Abstract
Adults produce left-lateralized N170 responses to visual words relative to control stimuli, even within tasks that do not require active reading. This specialization begins in preschoolers as a right-lateralized N170 effect. We investigated whether this developmental shift reflects an early learning phenomenon, such as attaining visual familiarity with a script, by training adults in an artificial script and measuring N170 responses before and afterward. Training enhanced the N170 response, especially over the right hemisphere. This suggests N170 sensitivity to visual familiarity with a script before reading becomes sufficiently automatic to drive left-lateralized effects in a shallow encoding task.
Background
During reading, skilled adult readers rely on specialized tuning of visual brain areas that specifically support processing of a familiar written language (McCandliss, Cohen, & Dehaene, 2003). Evidence from neurophysiological studies suggests that such specialized responses in the visual brain occur within 200 ms after word presentation. A robust finding is an enhanced N170 component1 of the event-related potential (ERP) especially at posterior electrodes over the left hemisphere in response to words compared to low-level visual control stimuli like strings of symbols or forms (Bentin, Mouchetant-Rostaing, Giard, Echallier, & Pernier, 1999; Brem et al., 2005; Maurer, Brandeis, & McCandliss, 2005; Maurer, Brem, Bucher, & Brandeis, 2005; Maurer, Zevin, & McCandliss, 2008; Zhang, Begleiter, Porjesz, & Litke, 1997). Although this specialization for print is statistically most robust around the N170 peak (Maurer, Brandeis et al., 2005), it may also extend into the subsequent component until about 300 ms (Maurer, Brem et al, 2005). A similar specialization is found in the corresponding magnetic field (Eulitz et al., 2000; Shirahama, Ohta, Takashima, Matsushima, & Okubo, 2004; Tarkiainen, Helenius, Hansen, Cornelissen, & Salmelin, 1999). Specialization for print reflects largely implicit processes, as it is evident even under minimal visual encoding task demands such as one-back repetition detection tasks (Maurer, Brandeis et al., 2005; Maurer, Brem et al., 2005) that carry no explicit task demands for associating the visual stimuli with linguistic information. Intracranial and magnetic field recordings suggest word-specific sources corresponding to the N170 specialization in inferior occipito-temporal cortex, mainly in the left hemisphere (Allison, McCarthy, Nobre, Puce, & Belger, 1994; Tarkiainen et al., 1999).
A similar specialization in the N170 has been found for faces (Bentin, Allison, Puce, & Perez, 1996) and for objects of individual expertise (e.g. for birds in bird-experts, Tanaka & Curran, 2001) and has been related to experience-dependent changes in visual expertise (Gauthier, Curran, Curby, & Collins, 2003). Thus, the notion of visual expertise may also account for the N170 specialization for written words. Interestingly however, the N170 response is typically bilateral or right-lateralized for faces and expertise-objects (Bentin et al., 1996; Rossion, Joyce, Cottrell, & Tarr, 2003; Tanaka & Curran, 2001), but left-lateralized for words (Bentin et al., 1999; Maurer, Brandeis et al., 2005; Maurer, Brem et al., 2005; Maurer et al., 2008; Rossion et al., 2003). Left-lateralization of the N170 in response to orthographic stimuli is not limited to alphabetic scripts and has been shown to occur in other writing systems with a different organization, such as Japanese (Maurer et al., 2008) and Chinese (Wong, Gauthier, Woroch, DeBuse, & Curran, 2005) perhaps reflecting automatic mapping between orthography and left-lateralized language regions that may accrue at several levels of organization.
One explanation for the left-lateralization of the N170 for visual words is the phonological mapping hypothesis, which proposes that this left lateralization reflects automatic connections between a familiar script and left-lateralized phonological language processes (Maurer & McCandliss, 2007). Repeatedly pairing this class of stimuli with activation in left-lateralized temporal and frontal language regions may drive specialization and increased tuning of left occipito-temporal extrastriate visual region known as the Visual Word Form Area (McCandliss, et al., 2003) such that the simple presentation of these visual stimuli is sufficient to activate these left-lateralized regions. An important developmental extension to this hypothesis is that early in learning, before connections between script and phonological representations become automated, increased visual familiarity with a script alone may drive changes in N170 responses, but that this form of learning should drive bilateral or even right-lateralized N170 responses, as do other domains of visual expertise that do not involve associations with language per se.
Given that reading skills continue to improve throughout childhood, developmental studies provide crucial insights into the nature of the N170 expertise effects for reading and the dynamics of the emergence of left lateralization. Only a few ERP studies with children, however, have directly focused on rapid ERP responses to visual words vs. other classes of visual stimuli. One study conducted with pre-reading kindergarten children and literate adults showed that N170 specialization for print, exhibited by an early ERP sensitivity to the contrast between familiar words vs. unfamiliar symbol strings developed only after the start of reading training (Maurer, Brem et al., 2005). Interestingly, a subset of the kindergarten children with the greatest letter knowledge demonstrated a right-lateralized N170 effect (labeled N1 effect in that study to account for developmental latency shifts) sensitive for familiar print. The fact that the topography of this finding stands in contrast to the adults’ more left-lateralized N170 specialization raises a set of interesting questions regarding lateralization and the role of early print familiarity. Maurer and colleagues attributed this right-lateralized N170 effect to early gains in visual familiarity with a script (Maurer, Brem et al., 2005), as these children had not yet learned to read.
Importantly, the contrasting laterality effect for adults versus kindergarten pre-readers with letter knowledge was found using a very shallow encoding task (i.e. one-back repetition detection). Such tasks exert no explicit task demands to engage associations with phonology, but can be completed based solely on visual representations of stimuli. Thus the effects in adults may represent the impact of automating orthographic-phonological mappings associated with specialized tuning of the visual word form area. The children from that study were followed for 1.5 years as they received their initial year and a half of reading instruction. Over this time, they developed large amplitude and topographic differences in early ERP responses to words versus symbol strings, producing an N170 effect peaking at approximately 210 milliseconds, which was even larger than the N170 effect in adults (Maurer et al., 2006). Although the topography of this ERP effect was clearly bilateral in these children in 2nd grade, contrasts across the kindergarten and 2nd grade recordings indicated a change in the direction of increasing left lateralization (Maurer et al., 2007).
A similar developmental study investigated N170 sensitivity to visual word forms in 4-, 7- and 10- year-olds, while controlling for letter familiarity effects across groups. Posner & McCandliss (2000) examined ERP responses to familiar words in contrast to familiar letters organized as orthographically implausible consonant strings, thereby isolating perceptual expertise for visual word forms from familiarity with letters. Unlike the studies reviewed above, 4- and 7-year-olds showed no clear sensitivity to word forms relative to familiar script organized into consonant strings. 10-year-olds, however, started to exhibit some sensitivity in early ERP effects, emerging after 200 ms (Posner & McCandliss, 2000). These findings suggests a protracted development for reaching adult-like N170 sensitivity for visual word forms that emerges much later than the print familiarity effects described above (McCandliss, Posner, & Givon, 1997).
Taken together, developmental studies suggest that N170 changes associated with reading develop during early reading acquisition, and that the size (i.e. amplitude) of the N170 specialization for print peaks in the first years of reading training. Furthermore, the transition from initially right-lateralized to more left-lateralized specialization suggests that the nature of such learning effects change profoundly over the course of learning to read. Whereas in preschoolers the beginning N170 specialization is right-lateralized and is likely linked to visual familiarity, it is transformed to the characteristic left-lateralized type of expertise possibly through the protracted process of automating orthographic-phonological mappings and hence tuning left-lateralized extrastriate visual mechanisms to processing a script.
These developmental studies, however, provide little insight into whether the early right-lateralized N170 script effects, which emerge as children shift from pre-readers to early readers, reflect maturation of brain mechanisms that support N170 responses to familiar stimuli, or reflect the increase in script familiarity that typically occurs at this age. Similarly, contrasts between preschoolers exhibiting right-lateralized N170 script effects and expert adult readers showing left-lateralized N170 script effects provide little direct evidence to differentiate whether these effects are linked to learning dynamics versus maturation of visual system ERP responses.
Such issues can be more directly addressed by experimental designs that examine the impact of manipulating print familiarity on N170 topography. This can be achieved by studying the impact of short-term training in adults as they learn to read completely novel visual stimuli generated from an artificial writing system.
One advantage of an artificial orthography paradigm is that it can be used to explore phemonena previously demonstrated in young children that emerge during the early phases of learning to read, providing the opportunity to explore whether similar phenomena emerge in adults learning a novel script for the first time.
By studying short-term training, and measuring ERPs to these stimuli under the same shallow encoding condition of a one-back detection task previously used in the adult and child studies reviewed above, we are able to examine whether increased script familiarity leads to changes in N170 responses. Furthermore we can examine whether the topography of such changes is right-lateralized, which would parallel the early childhood findings and support an early familiarization account of such findings.
Under the phonological mapping hypothesis, it is the difference in the automaticity of links between orthography and phonology that drive the topographic differences between ERP responses in early childhood versus adulthood. Thus short-term training that increases script familiarity, yet falls far short of establishing automaticity, should elicit an increased right-lateralized N170 enhancement in adults similar to that seen in children, given a shallow encoding condition. Alternatively, if the increasingly left-lateralized N170 response that occurs over development is linked to maturational factors in the expression of familiarity effects, then short-term training should lead to increased left-lateralized N170 responses, similar to those that occur for highly trained English stimuli under shallow encoding task demands. Adult laboratory-based training experiments hold several advantages for examining such issues. The possibility of previous exposure to the experimental stimuli is logically ruled out, and thus effects related to the early training phase, prior to automaticity, can be directly examined under short-term training conditions. Indeed, similar adult training studies have been used to investigate neural changes during development of expertise for novel objects (e.g. “greebles”), revealing that under some training conditions, N170 responses can be modulated by short-term training experiences in adults (Rossion, Gauthier, Goffaux, Tarr, & Crommelinck, 2002). Similarly, several training studies have examined how brain processes are affected by learning novel visual stimuli within the domain of reading. Studies using fMRI report decreased activation in bilateral fusiform regions (Xue, Chen, Jin, & Dong, 2006; Xue & Poldrack, 2007) following training designed to focus only on visual perceptual learning in the absence of language associations. However, when training included linking visual stimuli with aspects of auditory language processing (Xue et al., 2006) activation increases in the same regions, especially within left-lateralized regions including the Visual Word Form Area. Due to the low time resolution of fMRI, however, the results of these studies are not conclusive about fast visual processes that are critical for fluent reading. One reading training study used ERP measures, which provide enhanced temporal resolution that enable the impact of training on early encoding mechanisms to be examined. McCandliss and colleagues (McCandliss et al, 1997) trained subjects to recognize familiar letters combined to form novel letter strings via orthographic patterns that were only slightly different from subjects’ native English. Interestingly, over the course of 50 hours of training to associate the novel pseudowords with line drawings depicting high-frequency English words, N170 responses to the trained pseudowords showed no training effects relative to the control stimuli, which presented equally familiar letters organized into familiar visual word or consonant strings. McCandliss et al. (1997) suggested that future ERP studies that present highly unfamiliar letter-like stimuli may be more sensitive to the effects of increased familiarity that occurs during short-term training. Thus, the present study employs an artificial orthography script that did not resemble familiar alphabetic writing, and creates whole-word characters by combining letter-like symbols vertically (Fig. 1, top). Using this artificial orthography, adult readers were exposed to the novel script by learning to associate novel symbols with auditorily presented English words over the course of a twenty minute active training block.
Figure 1. Stimuli.
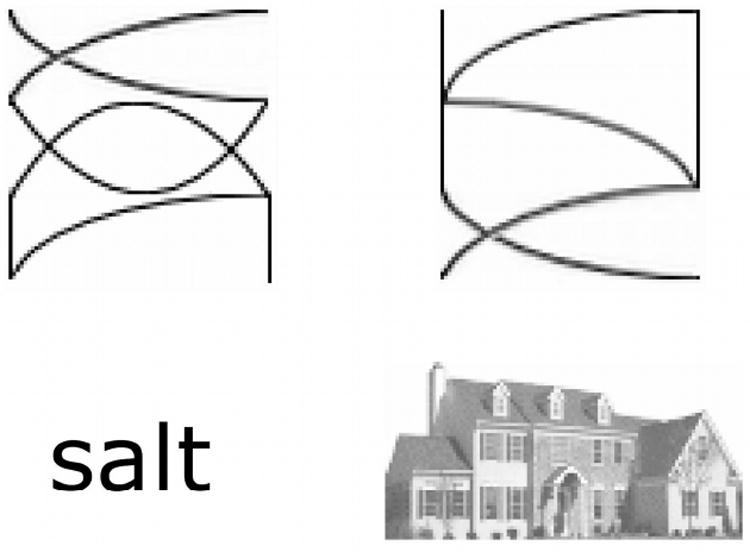
Before and after training participants detected immediate repetitions of artificial orthography symbols, of words, and of houses. Two types of artificial orthography symbols were presented: those that were trained (upper left) and transfer symbols (upper right) that were made up from elements of the trained symbols.
Subjects performed an identical battery of one-back repetition detection tasks with symbols and control stimuli (Fig. 1) before and after training, all within the same ERP recording session, which enabled direct statistical comparisons of training-related changes. The one-back task was selected for two central reasons. First, this very task has been employed in both adult and child studies of the N170 response to familiar scripts versus strings of unfamiliar symbols, and, as reviewed above, elicits right-lateralized N170 responses in early readers during childhood and left-lateralized N170 responses in skilled adult readers (Maurer, Brem et al., 2005; Maurer et al., 2006; Maurer et al., 2008). Thus examining how increasing familiarity of a novel script impacts ERP responses recorded during this shallow encoding task provides a means of testing the hypothesis that the right-lateralized N170 script effects in young children reflects familiarity rather than maturational effects. Secondly, this task is ideal for pre-post assessment of learning as the task can be performed efficiently with comparable reaction times and accuracy rates in the pre-test condition before any training on the novel characters has occurred (Maurer, Brem et al., 2005; Maurer et al., 2008).
To further explore the implicit versus strategic nature of the N170 training modulation as assessed by ERP responses in a one-back task, we contrasted two different strategic instructions preceding the training2. The differential strategic instructions are described in more detail in another paper within this special issue (Yoncheva et al., in this issue), in which effects of strategic instruction are specifically investigated using a reading verification task and analysis methods sensitive to subtle topographic changes in N170 lateralization.
Finally, the analysis plan of making direct comparisons between pre and post training observations motivated additional aspects of the study design, such as the addition of control stimulus conditions to determine whether changes in ERP responses were attributable to more general factors that might differ between pre and post recording epochs (i.e. task familiarization, fatigue, physical aspects of EEG recording that may change over a session, etc.). To address these issues, pre-post assessment blocks also contained two classes of stimuli that were not the subject of training: houses and written English words. Pre-post training changes for symbols were only considered to reflect training-related activity when they differed from those observed for the word and house stimuli in the control conditions. In addition, to examine whether learning effects related to the artificial orthography were accruing at the level of script familiarity versus specific word characters, an additional set of artificial orthography stimuli were created from the same elements, but were not included in the training (transfer symbols).
Methods
Subjects
Criteria for the study included right-handed, native English speakers, between 18 and 36 years old, reading ability within 2 SD of average performance on the TOWRE (Torgesen, Wagner, & Rashotte, 1999) reading test. Out of an initial 55 subjects who participated in this study 13 subjects were excluded because of a low training performance (< 80% accuracy at matching trained characters with trained words), and 12 subjects were excluded because of low signal-to-noise ratios in the ERP data in the one-back task (Maurer, Brandeis et al., 2005). The remaining 30 subjects (15 female, mean age 25 years, all right-handed) passed all inclusion criteria.
Stimuli and training
During a training phase, which lasted approximately 20 minutes, the participants were presented with 16 symbol-word pairs (visual-auditory), with each pair repeated 20 times. Participants were trained in one of three different training sets, which were closely matched and counterbalanced between subjects and training groups (see Yoncheva et al., in this issue). A symbol was shown for 2334 ms on a computer monitor. 1334 ms after the appearance of the symbol a spoken word (mean duration = 600 ms, SD = 55 ms) was played over headphones. The auditory stimuli were monosyllabic (CVC), English words spoken in a female voice. Auditory words were chosen from the set of phonological combinations possible from combining the letter-like symbols, without regard for how such words were spelled in English. Average spoken word frequency of the selected words was 106 (SD ±493) per million (CELEX database; N-watch program, Davis, 2005). We chose English words instead of meaningless pseudowords to minimize learning in the auditory modality and to facilitate learning during the short training session by enabling subjects to take advantage of semantic associations of known words in their efforts to establish mappings between whole characters and known words in the whole word condition (see below).
Each symbol-word pair was preceded by a face stimulus with either a fearful or a neutral expression for 300 ms in a fashion completely unrelated to the task and stimulus conditions of the training task. These incidental face stimuli were included to examine the effects of facial expression on ERP responses for the purposes of another study, the data for which are reported elsewhere (Blau, Maurer, Tottenham, & McCandliss, 2007).
While the training procedures and stimuli were identical for all participants, the instruction that preceded training was manipulated to influence subjects’ strategic approach to the training task: half of the subjects (n=15) were asked to associate the entire characters and the auditory words (“whole-word” group), while the other half of the subjects (n=15) was explicitly instructed to focus on hidden grapheme-phoneme correspondences (“grapheme-phoneme” group) between parts of the characters and parts of the spoken words. The latter instruction was possible because all symbols and words consisted of 3 parts (graphemes/phonemes) and all symbols were constructed according to the same grapheme-phoneme conversion rules (for a more detailed description see the paper by Yoncheva et al. in this issue). Thus, although potential training strategy effects are analyzed and reported, the experiment was designed such that training main effects could be examined as well, as each training condition contained identical visual and auditory stimuli presented in an identical fashion.2 We did not expect to find large effects of strategic instruction on ERP responses within the one-back task, as this task does not explicitly require associating visual stimuli with words or phonemes, and 20 minutes of training is likely insufficient to produce automatic responses that differentiate different reading strategies. Effects of strategy during training were more likely to occur during a reading verification task which explicitly requires reading (see Yoncheva et al. in this issue).
One-back task
To investigate main effects of training we applied the same one-back repetition detection task with the identical stimuli before and after training with the subjects pressing a button for immediate stimulus repetitions (Maurer, Brandeis et al., 2005; Maurer, Brem et al., 2005; Maurer et al., 2007; Maurer et al., 2006; Maurer et al., 2008). As noted above, the minimal encoding demands of this task allow the same task instructions to be applied to several classes of stimuli including completely unfamiliar stimuli, and ERP responses under this task may be sensitive to both early familiarity effects as well as expertise effects associated with expert levels of visual word form processing. 16 trained symbols, 16 transfer symbols, 16 English words, and 16 houses were presented 4 times as non-targets in separate blocks segregated by stimulus type. In addition, each stimulus appeared once as a target. The transfer symbols were built from the same grapheme/phoneme elements as used for the trained symbols, but made up words that were not trained. The printed English word stimuli were mono-syllabic four letter words, with a written word frequency of 41 (SD ±26) per million (CELEX database; N-watch program, Davis, 2005). The symbols subtended 2.4 × 2.6 degrees of visual angle, the words 2 × 0.8, and the houses 3.5 × 1.8. The stimuli were shown for 683 ms, separated by a fixation cross lasting randomly between 900 and 1200 ms. Thus, running-time of the one-back repetition detection task was approximately 9 minutes (2 × 9 minutes for pre and post experiments). Participants were allowed to take short breaks between experiments or during training, if needed.
ERP recording and processing
The 128-channel EEG was recorded using a geodesic sensor net (GSN200; Tucker, 1993) with a Cz reference. Data were sampled at 250 Hz/channel with filter settings 0.1-100 Hz. Impedance was below 50 kΩ (Ferree, Luu, Russell, & Tucker, 2001). Using BESA software, channels with excessive artifacts were spline interpolated, and eye blinks were corrected (multiple source eye correction method according to Berg & Scherg, 1994). The data then were digitally bandpass filtered (0.3-30 Hz), segmented (-150-850ms), artifact rejected (± 100uV), and averaged according to non-target stimuli separately for the four conditions. Using Brain Vision Analyzer software, the averaged data were re-referenced to average reference enabling the use of the recording reference as 129th channel (Lehmann & Skrandies, 1980) and baseline corrected (-150 to 0 ms). Pre-post difference ERPs were computed for each stimulus condition by subtracting the ERP before training from the ERP after training. After computing Global Field Power (GFP) (Lehmann & Skrandies, 1980), grand means were computed for all four pre-post difference ERPs and for the 4 stimulus conditions separately before and after training.
Statistical analyses
ERP training effects were analyzed by combining a two-step analysis strategy with an ERP mapping approach (Lehmann & Skrandies, 1980; Brandeis & Lehmann, 1986; Michel et al., 2001), which identifies ERP segments of interest based on stable map topographies (“microstates”) rather than based on peaks in waveforms.
In a first step of the analysis, we identified time windows during which learning effects occurred for the trained symbols by computing a Topographic Analysis of Variance (TANOVA) on raw maps for each time point in the ERP (-150 to 750 ms) across all subjects. To account for multiple comparisons across the entire time range we considered a pre-post difference to indicate a significant training effect, if it lasted for at least 3 consecutive samples at a threshold of p<0.01 (the joint probability of p<0.01 in 3 consecutive time frames, i.e. 0.01*0.01*0.01, is lower than an equivalent Bonferroni-corrected p-value, i.e. 0.05/225). TANOVA on raw maps detects all systematic amplitude differences between two maps based on a nonparametric randomization test (Holmes, Blair, Watson, & Ford, 1996) on the GFP of difference maps (Lehmann & Skrandies, 1980; Strik, Fallgatter, Brandeis, & Pascual-Marqui, 1998), i.e. between pre and post maps in the present case. The time point-wise TANOVA approach has been used in many previous studies to identify time segments during which two conditions were processed differently (e.g. Maurer, Bucher, Brem, & Brandeis, 2003; Schulz et al., 2008; Murray et al., 2004), or during which changes over time occurred due to training (e.g. Stein, Dierks, Brandeis, Wirth, Strik, & Koenig, 2006). Note that TANOVA differences between raw maps can result from differences in map strength (even with similar topographies) or from differences in topography (even with similar GFP), which was investigated in more detail later in the analysis procedure. Based on the ERP mapping approach we were averaging across a TANOVA segment, given that topographies of the training effects were stable throughout the segment. Stability of the topographies was ensured by computing Global Dissimilarity (GD, Lehmann & Skrandies, 1980) for the post-pre difference ERP. GD is computed as the GFP of the difference between normalized maps at subsequent time points and thus is large when topographies change and small when they remain similar.
While this first step of the analysis allowed us to focus on training effects for the trained symbols by identifying time segments with robust pre-post differences, in a second step we tested whether these training effects 1) would be absent for words and houses (to exclude testing order effects), 2) would generalize to transfer symbols, and 3) would be left- or right-lateralized.
The 3 measures used were map strength (GFP), map topography (topographic 3D centroids), and lateralization (left- and right-hemispheric topographic peak channels, i.e. the posterior channel pair at homologue left and right hemisphere sites with the largest training effect averaged across the pair) of the difference between pre and post training (post minus pre) averaged across the time segments identified in the first analysis step (TANOVA).
The positive 3D centroid (Brandeis et al., 1994; Maurer et al., 2003) is the voltage-weighted average of the locations of all electrodes showing positive values; the negative 3D centroid is the analogous computed for electrodes with negative values. Centroid locations are defined in Talairach space by x- (left-right), y- (posterior-anterior), and z- (inferior-superior) coordinates. As 3D centroids are a purely topographic measure independent of GFP, and as they are computed based on all 129 electrodes, they can be used to verify whether lateralization effects at a subset of selected electrodes also hold for entire, normalized maps.
We then ran 3 repeated measure MANOVAs on GFP, centroids, and lateralization with “stimulus-condition” as within-subject factor (words vs. trained symbols vs. transfer symbols vs. houses). For the lateralization analysis there was an additional “hemisphere” within-subject factor. For the centroid analysis an additional “polarity” within-subject factor was used (and reported in interaction effects), and the 3 coordinate axes (x, y, z) were treated as multivariate dependent measures (for the centroid method see also Maurer, Brandeis et al., 2005; Maurer, Brem et al., 2005).
Behavioral responses to targets were analyzed in 2 repeated measure MANOVAs on accuracy and reaction time with “time” (pre vs. post) and “stimulus-condition” as within-subject factors (words vs. trained symbols vs. transfer symbols vs. houses). Due to a software error the behavioral responses to word targets were lost in 4 subjects, and these subjects were excluded from behavioral analyses including the word condition. As no data were lost for the critical symbol conditions, the ERP analyses were run with all 30 subjects. However, the ERP MANOVA analyses that included the word condition were additionally recomputed with only those 26 subjects having complete behavioral data.
Results
Behavioral results
As listed in table 1, the participants were more accurate in detecting targets after training than before training (time, F(1,25)=16.1, p<0.001). This was the case for all conditions except for words (time x stimulus-condition, F(3,23)=4.6, p<0.05), for which target detection was superior to the other conditions at both occasions reflected in the stimulus condition main effect (stimulus-condition, F(3,23)=20.0, p<0.001). The reaction time analysis yielded no significant effects (all p>0.13).
Table 1.
Behavioral one back detection [mean (SD)]
| Accuracy (%) | reaction time (ms) | |||
|---|---|---|---|---|
| pre | post | pre | post | |
| words | 91.7 (21.5) | 94.1 (19.6) | 616 (169) | 588 (118) |
| trained symbols | 79.3 (20.4) | 89.3 (18.4) | 639 (134) | 610 (124) |
| transfer symbols | 79.4 (20.7) | 89.1 (18.8) | 630 (126) | 630 (119) |
| houses | 81.8 (21.0) | 90.3 (18.6) | 628 (112) | 603 (93) |
ERP time segment with training effects
In a first step, a Topographic Analysis of Variance (TANOVA) on raw ERP maps was computed for each time point comparing the response elicited by the trained artificial orthography stimuli before and after the training phase.
Training effects (p<0.01) were observed between 160 and 380 ms after stimulus presentation and encompassed the N170 component (Fig. 2A and B). As shown in Fig. 2C, this time segment corresponded to a broad Global Field Power (GFP) peak and to a flat Global Dissimilarity curve in the pre-post ERP difference. The flat Global Dissimilarity curve indicates similar consecutive topographies throughout the whole segment, as illustrated in Fig. 2D.
Figure 2. Point-to-point training effects for trained symbols.
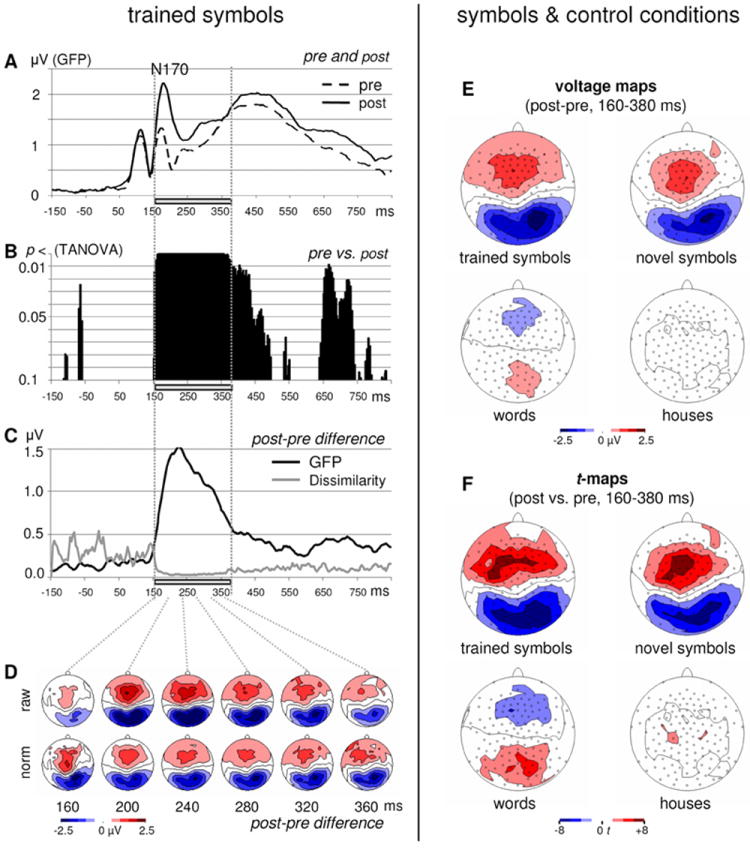
Global Field Power (GFP) in response to trained symbols was larger in the N170 and showed a different morphology between 200 and 350ms after training compared to before training (A). Results of the point-to-point analysis (TANOVA) of the ERP maps showed significant changes (p<0.01) after training compared to before training from 160 to 380 ms (grey bar) starting at the level of the N170 component (B). The ERP map differences peak at 220 ms (GFP of the post-pre difference ERP, black line), and the low Global Dissimilarity values (Dissimilarity, grey line) during the segment with training effects (grey rectangle) indicate stable topographies throughout the whole segment (C). Map series of the post-minus pre difference ERP are sampled at 40 ms intervals and illustrate the stable posterior negative / anterior positive topographies during the time segment identified by the TANOVA approach (D). Whereas the map strength varied throughout the segment (raw; compare also GFP in C), the similarity of the topographies with their right-lateralized posterior negativity is evident in the normalized maps (norm: GFP = 1). The post-minus-pre difference maps (E) for the segment starting at the N170 (averaged values across 160 – 380 ms) illustrate the increase in negativity over posterior channels, particularly over the right hemisphere for symbols. These differences are absent for the control stimuli: they are much smaller for houses and show even the reversed polarity for words with an increase in posterior positivity and anterior negativity. Statistical post-vs-pre t-maps (F) illustrate that at posterior electrodes t-values are highest over the right hemisphere for the symbols. Note that the significant post-minus-pre effect for words shows a distribution with a reversed polarity (posterior positivity) compared to the effect for symbols.
Characterizing training effects
In a second step of the analysis, these training effects were characterized by testing whether they 1) would be absent for words and houses (to exclude testing order effects), 2) would generalize to transfer symbols, and 3) would be left- or right-lateralized. The 3 measures used in the MANOVAs were map strength (GFP), map topography (3D centroids), and lateralization (left and right hemispheric topographic peak channels) of the difference between pre and post training (post minus pre) averaged across the time segment that was identified in the first analysis step (160 - 380 ms).
GFP effects
The GFP analysis for the pre-post differences revealed that map strength depended upon the stimulus condition (stimulus-condition, F(3,27)=3.5, p<0.05; p<0.1 with the 26 subjects having complete behavioral data of the word condition). Within-subject contrasts for the stimulus-condition effect showed that GFP of the pre-post difference maps (Fig. 2E and F) did not differ between trained and transfer symbols (F<1), but was larger for trained symbols than for words (p<0.05; p<0.1 with the 26 subjects having complete behavioral data of the word condition) and for transfer symbols than for houses (p<0.01).
Lateralization effects
The lateralization analysis for the channels at the topographic peak (channels 66 and 85, see Figs. 3 and 4) of the pre-post difference map revealed that the amplitudes differed between the different types of stimuli (stimulus-condition, F(3,27)=25.7, p<0.001). These differences were more pronounced over the right than over the left hemisphere (stimulus-condition x hemisphere, F(3,27)=5.3, p<0.01). This stimulus-type-by-hemisphere interaction also modulated the additional main effect of hemisphere (F(1,29)=4.6, p<0.05). The within-subject contrasts for the stimulus-type-by-hemisphere interaction revealed that trained and transfer symbols did not differ (F<1), but that trained symbols differed from words (p<0.001) and transfer symbols differed from houses (p<0.05). t-tests vs. zero for the pre-post difference value for all 4 conditions separately, revealed increased negativities at both electrodes for trained and transfer symbols (all p’s<0.001), no significant change for houses (p’s>0.25), and increased positivities at both electrodes for words (left: p<0.01, right: p<0.001).
Figure 3. ERP waveforms at the topographic peak channels.
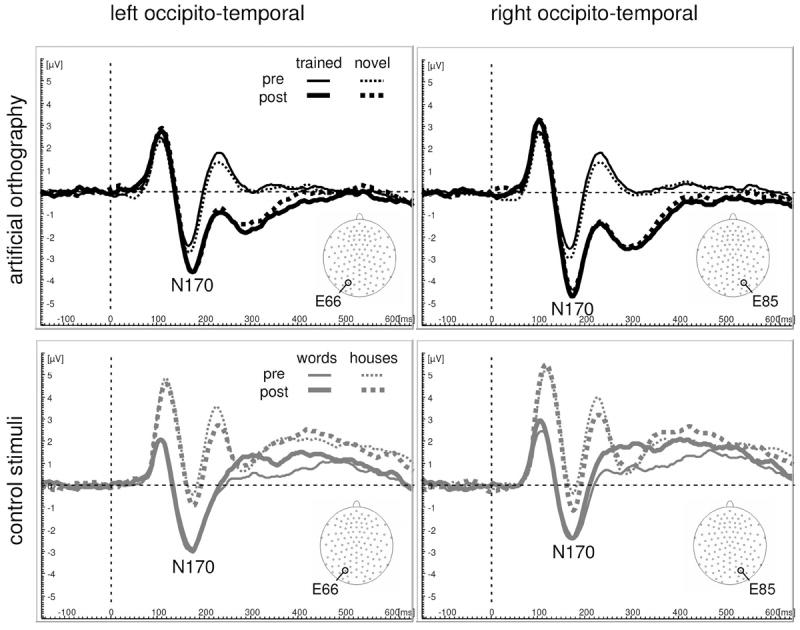
The waveforms over left and right occipito-temporal electrodes illustrate that between about 160 and 400 ms the ERP potentials in response to the artificial orthography symbols are more negative after training compared to before training. These training effects are stronger over the right hemisphere and are similar for both trained and transfer symbols. In contrast these differences are much smaller for houses and even positive for words starting at about 250 ms.
Centroid Effects
The centroid analysis yielded a “stimulus-condition” main effect (F(9,21)=7.5, p<0.001) and a “stimulus-condition-by-polarity” interaction (F(9,20)=8.9, p<0.001), indicating different topographic distribution of the negativities and positivities between the four conditions (see also Fig. 2E and F). The within-subject contrast revealed that, whereas the centroids of the pre-post difference map did not differ significantly between the trained and the transfer condition, they differed between words and trained symbols (x-axis: p<0.05; y-axis: p<0.001) and between transfer symbols and houses (x-axis: p<0.05; y-axis: p<0.001; z-axis: p<0.001).
In order to test lateralization effects of the pre-post difference more specifically we computed t-tests vs. zero for the centroid location on the x-axis separately for all 4 conditions. While the negative centroids were right-lateralized for trained (p<0.001) and transfer (p<0.01) symbols, they showed no significant lateralization effect for English visual words nor for houses (p’s>0.25). In addition, the positive centroids showed no significant lateralization effect in any of the 4 conditions (all p’s>0.25, except for trained symbols with p>0.12).
N170 peak
As the segment that showed training effects lasted longer than the N170 component (see also Fig. 3), we ran an additional test to explore whether the effects in the whole segment were representative for the effects in the N170 component. We thus computed an analogous MANOVA at the N170 peak latency (176 ms; defined as the GFP peak in the mean of the grand means across all 8 conditions) with the factors hemisphere (left vs. right occipito-temporal channels, i.e. channels 66 vs. 85), and stimulus-condition (words vs. trained symbols vs. transfer symbols vs. houses).
The effects in this analysis mirrored those of the analysis for the whole segment: the amplitudes of the pre-post difference map differed between different types of stimuli (stimulus-condition, F(3,27)=7.3, p<0.001), especially over the right hemisphere (stimulus-condition x hemisphere, F(3,27)=3.5, p<0.05). This interaction also modulated the hemisphere main effect (F(1,29)=5.8, p<0.05).
Discussion
The present study investigated how exposure to a novel script during short-term training in an artificial orthography modulates N170 responses. Moreover, we sought to characterize the specificity and lateralization of these effects in order to relate them to right-lateralized N170 effects previously reported for a subgroup of kindergartners as well as to long-term training effects indicated by the left-lateralized N170 response to familiar English words in adults. As reviewed in the introduction, previous results from kindergarten pre-readers demonstrated an interesting phenomenon of a right-lateralized N170-like effect for familiar script relative to unfamiliar control stimuli, which occurred in a one-back task and which was associated with letter knowledge (Maurer et al, 2005). Based on the latter finding it is likely that this early emerging right-lateralized N170 effect reflects script familiarity. To examine this phenomenon further, and to explore the notion that differences between N170 lateralization observed at different ages reflects learning rather than other factors such as maturation, the adult artificial orthography paradigm provides an important test of the hypothesis that experimental manipulations of familiarity in a highly novel script will lead to right-lateralized N170 responses.
Results showed a clear difference between pre and post training ERP responses for the artificial script stimuli. Thus, these results link the emergence of right-lateralized N170 responses to short-term learning, as revealed by the one-back task.
More specifically, our electrophysiological data reveal artificial orthography training effects across a 220-ms-segment which started with an increase of the initial N170 component. Throughout the entire training effect segment – indicated by significant post-minus-pre difference ERP – the spatial configuration of the training effect retained a stable N170-like topography (Brem et al., 2006; Brem et al., 2005; Maurer, Brandeis et al., 2005; Maurer, Brem et al., 2005) suggesting that the same neural activity reflected near the N170 onset remained stable until about 380 ms. The extension of the N170 training effect beyond the range of the initial N170 component is in agreement with many ERP findings of perceptual specialization for print. For example, Maurer, Brem, et al. (2005) reported an N170-like topographic effect that lasted until about 300 ms. A similar effect in the present study, however, was even more prolonged, which may be characteristic of an early phase of learning.
Importantly, this N170 increase did not occur for words or houses, which implies that the enhancement is not related to any main effect differences between pre and post data collection sessions, but rather due to training itself which was specific for the artificial symbols. Although a small positive increase was noted in the English word condition, this effect was in the opposite direction as the training effect, and did not effect ERP responses during the first 250 ms, potentially reflecting a word repetition priming effect (Zhang et al., 1997).
Furthermore, the generalization to transfer stimuli in the present study that consisted of the same elements as the trained stimuli, arranged differently, suggests increased script familiarity, as opposed to familiarity for individual visual word character stimuli. Although other implicit reading studies also revealed no N170 differences between familiar and unfamiliar word forms (i.e. words vs. pseudowords) in languages with consistent orthographic to phonological mappings (Maurer, Brem et al., 2005), such differences have been reported in English, which has more inconsistent mappings (Maurer, Brandeis et al., 2005) potentially reflecting reduced automaticity for grapheme-phoneme mappings relative to other unit sizes such as entire words. Nonetheless, we propose that the short term training carried out in this study falls far short of the magnitude of training necessary to produce automaticity in orthographic-phonological mappings at any unit size, and more likely reflects lower level familiarization with the novel script. Accordingly, while the participants in the present study became familiar with the visual appearance of the stimuli during cross-modal learning, one might expect similar training effects to occur in a one-back task after unimodal, visual discrimination learning. However, as we did not manipulate this factor in the present study this hypothesis remains to be tested.
Given the design of the training and transfer sets, familiarization with the novel script in this study is unlikely accruing at the level of entire word tokens, but likely accrued at a lower level of analysis, perhaps reflecting familiarity with recurring script features, entire letters, or bigram combinations (see Dehaene et al., 2005 for discussion). Although the study was not designed to definitively resolve which of these levels most likely mediates the transfer effects, it is worth noting that at the letter-level stimuli had nearly perfect overlap across training and transfer sets, while bigram units demonstrated less than 40% overlap3, suggesting that the most likely level of representation rests at the letter level or below.
Interestingly, the finding of right-lateralized N170 familiarity effects for the artificial script are quite distinct from the left-lateralized N170 enhancement effects typically seen in adults under the same one-back task demands for words written in a familiar script (compared to unfamiliar control stimuli: Maurer, Brandeis, et al., 2005; Maurer, Brem, et al., 2005; Maurer et al., 2008; and compared to a naïve control group in a cross-linguistic design: Maurer et al., 2008). One potential explanation for this difference, as suggested above, is that the one-back task places little explicit pressure on subjects to utilize the newly learned associations between familiar (or unfamiliar) script and linguistic codes. Subjects simply need to compare each visual stimulus to the previous one, perhaps based on memory traces associated with low-level visual encoding processes. Although these processes may be influenced by short term learning and visual familiarity with a script, such early learning effects might be distinguished from cognitive processes of reading that involve co-activation of script representations with language representations, such as the association between letter-like components of visual word forms and sub-syllabic phonology.
We propose that in these adults, experience with English script has rendered associations between orthography and phonology sufficiently automatic to be activated within the context of the one-back task, i.e. resulting in implicit reading (Maurer, Brem, et al., 2005). Such effects are unlikely to have developed for children in the earliest phases of reading when they know only a few letter names. Similarly, such effects may not have yet developed for adults viewing the novel script following short term training. To explore this explanation further, training-induced N170 enhancement effects that emerge under the minimal visual encoding demands of the one-back task can be contrasted with those that emerge under a reading verification task (Yoncheva et al. in this issue). Unlike the one-back task, the reading verification task explicitly requires subjects to attempt to associate each visual word character with learned linguistic information as they prepare for a cross-modal matching judgment on a subsequently presented auditory word. When ERPs were collected under this task, subjects who were trained to attend to the associations between letter-like symbols hidden within the word characters and sub-syllabic phonological features of the auditory words (the “grapheme-phoneme” focus group) produced a left-lateralized N170 training effect. When these same subjects viewed these same trained stimuli under the one-back task instructions, however, no such left-lateralized training effect emerged. Taking these results together with the left-lateralized N170 effect for English, a pattern begins to emerge that suggests that early in learning, left-lateralized N170 effects may be observed, but only under conditions which explicitly focus attention upon associations between orthography and phonology, and that later in training, such effects may become sufficiently automated to drive left-lateralized responses even under the minimal visual encoding demands of the one-back task. In keeping with this pattern, when subjects become familiarized with a novel script, but fail to notice and practice associations between letter-like symbols and sub-syllabic phonology, as in the case of the “whole word” focus group, no left-lateralized N170 effects emerged, even under the reading verification task.
Collectively, the results of the current one-back task demonstrate an important limit on the nature of the learning reported in the Yoncheva et al. (same issue) study. While left-lateralized N170 effects may by induced by short term training that emphasizes grapheme-phoneme associations, demonstration of such effects may be dependent on explicit task demands, and thus may reflect top-down attention to grapheme-phoneme associations that are not necessarily induced by the one-back task. Eliciting left-lateralized N170 effects in the one-back task may require much greater amounts of experience associating a visual script with phonology, perhaps approaching the amount of experience that leads to the late emergence of left-lateralized N170 responses in children learning to read their native language.
The right-lateralized increase in the present study contrasts with at least one earlier finding of a familiarization effect with a different novel script leading to increased left-lateralized N170 responses (Brem et al., 2005). This study used more string-like pseudofont stimuli that contained a high overlap of visual features to the roman alphabet, and like most alphabetic languages, this script formed word-like units by arranging the letter-like stimuli into horizontal strings with spacing similar to the subjects’ native script (Brem et al., 2005). Familiarization effects in this earlier study may indicate that the fast brain processes that are specialized for alphabetic reading can generalize to similar string-like scripts, but not to artificial orthography stimuli with a very different visual structure, such as the script employed in the present study. Given our current goal of examining parallels between early childhood learning effects and adult learning, adopting scripts that are completely novel in many dimensions may help to minimize the impact of adults’ previously learned script, thereby enabling a closer comparison between childhood and adulthood learning.
Interestingly, the topography of the right-lateralized N170 script training effect bears a resemblance to the topography of N170 training effects in studies that examine the impact of extensive training on N170 responses to novel visual objects (Rossion et al., 2002). Previous studies have reported right-lateralized or bilateral visual expertise effects for novel objects and for faces (Bentin et al., 1996; Rossion et al., 2002; Rossion et al., 2003; Tanaka & Curran, 2001). This may suggest that the visual familiarity effects in the present study draw on pre-existing expertise networks developed for other forms of object recognition (McCandliss et al, 2003, Nelson et al., in press). A similar process might occur in pre-reading children encountering their first script. These initially recruited right-lateralized processes may become increasingly left-lateralized as reading skills progress under the influence of left-lateralized higher order language processes.
In contrast to the emergence of right-lateralized N170 responses to a familiar script, the development of left-lateralized N170 effects may be rather protracted as indicated by studies with children. The visual word N170 component in children was still bilateral (Brandeis, Vitacco, & Steinhausen, 1994; Schulz et al., 2008) in 11-year-old children, and even right-lateralized in 10-year-olds (Spironelli & Angrilli, in press). This development may even be modulated by additional factors such as gender (see Anonymous II in this issue) or learning strategies (see Yoncheva et al., in this issue). In a longitudinal developmental study the N170 specialization for words was still bilateral in 2nd grade, but the increase from kindergarten to 2nd grade was stronger over the left-hemisphere than over the right hemisphere (Maurer et al., 2007), indicating that not only the amplitude of the N170 specialization, but also its lateralization plays an important role in the first years of reading acquisition. This is in agreement with earlier models of learning to read (Orton, 1937; Bakker, 1990) proposing that visual word processing shifts from the right to left hemisphere as reading skills increase, and also with earlier results from ERP (Licht, Bakker, Kok, & Bouma, 1992) and fMRI studies (Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003).
In conclusion, the parallel findings that emerge between the longitudinal approach of the developmental ERP studies and the experimental approach of this adult artificial script training study converge to support the observation that early ERP responses to newly learned scripts likely reflect script familiarity at a level of learning that does not yet automatically engage orthographic-phonological mappings.
Acknowledgments
This research was supported by the Swiss National Science Foundation (Fellowship for Prospective Researchers: UM), and the US National Science Foundation (NSF 529112).
This research was supported by the Swiss National Science Foundation (Fellowship for Prospective Researchers: UM), the National Science Foundation (REC-0337715: BDM), and the John Merck Scholars Program in the Biology of Developmental Disabilities in Children (BDM). We thank Dr. Mike Worden for technical assistance with the EEG system.
Footnotes
The N170 component typically peaks between 150 and 200 ms and shows a topography of a bifocal posterior negativity and of a central positivity. In some ERP studies the N170 is also labeled N1 or N150, or even P150, if referred to the positive pole of the topographic distribution.
Including the instruction as between-subject factor (grapheme-phoneme vs. whole-word) in the analyses on ERP training effects yielded no significant modulation of instruction (neither main effects nor interactions in the MANOVAs), as expected considering the implicit nature of the one-back task. Thus, the data were collapsed across the groups in this paper to facilitate a more straightforward presentation.
It is also possible that some learning accrued at the level of two-letter combinations (bigrams) which would be intermediate to the level of entire word characters and the letter level and below. As the stimulus sets were not designed to exclude this possibility, a post-hoc analysis of bigram overlap between trained and transfer items was conducted. Of the unique bigrams appearing in the training set, 51% also appeared in the transfer set (as opposed to 100% overlap for the letters). Considering each bigram repetition as a unique token, the overlap was 39% (as opposed to 92% for the letters).
References
- Allison T, McCarthy G, Nobre A, Puce A, Belger A. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cerebral Cortex. 1994;4(5):544–554. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- Bakker DJ. Neuropsychological treatment of dyslexia. New York: Oxford University Press; 1990. [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Mouchetant-Rostaing Y, Giard MH, Echallier JF, Pernier J. ERP manifestations of processing printed words at different psycholinguistic levels: time course and scalp distribution. Journal of Cognitive Neuroscience. 1999;11(3):235–260. doi: 10.1162/089892999563373. [DOI] [PubMed] [Google Scholar]
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalography and Clinical Neurophysiology. 1994;90(3):229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Bitan T, Manor D, Morocz IA, Karni A. Effects of alphabeticality, practice and type of instruction on reading an artificial script: an fMRI study. Brain Research: Cognitive Brain Research. 2005;25(1):90–106. doi: 10.1016/j.cogbrainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Blau VC, Maurer U, Tottenham N, McCandliss BD. The face-specific N170 component is modulated by emotional facial expression. Behavioral Brain Functions. 2007;3:7. doi: 10.1186/1744-9081-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis D, Lehmann D. Event-related potentials of the brain and cognitive processes: approaches and applications. Neuropsychologia. 1986;24(1):151–168. doi: 10.1016/0028-3932(86)90049-7. [DOI] [PubMed] [Google Scholar]
- Brandeis D, Vitacco D, Steinhausen HC. Mapping brain electric micro-states in dyslexic children during reading. Acta Paedopsychiatrica. 1994;56(3):239–247. [PubMed] [Google Scholar]
- Brem S, Bucher K, Halder P, Summers P, Dietrich T, Martin E, et al. Evidence for developmental changes in the visual word processing network beyond adolescence. Neuroimage. 2006;29(3):822–837. doi: 10.1016/j.neuroimage.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Brem S, Lang-Dullenkopf A, Maurer U, Halder P, Bucher K, Brandeis D. Neurophysiological signs of rapidly emerging visual expertise for symbol strings. Neuroreport. 2005;16(1):45–48. doi: 10.1097/00001756-200501190-00011. [DOI] [PubMed] [Google Scholar]
- Davis CJ. N-watch: a program for deriving neighborhood size and other psycholinguistic statistics. Behavior Research Methods. 2005;37(1):65–70. doi: 10.3758/bf03206399. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends in Cognitive Sciences. 2005;9(7):335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Eulitz C, Eulitz H, Maess B, Cohen R, Pantev C, Elbert T. Magnetic brain activity evoked and induced by visually presented words and nonverbal stimuli. Psychophysiology. 2000;37(4):447–455. [PubMed] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology. 2001;112(3):536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Curran T, Curby KM, Collins D. Perceptual interference supports a non-modular account of face processing. Nature Neuroscience. 2003;6(4):428–432. doi: 10.1038/nn1029. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Sakai KL. Learning letters in adulthood: direct visualization of cortical plasticity for forming a new link between orthography and phonology. Neuron. 2004;42(2):311–322. doi: 10.1016/s0896-6273(04)00196-5. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. Journal of Cerebral Blood Flow and Metabolism. 1996;16(1):7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalography and Clinical Neurophysiology. 1980;48(6):609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Licht R, Bakker DJ, Kok A, Bouma A. Grade-related changes in event-related potentials (ERPs) in primary school children: differences between two reading tasks. Journal of Clinical and Experimental Neuropsychology. 1992;14(2):193–210. doi: 10.1080/01688639208402823. [DOI] [PubMed] [Google Scholar]
- Maurer U, Brandeis D, McCandliss B. Fast, visual specialization for reading in English revealed by the topography of the N170 ERP response. Behavioral Brain Functions. 2005;1(1):13. doi: 10.1186/1744-9081-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Brem S, Bucher K, Brandeis D. Emerging neurophysiological specialization for letter strings. Journal of Cognitive Neuroscience. 2005;17(10):1532–1552. doi: 10.1162/089892905774597218. [DOI] [PubMed] [Google Scholar]
- Maurer U, Brem S, Bucher K, Kranz F, Benz R, Steinhausen H-C, et al. Impaired tuning of a fast occipito-temporal response for print in dyslexic children learning to read. Brain. 2007;130:3200–3210. doi: 10.1093/brain/awm193. [DOI] [PubMed] [Google Scholar]
- Maurer U, Brem S, Kranz F, Bucher K, Benz R, Halder P, et al. Coarse neural tuning for print peaks when children learn to read. Neuroimage. 2006;33(2):749–758. doi: 10.1016/j.neuroimage.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Maurer U, McCandliss BD. The development of visual expertise for words: the contribution of electrophysiology. In: Grigorenko EL, Naples AJ, editors. Single-word reading: biological and behavioral perspectives. Mahwah, NJ: Lawrence Erlbaum Associates; 2007. [Google Scholar]
- Maurer U, Bucher K, Brem S, Brandeis D. Development of the automatic mismatch response: from frontal positivity in kindergarten children to the mismatch negativity. Clinical Neurophysiology. 2003;114(5):808–817. doi: 10.1016/s1388-2457(03)00032-4. [DOI] [PubMed] [Google Scholar]
- Maurer U, Zevin JD, McCandliss BD. Left-lateralized N170 effects of visual expertise in reading: evidence from Japanese syllabic and logographic scripts. Journal of Cognitive Neuroscience. 2008;20(10):1878–1891. doi: 10.1162/jocn.2008.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Posner MI, Givon T. Brain plasticity in learning visual words. Cognitive Psychology. 1997;33(1):88–110. [Google Scholar]
- Michel CM, Thut G, Morand S, Khateb A, Pegna AJ, Grave de Peralta R, Gonzalez S, Seeck M, Landis T. Electric source imaging of human brain functions. Brain Research: Brain Research Reviews. 2001;36(2-3):108–118. doi: 10.1016/s0165-0173(01)00086-8. [DOI] [PubMed] [Google Scholar]
- Murray MM, Michel CM, Grave de Peralta R, Ortigue S, Brunet D, Gonzalez Andino S, Schnider A. Rapid discrimination of visual and multisensory memories revealed by electrical neuroimaging. Neuroimage. 2004;21(1):125–135. doi: 10.1016/j.neuroimage.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Nelson JR, Liu Y, Fiez J, Perfetti CA. Assimilation and accommodation patterns in ventral occipitotemporal cortex in learning a second writing system. Human Brain Mapping. doi: 10.1002/hbm.20551. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orton ST. Reading, Writing and Speech Problems in Children. London, UK: Chapman & Hall; 1937. [Google Scholar]
- Parviainen T, Helenius P, Poskiparta E, Niemi P, Salmelin R. Cortical sequence of word perception in beginning readers. Journal of Neuroscience. 2006;26(22):6052–6061. doi: 10.1523/JNEUROSCI.0673-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JD. Characterizing the neural mechanisms of skill learning and repetition priming: evidence from mirror reading. Brain. 2001;124(Pt 1):67–82. doi: 10.1093/brain/124.1.67. [DOI] [PubMed] [Google Scholar]
- Posner M, McCandliss BD. Brain circuitry during reading. In: Klein R, McMullen P, editors. Converging methods for understanding reading and dyslexia. Cambridge: MIT Press; 2000. pp. 305–337. [Google Scholar]
- Rossion B, Gauthier I, Goffaux V, Tarr MJ, Crommelinck M. Expertise training with novel objects leads to left-lateralized facelike electrophysiological responses. Psychological Science. 2002;13(3):250–257. doi: 10.1111/1467-9280.00446. [DOI] [PubMed] [Google Scholar]
- Rossion B, Joyce CA, Cottrell GW, Tarr MJ. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage. 2003 doi: 10.1016/j.neuroimage.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Schulz E, Maurer U, van der Mark S, Bucher K, Brem S, Martin E, Brandeis D. Impaired semantic processing during sentence reading in children with dyslexia: combined fMRI and ERP evidence. Neuroimage. 2008;41(1):153–168. doi: 10.1016/j.neuroimage.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Shirahama Y, Ohta K, Takashima A, Matsushima E, Okubo Y. Magnetic brain activity elicited by visually presented symbols and Japanese characters. Neuroreport. 2004;15(5):771–775. doi: 10.1097/00001756-200404090-00006. [DOI] [PubMed] [Google Scholar]
- Spironelli C, Angrilli A. Developmental aspects of automatic word processing: Language lateralization of early ERP components in children, young adults and middle-aged subjects. Biological Psychology. doi: 10.1016/j.biopsycho.2008.01.012. in press. [DOI] [PubMed] [Google Scholar]
- Stein M, Dierks T, Brandeis D, Wirth M, Strik W, Koenig T. Plasticity in the adult language system: a longitudinal electrophysiological study on second language learning. Neuroimage. 2006;33(2):774–783. doi: 10.1016/j.neuroimage.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Strik WK, Fallgatter AJ, Brandeis D, Pascual-Marqui RD. Three-dimensional tomography of event-related potentials during response inhibition: evidence for phasic frontal lobe activation. Electroencephalography and Clinical Neurophysiology. 1998;108(4):406–413. doi: 10.1016/s0168-5597(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Curran T. A neural basis for expert object recognition. Psychological Science. 2001;12(1):43–47. doi: 10.1111/1467-9280.00308. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122(Pt 11):2119–2132. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. TOWRE - Test of word reading efficiency. Austin, Texas: PRO-ED; 1999. [Google Scholar]
- Tucker DM. Spatial sampling of head electrical fields: the geodesic sensor net. Electroencephalography and Clinical Neurophysiology. 1993;87(3):154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;18:18. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Wong AC, Gauthier I, Woroch B, DeBuse C, Curran T. An early electrophysiological response associated with expertise in letter perception. Cognitive, Affective, and Behavioral Neuroscience. 2005;5(3):306–318. doi: 10.3758/cabn.5.3.306. [DOI] [PubMed] [Google Scholar]
- Xue G, Chen C, Jin Z, Dong Q. Language experience shapes fusiform activation when processing a logographic artificial language: an fMRI training study. Neuroimage. 2006;31(3):1315–1326. doi: 10.1016/j.neuroimage.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Xue G, Poldrack RA. The neural substrates of visual perceptual learning of words: implications for the visual word form area hypothesis. Journal of Cognitive Neuroscience. 2007;19(10):1643–1655. doi: 10.1162/jocn.2007.19.10.1643. [DOI] [PubMed] [Google Scholar]
- Yoncheva YN, Blau VC, Maurer U, McCandliss BD. N170 in learning to read a novel script: the impact of attending to phonology on lateralization. Developmental Neuropsychology, current issue [Google Scholar]
- Zhang XL, Begleiter H, Porjesz B, Litke A. Visual object priming differs from visual word priming: an ERP study. Electroencephalogry and Clinical Neurophysiology. 1997;102(3):200–215. doi: 10.1016/s0013-4694(96)95172-3. [DOI] [PubMed] [Google Scholar]


