shows fractional muscle 2[3H]DG uptake in sedentary and exercising mice with varying degrees of muscle GLUT4 and HK II expression. Several significant points emerge: (1) GLUT4 overexpression increases fractional muscle 2[3H]DG uptake in sedentary mice, whereas HK II overexpression does not; (2) GLUT4 overexpression does not further increase the stimulatory effect of exercise on fractional 2[3H]DG uptake; (3) muscle can tolerate a 50% reduction in GLUT4 expression without compromising sedentary and exercise-stimulated fractional 2[3H]DG uptake, provided that the phosphorylation barrier is not reduced by HK II overexpression; and (4) HK II overexpression increases the maximum velocity (Vmax) of fractional 2[3H]DG uptake during exercise. The shift in control from transport to phosphorylation should not be interpreted as meaning that GLUT4 is not important in the regulation of muscle glucose uptake. GLUT4 translocation is the fulcrum that distributes the balance of control between the three steps that control muscle glucose uptake. It is the sensitive regulation of GLUT4 translocation that shifts the burden of control from glucose transport to phosphorylation.
Fig. 5.
Fractional 2-deoxyglucose (2[3H]DG) uptake in the gastrocnemius of mice expressing 0-, 0.5-, 1.0- and ∼3.5-fold wild-type GLUT4 levels during exercise or in the sedentary state. GLUT4 only affected fractional 2[3H]DG uptake in the sedentary state when it was overexpressed. However, the absence of GLUT4 caused a marked attenuation of fractional 2[3H]DG uptake during exercise regardless of whether HK II was overexpressed. HK II overexpression had no effect on the response during saline infusion but increased the exercise. Data points are means ± s.e.m. of 8–11 in vivo mouse experiments. Modified from Wasserman (Wasserman, 2009).
Determining the functional roles of AMPK and NOS signaling
In recent years, the roles of nitric oxide synthase (NOS) (Bradley et al., 1999; Higaki et al., 2001; Lee-Young et al., 2010; McConell et al., 2006; McConell and Wadley, 2008; Shearer et al., 2004) and AMP-activated protein kinase (AMPK) (Canto et al., 2010; Jensen et al., 2008; Jensen et al., 2009; Lee-Young et al., 2009; Maarbjerg et al., 2009; Mu et al., 2001; Shearer et al., 2004) in the regulation of glucose uptake by working muscle has received considerable attention. Studies in vivo show extensive interaction between the NOS and AMPK signaling pathways in working muscle (Lee-Young et al., 2010; Lee-Young et al., 2009). We have assessed how these signaling pathways affect barriers to muscle glucose uptake in vivo. Mice expressing a dominant-negative mutation of the AMPKα2 subunit within skeletal muscle have an impaired Rg response to exercise when compared with wild-type littermates exercising at the same relative work rate. Surprisingly, however, this decrease appears to have been due not to impaired glucose transport, but rather impaired glucose delivery (Lee-Young et al., 2009). This result is consistent with the demonstration that the AMPKα2 mutant mice had a reduction in muscle NOS activity during exercise (Lee-Young et al., 2009), whereas glucose transporter activity was unaffected (Maarbjerg et al., 2009). Mice with a global endothelial NOS deletion also have a marked decrease in glucose delivery to working muscle, largely due to decreased blood flow (Lee-Young et al., 2010). Remarkably, despite this decrease, glucose influx is greatly accelerated, because of the rapid decline in muscle metabolic state (Lee-Young et al., 2010). The ability to adapt to reduced glucose delivery and sustain muscle glucose uptake reflects the powerful nature of distributed control of muscle glucose uptake. It is notable that endothelial NOS deletion results in impaired mitochondrial function. One can speculate that the increased reliance on glycolysis for energy production (as evidence by marked increases in plasma lactate levels) reduces G6P concentration. It can then be hypothesized that the reduction of G6P reduces the control coefficient for glucose phosphorylation, permitting high rates of glucose uptake even in the presence of low glucose delivery rates.
Control of insulin-stimulated muscle glucose influx
Determining the functional roles of glucose delivery, transport and phosphorylation
The use of isotopic analogs was applied to the concept of countertransport, as described above, to delineate barriers to insulin-stimulated muscle glucose influx during hyperinsulinemic, euglycemic clamps (insulin clamps) with physiological doses of insulin in unstressed rats (Halseth et al., 1998; Halseth et al., 2000; Halseth et al., 2001; O'Doherty et al., 1998). The countertransport method showed that insulin decreases the muscle membrane glucose gradient in a dose-dependent manner, reflecting a shift in control of muscle glucose uptake away from glucose transport to delivery and/or phosphorylation. These results were also consistent with those obtained by muscle interstitial microdialysis (Holmang et al., 1998; Rosdahl et al., 1998).
Examination of insulin-stimulated glucose flux control using unstressed, transgenic mice during insulin clamps performed at high physiological insulin levels showed remarkable agreement with results obtained using the countertransport method in insulin-clamped mice. Consistent with predictions of the countertransport approach, HKTg mice (deletion of RPhos in Fig. 2) had increased insulin-stimulated Rg values (Fueger et al., 2004a; Halseth et al., 1999), whereas GLUT4Tg mice (deletion of RTransport in Fig. 2) did not (Fueger et al., 2004b). Control of muscle glucose uptake using the three-step process showed that the control coefficient for glucose transport fell to near zero. Thus, as with exercise, physiological insulin stimulation results in increased GLUT4 translocation and a precipitous fall in the control coefficient for transport. The difference between insulin stimulation and exercise is that with insulin, control of muscle glucose uptake is shared between glucose delivery and phosphorylation, whereas during exercise the onus of control rests with the phosphorylation step. Again, these results are the same as those obtained using the countertransport approach in rats.
Mice with a heterozygous or homozygous deletion of GLUT4 and mice with a heterozygous HK II deletion extended results obtained in insulin-clamped GLUT4 and HK II overexpressing mice. A reduction in HK II impairs whole-body insulin sensitivity and heart Rg, but not Rg of the gastrocnemius muscle (Fueger et al., 2007a). As was the case with exercise, a 50% reduction in GLUT4 did not impair Rg in insulin-stimulated states (Fueger et al., 2004b). Muscle can tolerate a 50% reduction in GLUT4 during insulin stimulation provided that the barrier to glucose phosphorylation is not removed by HK II overexpression (Fueger et al., 2004b). However, in the absence of a barrier to glucose phosphorylation, membrane glucose transport becomes a limitation to muscle glucose uptake (Fig. 6).
Fig. 6.
Resistance to glucose phosphorylation and the impact of a 50% reduction in GLUT4 on the index of skeletal muscle glucose uptake (Rg) during the steady-state period of a 4.0 mU kg–1 min–1 insulin clamp. The absence of a single GLUT4 allele (GLUT4+/–) in mice does not affect Rg during physiological insulin stimulation when resistance to phosphorylation is high. However, it leads to a marked reduction in Rg when the resistance to glucose phosphorylation is reduced by HK II overexpression. ‡P<0.05 compared with Rg in wild-type (WT) mice. *P<0.05 compared with Rg in mice overexpressing HK II (HKTg). Data are from Fueger et al. (Fueger et al., 2004b).
The fractional skeletal muscle 2[3H]DG uptake in saline-infused and insulin-clamped mice with varying degrees of muscle GLUT4 and HK II expression is summarized in Fig. 7. The relationships of these proteins to muscle 2[3H]DG uptake in insulin-clamped mice is generally similar to the response in exercised mice (Fig. 5). It can be readily seen that: (1) GLUT4, but not HK II, overexpression increases fractional muscle 2[3H]DG uptake in saline-infused mice; (2) GLUT4 overexpression does not increase the stimulatory effect of a physiological increase in insulin on fractional 2[3H]DG uptake; (3) muscle can tolerate a 50% reduction in GLUT4 expression without affecting insulin-stimulated fractional 2[3H]DG uptake; and (4) HK II overexpression increases the Vmax of fractional 2[3H]DG uptake during an insulin clamp conducted at physiological hyperinsulinemia. We interpret these findings to mean that the sensitive regulation of GLUT4 translocation increases glucose membrane permeability to the point where the onus of control shifts from glucose transport in the saline-infused state to glucose phosphorylation (and glucose delivery) under insulin-clamped conditions.
Fig. 7.
Fractional 2-deoxyglucose (2[3H]DG) uptake in the gastrocnemius of mice expressing 0-, 0.5-, 1.0- and ∼3.5-fold wild-type GLUT4 levels during the steady-state period of a 4.0 mU kg–1 min–1 insulin clamp or during an equal duration saline infusion. HK II content was either normal (WT) or the protein was overexpressed. GLUT4 only affected fractional 2[3H]DG uptake during saline infusion when it was overexpressed. However, the absence of GLUT4 caused a marked attenuation of fractional 2[3H]DG uptake during insulin stimulation regardless of whether HK II was overexpressed. HK II overexpression had no effect on the response during saline infusion but increased the maximal response to insulin. Data points are means ± s.e.m. of 8–11 in vivo mouse experiments. Modified from Wasserman (Wasserman, 2009).
Barriers to muscle glucose uptake in the diet-induced insulin-resistant state
Insulin resistance can be associated with deficits in muscle blood flow (Clerk et al., 2007; Duplain et al., 2001; Inyard et al., 2009; Laakso et al., 1992), membrane glucose transport (Han et al., 1995; Liu et al., 1996; Zierath et al., 1997) and intracellular capacity to phosphorylate glucose (Bonadonna et al., 1996; Braithwaite et al., 1995; Pendergrass et al., 1998; Williams et al., 2001). We delineated the steps that cause the functional impairment in muscle glucose uptake by applying the countertransport method to rats fed a high-fat diet. Results showed that extracellular and intracellular resistances were the two chief causes of the resistance of muscle glucose uptake to insulin. This is not to say that transport is normal. Evidence clearly shows that glucose transport is defective with diet-induced insulin resistance (Liu et al., 1996). The countertransport experiments show, however, that the primary functional limitations are in glucose delivery and phosphorylation in this model. The pathogenesis of insulin resistance is further complicated when one considers that extracellular barriers to glucose delivery are also apt to be barriers to insulin delivery. Impaired insulin delivery will impact muscle glucose uptake and metabolic regulation in general if the barrier to insulin delivery is sufficiently large.
Mice of the C57Bl/6J strain develop insulin resistance on a high-fat diet (Surwit et al., 1988) and were used to determine the functional deficits that make muscle insulin resistant. The hypothesis that HK II overexpression could correct insulin-stimulated muscle glucose uptake in mice fed a high-fat diet was tested. In contrast to the marked effect of HK II overexpression on insulin-stimulated muscle glucose uptake in mice fed chow, there was no effect in mice fed the high-fat diet (Fueger et al., 2004a) (Fig. 8). This suggested that the glucose phosphorylation barrier was not the functional limitation causing insulin resistance to muscle glucose uptake. The countertransport data showed that extracellular resistance was the chief site of resistance to muscle glucose uptake. To further test this finding, we treated mice fed the high-fat diet for 3 months with the PDE5a inhibitor, sildenafil (Ayala et al., 2007). PDE5a is expressed in vascular smooth muscle and causes breakdown of cyclic guanosine monophosphate (cGMP). cGMP signals relaxation of vascular smooth muscle. Inhibition of PDE5a with sildenafil treatment results in increased cGMP levels and decreased vascular resistance (reduction in RExtracell in Fig. 2). We showed that this compound increases insulin-stimulated muscle Rg in mice fed the high-fat diet and it did so without improving muscle insulin signaling (Fig. 9). This supports the theory that inhibition of PDE5a acts by lowering the barrier to glucose delivery and, by doing so, decreases insulin resistance. One would predict that sildenafil increases insulin delivery to the muscle as well. The fact that insulin signaling was not enhanced would suggest that the improved Rg response was not due to greater insulin availability. Sildenafil treatment had other consequences, including increased energy expenditure and decreased body weight. So although sildenafil is effective at improving insulin-stimulated muscle glucose uptake in the insulin-resistant mouse, more work is required before the mechanism of action can be fully defined (Ayala et al., 2007).
Fig. 8.
Glucose metabolic index measured using 2-deoxyglucose (2[3H]DG) was measured for the gastrocnemius and superficial vastus lateralis (SVL) during the last 30 min of a 120-min saline infusion or hyperinsulinemic-euglycemic clamp (4.0 mU kg–1 min–1) experiment on conscious, unrestrained mice fasted for 5 h. Wild-type (WT) or HK II overexpressing (HKTg) mice were fed either a standard diet or a high-fat diet up to the age of 4 months of age and fasted for 5 h. Data are means ± s.e.m. for 7–14 mice per group. *P<0.05 vs saline condition; †P<0.05 vs WT standard diet; ‡P<0.05 vs HKTg standard diet. Data are from Fueger et al. (Fueger et al., 2004b). Figure reproduced from Wasserman (Wasserman, 2009).
Fig. 9.
Hyperinsulinemic-euglycemic clamps on conscious, unrestrained C57Bl/6J mice fasted for 5 h and chronically treated with either vehicle or sildenafil plus arginine subcutaneously for 3 months. The the index of skeletal muscle glucose uptake (Rg) measured using 2[3H]DG is shown for the soleus, gastrocnemius and superficial vastus lateralis (SVL). Data are the means ± s.e.m. for 7–8 mice per group. *P<0.05 vs vehicle. Data are from Ayala et al. (Ayala et al., 2007). Figure reproduced from Wasserman (Wasserman, 2009).
Conclusions
For decades, the presumption by researchers was that muscle glucose uptake is rate limited by membrane transport. This conclusion was based almost entirely on studies conducted in vitro. In the whole organism, the paradigm for control of glucose flux into muscle is much different. This paradigm posits that muscle glucose uptake is under distributed control by processes that control glucose delivery to, membrane transport into and phosphorylation within muscle. This model recognizes that the liver and gut are also important determinants of muscle glucose uptake as they maintain glucose concentrations and sustain muscle glucose delivery. Moreover, feedback inhibition of HK II by G6P provides a means by which the distribution of control is transferred to pathways downstream of glucose phosphorylation (i.e. glycogen synthesis and glycolysis). This enables the linkage of energy metabolism/storage with glucose flux. Here we show, using isotopic methods and transgenic/mutant mice, that each of these three steps can be formidable barriers to muscle glucose uptake under physiological conditions. The therapeutic implication is that one or more of these steps should be effective targets for treatment of glucose intolerance and insulin resistance. Here we showed the importance of a therapy that targets the glucose delivery step.
Footnotes
The authors' research described in this review was funded by NIH grants DK54902, DK50277 and DK59637. Deposited in PMC for release after 12 months.
References
- Ayala J. E., Bracy D. P., McGuinness O. P., Wasserman D. H. (2006). Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55, 390-397 [DOI] [PubMed] [Google Scholar]
- Ayala J. E., Bracy D. P., Julien B. M., Rottman J. N., Fueger P. T., Wasserman D. H. (2007). Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 56, 1025-1033 [DOI] [PubMed] [Google Scholar]
- Berglund E. D., Li C. Y., Poffenberger G., Ayala J. E., Fueger P. T., Willis S. E., Jewell M. M., Powers A. C., Wasserman D. H. (2008). Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes 57, 1790-1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund E. D., Lee-Young R. S., Lustig D. G., Lynes S. E., Donahue E. P., Camacho R. C., Meredith M. E., Magnuson M. A., Charron M. J., Wasserman D. H. (2009). Hepatic energy state is regulated by glucagon receptor signaling in mice. J. Clin. Invest. 119, 2412-2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berria R., Wang L., Richardson D. K., Finlayson J., Belfort R., Pratipanawatr T., De Filippis E. A., Kashyap S., Mandarino L. J. (2006). Increased collagen content in insulin-resistant skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 290, E560-E565 [DOI] [PubMed] [Google Scholar]
- Bonadonna R., Prato S. D., Bonora E., Saccomani M., Gulli G., Natali A., Frascerra S., Pecori N., Ferrannini E., Bier D., et al. (1996). Roles of glucose transport and glucose phosphorylation in muscle insulin resistance of NIDDM. Diabetes 45, 915-925 [DOI] [PubMed] [Google Scholar]
- Bradley S. J., Kingwell B. A., McConell G. K. (1999). Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes 48, 1815-1821 [DOI] [PubMed] [Google Scholar]
- Braithwaite S. S., Palazuk B., Colca J. R., Edwards C. W., Hofmann C. (1995). Reduced expression of hexokinase II in insulin-resistant diabetes. Diabetes 44, 43-48 [DOI] [PubMed] [Google Scholar]
- Canto C., Jiang L. Q., Deshmukh A. S., Mataki C., Coste A., Lagouge M., Zierath J. R., Auwerx J. (2010). Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 11, 213-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerk L. H., Vincent M. A., Barrett E. J., Lankford M. F., Lindner J. R. (2007). Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensin-converting enzyme inhibition. Am. J. Physiol. Endocrinol. Metab. 293, E1804-E1809 [DOI] [PubMed] [Google Scholar]
- Coker R. H., Krishna M. G., Lacy D. B., Bracy D. P., Wasserman D. H. (1997). Role of hepatic alpha- and beta-adrenergic receptor stimulation on hepatic glucose production during heavy exercise. Am. J. Physiol. 273, E831-E838 [DOI] [PubMed] [Google Scholar]
- Coker R. H., Lacy D. B., Krishna M. G., Wasserman D. H. (1999). Splanchnic glucagon kinetics in exercising alloxan-diabetic dogs. J. Appl. Physiol. 86, 1626-1631 [DOI] [PubMed] [Google Scholar]
- Duplain H., Burcelin R., Sartori C., Cook S., Egli M., Lepori M., Vollenweider P., Pedrazzini T., Nicod P., Thorens B., et al. (2001). Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104, 342-345 [DOI] [PubMed] [Google Scholar]
- Etgen G. J., Jr, Wilson C. M., Jensen J., Cushman S. W., Ivy J. L. (1996). Glucose transport and cell surface GLUT-4 protein in skeletal muscle of the obese Zucker rat. Am. J. Physiol. 271, E294-E301 [DOI] [PubMed] [Google Scholar]
- Febbraio M. A., Hiscock N., Sacchetti M., Fischer C. P., Pedersen B. K. (2004). Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53, 1643-1648 [DOI] [PubMed] [Google Scholar]
- Fueger P. T., Heikkinen S., Bracy D. P., Malabanan C. M., Laakso M., Wasserman D. H. (2003). Hexokinase II partial knockout impairs exercise-stimulated muscle glucose uptake in oxidative muscles of mice. Am. J. Physiol. 285, 958-963 [DOI] [PubMed] [Google Scholar]
- Fueger P. T., Bracy D. P., Malabanan C. M., Pencek R. R., Granner D. K., Wasserman D. H. (2004a). Hexokinase II overexpression improves exercise-stimulated but not insulin-stimulated muscle glucose uptake in high-fat-fed C57BL/6J mice. Diabetes 53, 306-314 [DOI] [PubMed] [Google Scholar]
- Fueger P. T., Hess H. S., Bracy D. P., Pencek R. R., Posey K. A., Charron M. J., Wasserman D. H. (2004b). Regulation of insulin-stimulated muscle glucose uptake in the conscious mouse: role of glucose transport is dependent on glucose phosphorylation capacity. Endocrinology 145, 4912-4916 [DOI] [PubMed] [Google Scholar]
- Fueger P. T., Hess H. S., Posey K. A., Bracy D. P., Pencek R. R., Charron M. J., Wasserman D. H. (2004c). Control of exercise-stimulated muscle glucose uptake by GLUT4 is dependent on glucose phosphorylation capacity in the conscious mouse. J. Biol. Chem. 279, 50956-50961 [DOI] [PubMed] [Google Scholar]
- Fueger P. T., Lee-Young R. S., Shearer J., Bracy D. P., Heikkinen S., Laakso M., Rottman J. N., Wasserman D. H. (2007a). Phosphorylation barriers to skeletal and cardiac muscle glucose uptakes in high-fat fed mice: studies in mice with a 50% reduction of hexokinase II. Diabetes 56, 2476-2484 [DOI] [PubMed] [Google Scholar]
- Fueger P. T., Li C. Y., Ayala J. E., Shearer J., Bracy D. P., Charron M. J., Rottman J. N., Wasserman D. H. (2007b). Glucose kinetics and exercise tolerance in mice lacking the GLUT4 glucose transporter. J. Physiol. 582, 801-812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funai K., Schweitzer G. G., Sharma N., Kanzaki M., Cartee G. D. (2009). Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 297, E242-E251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear L. J., Hirshman M. F., King P. A., Thompson C. M., Horton E. D., Horton E. S. (1990). Skeletal muscle plasma membrane glucose transport and glucose transporters after exercise. J. Appl. Physiol. 68, 193-198 [DOI] [PubMed] [Google Scholar]
- Halseth A. E., Bracy D. P., Wasserman D. H. (1998). Limitations to exercise- and maximal insulin-stimulated muscle glucose uptake. J. Appl. Physiol. 85, 2305-2313 [DOI] [PubMed] [Google Scholar]
- Halseth A., Bracy D., Wasserman D. (1999). Overexpression of hexokinase II increases insulin-and exercise-stimulated muscle glucose uptake in vivo. Am. J. Physiol. 276, E70-E77 [DOI] [PubMed] [Google Scholar]
- Halseth A. E., Bracy D. P., Wasserman D. H. (2000). Limitations to basal and insulin-stimulated skeletal muscle glucose uptake in the high-fat-fed rat. Am. J. Physiol. Endocrinol. Metab. 279, E1064-E1071 [DOI] [PubMed] [Google Scholar]
- Halseth A. E., Bracy D. P., Wasserman D. H. (2001). Functional limitations to glucose uptake in muscles comprised of different fiber types. Am. J. Physiol. Endocrinol. Metab. 280, E994-E999 [DOI] [PubMed] [Google Scholar]
- Han X. X., Handberg A., Petersen L. N., Ploug T., Galbo H. (1995). Stability of GLUT-1 and GLUT-4 expression in perfused rat muscle stimulated by insulin and exercise. J. Appl. Physiol. 78, 46-52 [DOI] [PubMed] [Google Scholar]
- Higaki Y., Hirshman M. F., Fujii N., Goodyear L. J. (2001). Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes 50, 241-247 [DOI] [PubMed] [Google Scholar]
- Hirsch I. B., Marker J. C., Smith L. J., Spina R. J., Parvin C. A., Holloszy J. O., Cryer P. E. (1991). Insulin and glucagon in prevention of hypoglycemia during exercise in humans. Am. J. Physiol. 260, E695-E704 [DOI] [PubMed] [Google Scholar]
- Holmang A., Muller M., Andersson O. K., Lonnroth P. (1998). Minimal influence of blood flow on interstitial glucose and lactate-normal and insulin-resistant muscle. Am. J. Physiol. 274, E446-E452 [DOI] [PubMed] [Google Scholar]
- Hoppeler H., Weibel E. R. (1998). Limits for oxygen and substrate transport in mammals. J. Exp. Biol. 201, 1051-1064 [DOI] [PubMed] [Google Scholar]
- Inyard A. C., Chong D. G., Klibanov A. L., Barrett E. J. (2009). Muscle contraction, but not insulin, increases microvascular blood volume in the presence of free fatty acid-induced insulin resistance. Diabetes 58, 2457-2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T. E., Schjerling P., Viollet B., Wojtaszewski J. F., Richter E. A. (2008). AMPK alpha1 activation is required for stimulation of glucose uptake by twitch contraction, but not by H2O2, in mouse skeletal muscle. PLoS ONE 3, e2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T. E., Wojtaszewski J. F., Richter E. A. (2009). AMP-activated protein kinase in contraction regulation of skeletal muscle metabolism: necessary and/or sufficient? Acta Physiol. (Oxf.) 196, 155-174 [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. (1995). The control of flux: 21 years on. Biochem. Soc. Trans. 23, 341-366 [DOI] [PubMed] [Google Scholar]
- Katz A., Broberg S., Sahlin K., Wahren J. (1986). Leg glucose uptake during maximal dynamic exercise in humans. Am. J. Physiol. 251, E65-E70 [DOI] [PubMed] [Google Scholar]
- Katz A., Sahlin K., Broberg S. (1991). Regulation of glucose utilization in human skeletal muscle during moderate dynamic exercise. Am. J. Physiol. 260, E411-E415 [DOI] [PubMed] [Google Scholar]
- Kramer H. F., Witczak C. A., Taylor E. B., Fujii N., Hirshman M. F., Goodyear L. J. (2006). AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J. Biol. Chem. 281, 31478-31485 [DOI] [PubMed] [Google Scholar]
- Kramer H. F., Taylor E. B., Witczak C. A., Fujii N., Hirshman M. F., Goodyear L. J. (2007). Calmodulin-binding domain of AS160 regulates contraction- but not insulin-stimulated glucose uptake in skeletal muscle. Diabetes 56, 2854-2862 [DOI] [PubMed] [Google Scholar]
- Kraniou G. N., Cameron-Smith D., Hargreaves M. (2006). Acute exercise and GLUT4 expression in human skeletal muscle: influence of exercise intensity. J. Appl. Physiol. 101, 934-937 [DOI] [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Brechtel G., Baron A. D. (1992). Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41, 1076-1083 [DOI] [PubMed] [Google Scholar]
- Lavoie C., Ducros F., Bourque J., Langelier H., Chiasson J. L. (1997). Glucose metabolism during exercise in man: the role of insulin and glucagon in the regulation of hepatic glucose production and gluconeogenesis. Can. J. Physiol. Pharmacol. 75, 26-35 [DOI] [PubMed] [Google Scholar]
- Lee-Young R. S., Griffee S. R., Lynes S. E., Bracy D. P., Ayala J. E., McGuinness O. P., Wasserman D. H. (2009). Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J. Biol. Chem. 284, 23925-23934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Young R. S., Ayala J. E., Hunley C. F., James F. D., Bracy D. P., Kang L., Wasserman D. H. (2010). Endothelial nitric oxide synthase is central to skeletal muscle metabolic regulation and enzymatic signaling during exercise in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1399-R1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenquist J. E., Mueller G. L., Cherrington A. D., Keller U., Chiasson J. L., Perry J. M., Lacy W. W., Rabinowitz D. (1977). Evidence for an important role of glucagon in the regulation of hepatic glucose production in normal man. J. Clin. Invest. 59, 369-374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lins P. E., Wajngot A., Adamson U., Vranic M., Efendic S. (1983). Minimal increases in glucagon levels enhance glucose production in man with partial hypoinsulinemia. Diabetes 32, 633-636 [DOI] [PubMed] [Google Scholar]
- Liu S., Baracos V. E., Quinney H., Clandinin M. T. (1996). Dietary fat modifies exercise-dependent glucose transport in skeletal muscle. J. Appl. Physiol. 80, 1219-1224 [DOI] [PubMed] [Google Scholar]
- Maarbjerg S. J., Jørgensen S. B., Rose A. J., Jeppesen J., Jensen T. E., Treebak J. T., Birk J. B., Schjerling P., Wojtaszewski J. F., Richter E. A. (2009). Genetic impairment of AMPKalpha2 signaling does not reduce muscle glucose uptake during treadmill exercise in mice. Am. J. Physiol. Endocrinol. Metab. 297, E924-E934 [DOI] [PubMed] [Google Scholar]
- MacLean D. A., Bangsbo J., Saltin B. (1999). Muscle interstitial glucose and lactate levels during dynamic exercise in humans determined by microdialysis. J. Appl. Physiol. 87, 1483-1490 [DOI] [PubMed] [Google Scholar]
- McConell G. K., Wadley G. D. (2008). Potential role of nitric oxide in contraction-stimulated glucose uptake and mitochondrial biogenesis in skeletal muscle. Clin. Exp. Pharmacol. Physiol. 35, 1488-1492 [DOI] [PubMed] [Google Scholar]
- McConell G. K., Huynh N. N., Lee-Young R. S., Canny B. J., Wadley G. D. (2006). L-Arginine infusion increases glucose clearance during prolonged exercise in humans. Am. J. Physiol. Endocrinol. Metab. 290, E60-E66 [DOI] [PubMed] [Google Scholar]
- Moates J. M., Lacy D. B., Goldstein R. E., Cherrington A. D., Wasserman D. H. (1988). Metabolic role of the exercise-induced increment in epinephrine in the dog. Am. J. Physiol. 255, E428-E436 [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Regen D. M., Park C. R. (1964). Identification of a mobile carrier-mediated sugar transport system in muscle. J. Biol. Chem. 239, 369-374 [PubMed] [Google Scholar]
- Mu J., Brozinick J. T., Valladares O., Bucan M., Birnbaum M. J. (2001). A role of AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 7, 1085-1094 [DOI] [PubMed] [Google Scholar]
- O’Doherty R. M., Bracy D. P., Osawa H., Wasserman D. H., Granner D. K. (1994). Rat skeletal muscle hexokinase II mRNA and activity are increased by a single bout of acute exercise. Am. J. Physiol. 266, E171-E178 [DOI] [PubMed] [Google Scholar]
- O’Doherty R. M., Bracy D. P., Granner D. K., Wasserman D. H. (1996). Transcription of the rat skeletal muscle hexokinase II gene is increased by acute exercise. J. Appl. Physiol. 81, 789-793 [DOI] [PubMed] [Google Scholar]
- O’Doherty R. M., Halseth A. E., Granner D. K., Bracy D. P., Wasserman D. H. (1998). Analysis of insulin-stimulated skeletal muscle glucose uptake in conscious rat using isotopic glucose analogs. Am. J. Physiol. 274, E287-E296 [DOI] [PubMed] [Google Scholar]
- Pendergrass M., Koval J., Vogt C., Yki-Jarvinen H., Iozzo P., Pipek R., Ardehali H., Printz R., Granner D. K., DeFronzo R. A., et al. (1998). Insulin-induced hexokinase II expression is reduced in obesity and NIDDM. Diabetes 47, 387-394 [DOI] [PubMed] [Google Scholar]
- Petersen H. A., Fueger P. T., Bracy D. P., Wasserman D. H., Halseth A. E. (2003). Fiber type-specific determinants of Vmax for insulin-stimulated muscle glucose uptake in vivo. Am. J. Physiol. Endocrinol. Metab. 284, E541-E548 [DOI] [PubMed] [Google Scholar]
- Pilegaard H., Osada T., Andersen L. T., Helge J. W., Saltin B., Neufer P. D. (2005). Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism 54, 1048-1055 [DOI] [PubMed] [Google Scholar]
- Ploug T., Galbo H., Richter E. A. (1984). Increased muscle glucose uptake during contractions: no need for insulin. Am. J. Physiol. 247, E726-E731 [DOI] [PubMed] [Google Scholar]
- Ploug T., Galbo H., Ohkuwa T., Tranum-Jensen J., Vinten J. (1992). Kinetics of glucose transport in rat skeletal muscle membrane vesicles: effects of insulin and contractions. Am. J. Physiol. 262, E700-E711 [DOI] [PubMed] [Google Scholar]
- Ren J. M., Semenkovich C. F., Gulve E. A., Gao J., Holloszy J. O. (1994). Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J. Biol. Chem. 269, 14396-14401 [PubMed] [Google Scholar]
- Richardson D. K., Kashyap S., Bajaj M., Cusi K., Mandarino S. J., Finlayson J., DeFronzo R. A., Jenkinson C. P., Mandarino L. J. (2005). Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J. Biol. Chem. 280, 10290-10297 [DOI] [PubMed] [Google Scholar]
- Richter E. A., Ploug T., Galbo H. (1985). Increased muscle glucose uptake after exercise. No need for insulin during exercise. Diabetes 34, 1041-1048 [DOI] [PubMed] [Google Scholar]
- Richter E. A., Jensen P., Kiens B., Kristiansen S. (1998). Sarcolemmal glucose transport and GLUT-4 translocation during exercise are diminished by endurance training. Am. J. Physiol. 274, E89-E95 [DOI] [PubMed] [Google Scholar]
- Roberts T. J., Weber J. M., Hoppeler H., Weibel E. R., Taylor C. R. (1996). Design of the oxygen and substrate pathways. II. Defining the upper limits of carbohydrate and fat oxidation. J. Exp. Biol. 199, 1651-1658 [DOI] [PubMed] [Google Scholar]
- Rosdahl H., Lind L., Millgard J., Lithell H., Ungerstedt U., Henriksson J. (1998). Effect of physiological hyperinsulinemia on blood flow and interstitial glucose concentration in human skeletal muscle and adipose tissue studied by microdialysis. Diabetes 47, 1296-1301 [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Goodyear L. J. (2002). Intracellular signaling in contracting skeletal muscle. J. Appl. Physiol. 93, 369-383 [DOI] [PubMed] [Google Scholar]
- Shearer J., Fueger P. T., Bracy D. P., Rottman J. N., Clanton J. A., Wasserman D. H. (2004). AMP Kinase-induced skeletal muscle glucose but not LCFA uptake is dependent on nitric oxide. Diabetes 53, 1429-1435 [DOI] [PubMed] [Google Scholar]
- Surwit R. S., Kuhn C. M., Cochrane C., McCubbin J. A., Feinglos M. N. (1988). Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37, 1163-1167 [DOI] [PubMed] [Google Scholar]
- Taylor C. R., Weibel E. R., Weber J. M., Vock R., Hoppeler H., Roberts T. J., Brichon G. (1996). Design of the oxygen and substrate pathways. I. Model and strategy to test symmorphosis in a network structure. J. Exp. Biol. 199, 1643-1649 [DOI] [PubMed] [Google Scholar]
- Vock R., Hoppeler H., Claassen H., Wu D. X., Billeter R., Weber J. M., Taylor C. R., Weibel E. R. (1996a). Design of the oxygen and substrate pathways. VI. structural basis of intracellular substrate supply to mitochondria in muscle cells. J. Exp. Biol. 199, 1689-1697 [DOI] [PubMed] [Google Scholar]
- Vock R., Weibel E. R., Hoppeler H., Ordway G., Weber J. M., Taylor C. R. (1996b). Design of the oxygen and substrate pathways. V. Structural basis of vascular substrate supply to muscle cells. J. Exp. Biol. 199, 1675-1688 [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Holloszy J. O. (1985). Activation of glucose transport in diabetic muscle: responses to contraction and insulin. Am. J. Physiol. 249, C233-C237 [DOI] [PubMed] [Google Scholar]
- Wasserman D. H. (1995). Regulation of glucose fluxes during exercise in the postabsorptive state. Annu. Rev. Physiol. 57, 191-218 [DOI] [PubMed] [Google Scholar]
- Wasserman D. H. (2009). Four grams of glucose. Am J. Physiol. Endocrinol. Metab. 296, E11-E21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman D. H., Halseth A. E. (1998). An overview of muscle glucose uptake during exercise. Sites of regulation. Adv. Exp. Med. Biol. 441, 1-16 [DOI] [PubMed] [Google Scholar]
- Wasserman D. H., Lickley H. L., Vranic M. (1984). Interactions between glucagon and other counterregulatory hormones during normoglycemic and hypoglycemic exercise in dogs. J. Clin. Invest. 74, 1404-1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman D. H., Lickley H. L., Vranic M. (1985). Important role of glucagon during exercise in diabetic dogs. J. Appl. Physiol. 59, 1272-1281 [DOI] [PubMed] [Google Scholar]
- Wasserman D. H., Spalding J. A., Bracy D., Lacy D. B., Cherrington A. D. (1989a). Exercise-induced rise in glucagon and ketogenesis during prolonged muscular work. Diabetes 38, 799-807 [DOI] [PubMed] [Google Scholar]
- Wasserman D. H., Spalding J. A., Lacy D. B., Colburn C. A., Goldstein R. E., Cherrington A. D. (1989b). Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. Am. J. Physiol. 257, E108-E117 [DOI] [PubMed] [Google Scholar]
- Wasserman D. H., Williams P. E., Lacy D. B., Goldstein R. E., Cherrington A. D. (1989c). Exercise-induced fall in insulin and hepatic carbohydrate metabolism during muscular work. Am. J. Physiol. 256, E500-E509 [DOI] [PubMed] [Google Scholar]
- Wasserman D. H., Williams P. E., Lacy D. B., Bracy D., Cherrington A. D. (1990). Hepatic nerves are not essential to the increase in hepatic glucose production during muscular work. Am. J. Physiol. 259, E195-E203 [DOI] [PubMed] [Google Scholar]
- Wasserman D. H., Geer R. J., Rice D. E., Bracy D., Flakoll P. J., Brown L. L., Hill J. O., Abumrad N. N. (1991). Interaction of exercise and insulin action in humans. Am. J. Physiol. 260, E37-E45 [DOI] [PubMed] [Google Scholar]
- Wasserman D. H., Mohr T., Kelly P., Lacy D. B., Bracy D. (1992). The impact of insulin-deficiency on glucose fluxes and muscle glucose metabolism during exercise. Diabetes 41, 1229-1238 [DOI] [PubMed] [Google Scholar]
- Wasserman D. H., Lacy D. B., Bracy D. P. (1993). Relationship between arterial and portal vein immunoreactive glucagon during exercise. J. Appl. Physiol. 75, 724-729 [DOI] [PubMed] [Google Scholar]
- Wasserman K., Van Kessel A. L., Burton G. G. (1967). Interaction of physiological mechanisms during exercise. J. Appl. Physiol. 22, 71-85 [DOI] [PubMed] [Google Scholar]
- Weber J. M., Brichon G., Zwingelstein G., McClelland G., Saucedo C., Weibel E. R., Taylor C. R. (1996a). Design of the oxygen and substrate pathways. IV. Partitioning energy provision from fatty acids. J. Exp. Biol. 199, 1667-1674 [DOI] [PubMed] [Google Scholar]
- Weber J. M., Roberts T. J., Vock R., Weibel E. R., Taylor C. R. (1996b). Design of the oxygen and substrate pathways. III. Partitioning energy provision from carbohydrates. J. Exp. Biol. 199, 1659-1666 [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Taylor C. R., Hoppeler H. (1991). The concept of symmorphosis: a testable hypothesis of structure-function relationship. Proc. Natl. Acad. Sci. USA 88, 10357-10361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Taylor C. R., Weber J. M., Vock R., Roberts T. J., Hoppeler H. (1996). Design of the oxygen and substrate pathways. VII. Different structural limits for oxygen and substrate supply to muscle mitochondria. J. Exp. Biol. 199, 1699-1709 [DOI] [PubMed] [Google Scholar]
- Williams K. V., Price J. C., Kelley D. E. (2001). Interactions of impaired glucose transport and phosphorylation in skeletal muscle insulin resistance: a dose-response assessment using positron emission tomography. Diabetes 50, 2069-2079 [DOI] [PubMed] [Google Scholar]
- Witczak C. A., Jessen N., Warro D. M., Toyoda T., Fujii N., Anderson M. E., Hirshman M. F., Goodyear L. J. (2010). CaMKII regulates contraction- but not insulin-induced glucose uptake in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 298, E1150-E1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. R., Nadel E. R., Shaw J. H., Stephenson L. A., Wolfe M. H. (1986). Role of changes in insulin and glucagon in glucose homeostasis in exercise. J. Clin. Invest. 77, 900-907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath J. R., Houseknecht K. L., Gnudi L., Kahn B. B. (1997). High-fat feeding impairs insulin-stimulated GLUT4 recruitment via an early insulin-signaling defect. Diabetes 46, 215-223 [DOI] [PubMed] [Google Scholar]
- Zinker B. A., Lacy D. B., Bracy D., Jacobs J., Wasserman D. H. (1993a). Regulation of glucose uptake and metabolism by working muscle. An in vivo analysis. Diabetes 42, 956-965 [DOI] [PubMed] [Google Scholar]
- Zinker B. A., Lacy D. B., Bracy D. P., Wasserman D. H. (1993b). Role of glucose and insulin loads to the exercising limbs in glucose uptake and metabolism. J. Appl. Physiol. 74, 2915-2921 [DOI] [PubMed] [Google Scholar]