Abstract
Background: Evidence of an association between fish and meat consumption and risk of dementia is inconsistent and nonexistent in populations in developing countries.
Objective: The objective was to investigate associations between fish and meat consumption with dementia in low- and middle-income countries.
Design: One-phase cross-sectional surveys were conducted in all residents aged ≥65 y in 11 catchment areas in China, India, Cuba, the Dominican Republic, Venezuela, Mexico, and Peru. A total of 14,960 residents were assessed by using the 10/66 standardized protocol, which includes face-to-face interviews for dietary habits and a cross-culturally validated dementia diagnosis.
Results: Dietary intakes and the prevalence of dementia varied between sites. We combined site-specific Poisson regression prevalence ratios (PRs) for the association between fish and meat consumption and dementia in 2 fixed-effect model meta-analyses adjusted for sociodemographic and health characteristics and fish and meat consumption as appropriate. We found a dose-dependent inverse association between fish consumption and dementia (PR: 0.81; 95% CI: 0.72, 0.91) that was consistent across all sites except India and a less-consistent, dose-dependent, direct association between meat consumption and prevalence of dementia (PR: 1.19; 95% CI: 1.07, 1.31).
Conclusions: Our results extend findings on the associations of fish and meat consumption with dementia risk to populations in low- and middle-income countries and are consistent with mechanistic data on the neuroprotective actions of omega-3 (n–3) long-chain polyunsaturated fatty acids commonly found in fish. The inverse association between fish and prevalent dementia is unlikely to result from poorer dietary habits among demented individuals (reverse causality) because meat consumption was higher in those with a diagnosis of dementia.
INTRODUCTION
Dementia is a chronic and progressive age-related disease characterized by irreversible cognitive decline and functional impairment. Worldwide, >24 million people have dementia, two-thirds of whom live in low- and middle-income countries (LAMICs) (1, 2). Treatment and prevention of dementia in LAMICs remains a largely neglected topic (3, 4).
Oily fish consumption is potentially appropriate for both the primary and secondary prevention of dementia and has biological plausibility (5, 6). Oily fish are a rich source of omega-3 long-chain polyunsaturated fatty acids (n−3 LCPs), which have antiinflammatory, antioxidant, antiatherogenic (5), antiamyloid, and neuroprotective properties (7, 8). Evidence of a benefit of fish consumption on dementia risk is currently limited to developed countries. Most observational studies report an association of high fish intake with better cognition (9, 10) or lower risk of dementia (11–15), although these findings are not consistent across all studies (16, 17). A recent small randomized controlled trial in healthy Dutch adults found no benefit of n−3 LCP supplementation over 6 mo (18), further trials are underway (19, 20), and current evidence of a benefit on cognitive health is not convincing (21). Furthermore, fish foods may contain contaminants, such as mercury and dioxins, that might contribute to neurodegenerative disorders. The proposal that the benefits of fish intake outweigh the potential risks (22) cannot currently be extended to LAMICs because of the absence of region-specific data.
Although few studies present data on the association of meat intake with risk of dementia, reports of associations with constituents of meat, such as saturated fat and cholesterol, are more frequent. In a large cohort of French adults followed for 7 y, there was no association of reported meat intake with dementia risk (11). Cross-sectional studies have reported a direct association of cholesterol with risk of cognitive impairment (10, 23), although this is not supported by longitudinal data (24) and gene-nutrient interactions are suggested (25). A direct association of saturated fat intake with cognitive decline (26, 27), vascular dementia (28), and Alzheimer disease (12) has been found in some, but not all (29), cohorts.
The current data were derived from the 10/66 population-based studies on dementia and aging in 10 LAMICs. The objectives of the current report were as follows: 1) to describe fish and meat intakes and their relation to the health and sociodemographic characteristics of older people across countries, 2) to test the hypotheses that dietary fish is inversely associated and dietary meat is directly associated with prevalent dementia, and 3) to test the consistency of the country-specific hypothesized inverse association of fish and dementia with control for the relevant confounders and after having disaggregated the potential concomitant opposing effect of meat consumption.
SUBJECTS AND METHODS
Study design
This was a cross-sectional catchment area one-phase survey of older people (aged ≥65 y) carried out at 11 sites: one urban and one rural in Peru, Mexico, China, and India and in urban sites only in Cuba, the Dominican Republic, and Venezuela. More affluent areas of the countries were avoided. Details on the catchment areas can be found on the 10/66 website (www.alz.co.uk/1066). Recruitment dates ranged from January 2003 to November 2007.
Participants
The chosen areas were mapped, and all residents aged ≥65 y were enumerated by means of door-knocking. Age was ascertained on the basis of self-report, documentation, and a relative's confirmation. No other inclusion or exclusion criteria were applied. Power calculations showed that a sample size of 2000 per country would allow an estimation of a typical dementia prevalence of 4.5% with a precision of ±0.9%.
Study protocol and operational procedures
The 10/66 Dementia Research Group standardized protocol was administered in full to all study participants (30). Development and validation of the 10/66 dementia diagnosis was previously successfully conducted in 25 LAMICs, including the 7 reported here (31). The participant interviews and assessments lasted from 2 to 3 h and were generally carried out at the participant's household. All materials, questionnaires, and assessments were translated into the local languages by bilingual local clinicians. In two 1-wk intensive training courses, held in London (United Kingdom) and Barcelona (Spain), the 10/66 London team trained the local principal investigators (PIs) on the 10/66 protocols and procedures. A detailed manual and a video training course were prepared on the physical and neurological examinations covering all aspects of the 10/66 protocol, the assessments, and the study procedures. Local interviewers were medical doctors in Cuba and China and lay interviewers (generally health workers) in all other centers. All received the same standard training on the 10/66 protocol and assessments. Moreover, the local PIs carried out periodic quality-control assessments and random checks throughout the data collection process. Data were recorded on paper data entry sheets and entered locally onto computers by using EpiData (32) files on which conditional skips and range checks were predefined. Data were then exported to SPSS (33) and STATA (34), cleaned, and combined into a single data set. The institutional review boards of the Institute of Psychiatry, KCL in London. and the institutional ethics committees in each of the countries that took part in this study approved the study protocol and its procedures.
Measures
The 10/66 protocol comprises questionnaires on participants' sociodemographic characteristics, health status, health behaviors, and risk factor exposures; physical and neurological examinations; and an extensive informant interview. In situations in which the participant was too cognitively impaired to answer questions, information was gathered from an informant. Details on measures and assessments are extensively described elsewhere (30). For the purposes of this study we considered the following variables:
1) Sociodemographic characteristics: sex, age, educational level, household living circumstances, and number of assets (motor vehicles, television, refrigerator and/or freezer, water utilities, electricity utilities, telephone, plumbed toilet, and plumbed bathroom).
2) Diagnosis of dementia: according to the 10/66 diagnostic algorithm (31).
3) Dietary habits: standardized questions on average weekly fish and meat intakes were measured in face-to-face interviews. Response options for “how often do you eat fish/meat in a week?” were “never,” “some days,” “most days,” and “every day.” The average daily portions of vegetables and fruit consumed were also recorded as was the alcohol units drunk per week.
4) Depressive episodes (mild, moderate, or severe): ascertained by using the Geriatric Mental State Examination (35) according to the International Classification of Diseases, 10th edition (ICD-10) (36).
5) Diastolic and systolic blood pressures: measured on 2 occasions, and the mean was calculated. Hypertension was defined according to the European Society of Hypertension (ESH) definition (average systolic blood pressure ≥140 mm Hg and/or average diastolic blood pressure ≥95 mm Hg) (37).
6) Self-reported chronic disease diagnoses: standardized questions (“have you ever been told by a doctor that you had a stroke/heart attack/angina or have diabetes?”) were asked for stroke, ischemic heart disease, and diabetes.
7) Smoking habits: ever smoked, current smoker, and lifetime smoking.
Data analysis
Participants' characteristics by country
We present descriptive statistics to illustrate the sociodemographic and health characteristics of participants and their weekly fish and meat intakes by country. On inspection of the data, we combined participants who reported fish or meat intakes on “most days” and “every day” given the small numbers in these groups.
We calculated correlations (Kendall's τ) between fish and meat consumption by country. We then used unadjusted ordered logistic regressions to measure the associations between fish and meat consumption (entered in the model as ordered categorical variables) and participants' health and sociodemographic characteristics after having dichotomized age (<75 y vs ≥75 y), educational level (no or very limited education vs at least completed primary school), smoking habits (never smoked vs ex- and current smokers), living arrangements (alone or with spouse only vs with ≥2 people), and number of assets (less vs >3 assets).
Association between prevalent dementia and diet
Dementia status was determined by applying the 10/66 diagnostic algorithm (31). To ascertain the risk of dementia associated with fish consumption we first calculated unadjusted robust prevalence ratios (PRs) with 95% CIs using Poisson regression, with control for household clustering and dietary intakes as continuous variables. Units of increase for all PRs are per fish and meat consumption level, year of age, education level (5 grades from none to tertiary), number of assets (0 to 7), number of servings of fruit and vegetables per week, and alcohol consumption (units/wk). The assumption was made that the levels of fish consumption had a natural ordering (low to high), with unknown distances between adjacent levels. We then performed the same analysis entering fish consumption in the model as an ordinal categorical variable. We carried out a likelihood ratio test to compare the 2 models by country. Because in none of the countries except China (χ2 = 4.03, P = 0.04) (Table 4) were the 2 models significantly different, PRs (with 95% CI) from the former model—interpreted as a test for trend—were considered and a linear effect of fish consumption on prevalent dementia was implied.
TABLE 4.
Prevalence ratios from robust Poisson regression models for the association of fish consumption with 10/66 dementia, with likelihood ratio tests for linearity and for test of hypothesis, by country1
| Prevalence ratio (95% CI) |
Chi-square test with df =
1 (P value) |
|||||
| Crude model | Model 12 | Model 23 | Model 34 | Linearity test | Test for the hypothesis | |
| Cuba | 0.67 (0.52, 0.88) | 0.86 (0.68, 1.08) | 0.83 (0.66, 1.04) | 0.81 (0.65, 1.02) | 2.41 (0.12) | 2.86 (0.09) |
| Dominican Republic | 0.74 (0.60, 0.91) | 0.77 (0.62, 0.94) | 0.78 (0.64, 0.95) | 0.80 (0.65, 1.00) | 0.59 (0.44) | 3.90 (0.05) |
| Peru | 0.83 (0.61, 1.14) | 0.87 (0.64, 1.20) | 0.84 (0.61, 1.14) | 0.76 (0.56, 1.05) | 1.32 (0.25) | 2.17 (0.14) |
| Venezuela | 1.11 (0.83, 1.49) | 0.92 (0.69, 1.23) | 0.92 (0.68, 1.26) | 0.87 (0.56, 1.34) | 0.02 (0.88) | 0.33 (0.57) |
| Mexico | 0.64 (0.49, 0.85) | 0.83 (0.64, 1.08) | 0.85 (0.65, 1.11) | 0.81 (0.62, 1.08) | 0.78 (0.38) | 2.52 (0.11) |
| China | 0.40 (0.26, 0.60) | 0.45 (0.31, 0.67) | 0.50 (0.36, 0.71) | 0.58 (0.39, 0.85) | 4.03 (0.04) | 6.94 (0.01) |
| India | 1.13 (0.84, 1.50) | 1.18 (0.88, 1.58) | 1.18 (0.88, 1.59) | 1.47 (0.92, 2.35) | 1.09 (0.30) | 2.40 (0.12) |
Prevalence ratios were adjusted for household clustering. Unit of increase in prevalence ratios are per fish, per meat consumption (none per week, some days, most days, or all days), per year, per level of education (5 levels from none to completed tertiary school), per number of assets (0 to 7), fruit and vegetable intake (servings/wk), and alcohol consumption (units/wk).
Adjusted for age, sex, educational level, and number of household assets.
As for model 1 plus family history of dementia; number of International Classification of Diseases, 10th edition, depressive symptoms; self-reported stroke; self-reported diabetes; self-reported coronary heart disease (including angina and myocardial infarction); smoking habit; living arrangements (live alone or only with spouse); and number of assets.
As for model 2 plus meat intake, alcohol consumption, and number of daily portions of fruit and vegetables.
We repeated the statistical procedure for meat consumption and again found no significant departures from linearity (Table 5). Therefore all PRs for the association between dementia and dietary meat intake were again calculated after meat intake was entered as a continuous variable in the Poisson regression models.
TABLE 5.
Prevalence ratios from robust Poisson regression models for the association of meat consumption with 10/66 dementia, with likelihood ratio tests for linearity and for test of hypothesis, by country1
| Prevalence ratio (95% CI) |
Chi-square test with df =
1 (P value) |
|||||
| Crude model | Model 12 | Model 23 | Model 34 | Linearity test | Test for the hypothesis | |
| Cuba | 1.28 (1.04, 1.58) | 1.28 (1.05, 1.55) | 1.22 (1.00, 1.48) | 1.31 (1.07, 1.60) | 1.40 (0.24) | 6.05 (0.01) |
| Dominican Republic | 1.11 (0.91, 1.37) | 1.14 (0.93, 1.4) | 1.12 (0.93, 1.36) | 1.19 (0.98, 1.45) | 0.04 (0.85) | 2.39 (0.12) |
| Peru | 1.52 (1.16, 1.99) | 1.45 (1.13, 1.88) | 1.40 (1.10, 1.78) | 1.41 (1.09, 1.81) | 0.70 (0.40) | 6.04 (0.01) |
| Venezuela | 1.36 (0.99, 1.87) | 0.83 (0.61, 1.13) | 0.84 (0.62, 1.14) | 0.91 (0.59–1.40) | 0.03 (0.86) | 0.04 (0.84) |
| Mexico | 1.07 (0.81, 1.41) | 1.17 (0.90, 1.52) | 1.19 (0.91, 1.54) | 1.24 (0.94, 1.64) | 0.61 (0.44) | 1.78 (0.18) |
| China | 0.67 (0.50, 0.90) | 0.72 (0.53, 0.97) | 0.67 (0.52, 0.88) | 0.95 (0.69, 1.31) | 1.04 (0.31) | 0.11 (0.74) |
| India | 1.04 (0.78, 1.39) | 1.02 (0.75, 1.38) | 1.01 (0.75, 1.36) | 0.77 (0.48, 1.21) | 0.03 (0.87) | 1.10 (0.29) |
Prevalence ratios were adjusted for household clustering. Unit of increase in prevalence ratios are per fish, per meat consumption (none per week, some days, most days, or all days), per year, per level of education (5 levels from none to completed tertiary school), per number of assets (0 to 7), fruit and vegetable intake (servings/wk), and alcohol consumption (units/wk).
Adjusted for age, sex, educational level, and number of household assets.
As for model 1 plus family history of dementia; number of International Classification of Diseases, 10th edition, depressive symptoms; self-reported stroke; self-reported diabetes; self-reported coronary heart disease (including angina and myocardial infarction); smoking habit; living arrangements (live alone or only with spouse); and number of assets.
As for model 2 plus meat intake, alcohol consumption, and number of daily portions of fruit and vegetables.
In 3 further models, we used Poisson regressions to generate adjusted PRs for the associations between prevalent dementia and weekly intakes of fish foods and meat. In model 1 we controlled for age, sex, and educational level. On the basis of the evidence in the literature, family history of dementia, self-reported chronic disease diagnoses (namely stroke, diabetes and coronary heart disease), ICD-10 depression, and smoking habits were included in model 2 along with living arrangements (living alone or with spouse only as opposed to living in multigenerational families) and number of assets (less vs >3 assets) as proxies of food availability and affordability. All variables were considered potential confounders, likely associated with both dietary habits and dementia status. Finally, to assess the independent effect of fish with control for other nutritional factors, we estimated model 3 in which the average daily number of fruit and vegetable portions and the weekly intake of meat (or fish as appropriate) and alcohol were added to the covariates of model 2. We carried out likelihood tests to test for departures from linearity and to test for the hypothesis of a linear association between dietary intake and dementia prevalence.
Finally, to summarize the associations between dietary fish, dietary meat, and prevalent dementia, we assumed that the true association was the same in all countries and combined the country-adjusted PRs from model 3 (see above) in 2 fixed-effect model meta-analytic forest plots. We did not use random-effect models because we wished to summarize the countries within this study rather than generalize to a hypothetical population of centers. A formal test for between-studies heterogeneity (Cochran's Q) was performed, and I2 Higgins values (38) were calculated (larger values meaning higher heterogeneity). Descriptive and analytic statistics were carried out by using release 1_7 of the 10/66 data set with STATA 9.2 software (StatCorp 2007, Stata Statistical Software: release 10; StataCorp, College Station, TX).
Role of the funding source
Study design, data collection and analysis, and interpretation of the findings were independent of all sponsors. All authors agreed on the contents of the article, and the ultimate decision and responsibility of submission lies with the corresponding author.
RESULTS
This article includes data on 14,960 participants from urban sites in Cuba, the Dominican Republic, Venezuela, and urban and rural sites in Peru, Mexico, China, and India. Response rates among eligible enumerated older people (≥65 y) ranged from 80% to 94% (Table 1) (2). Missing values for each variable are reported by country in Table 1.
TABLE 1.
Sociodemographic and health characteristics of participants by country1
| Country |
|||||||
| Variable | Cuba | Dominican Republic | Peru | Venezuela | Mexico | China | India |
| Sample size (n) | 2934 | 1999 | 1927 | 1939 | 1997 | 2162 | 1998 |
| Response rate (%) | 94 | 95 | 84 | 80 | 85 | 85 | 85 |
| Age [n (%)] | |||||||
| 65–69 y | 760 (25.9) | 533 (26.5) | 554 (28.7) | 823 (41.2) | 544 (27.12) | 699 (32.3) | 746 (37.3) |
| 70–74 y | 789 (26.9) | 520 (25.9) | 493 (25.5) | 469 (23.51) | 581 (29.02) | 658 (30.43) | 668 (33.4) |
| 75–79 y | 639 (21.8) | 397 (19.7) | 399 (20.6) | 346 (17.34) | 426 (21.28) | 456 (21.09) | 321 (16.1) |
| ≥80 y | 749 (25.5) | 561 (28.0) | 486 (25.2) | 309 (15.49) | 451 (22.53) | 349 (16.14) | 265 (13.2) |
| Missing values | 7 | 0 | 2 | 23 | 1 | 0 | 4 |
| Females (%) | 64.9 | 65.9 | 61.2 | 64.2 | 63.3 | 56.3 | 56.1 |
| Missing values (n) | 10 | 3 | 7 | 60 | 0 | 0 | 15 |
| Educational level [n (%)] | |||||||
| No education | 75 (2.5) | 392 (19.5) | 121 (6.3) | 158 (7.8) | 554 (27.7) | 811 (37.5) | 1088 (54.3) |
| Some education | 655 (22.3) | 1022 (50.8) | 231 (11.9) | 453 (22.5) | 864 (43.1) | 267 (12.4) | 429 (21.4) |
| Completed primary school | 979 (33.3) | 370 (18.4) | 727 (37.6) | 977 (48.4) | 351 (17.5) | 562 (26.0) | 328 (16.4) |
| Completed secondary school | 728 (24.7) | 135 (6.7) | 517 (26.7) | 271 (13.4) | 124 (6.2) | 380 (17.6) | 113 (5.6) |
| Completed tertiary school | 499 (16.9) | 73 (3.6) | 321 (16.6) | 96 (4.8) | 110 (5.5) | 142 (6.6) | 44 (2.2) |
| Missing values | 8 | 19 | 16 | 63 | 0 | 0 | 2 |
| Live alone or with spouse only [n (%)] | 706 (24.1) | 389 (19.3) | 274 (14.2) | 218 (10.8) | 525 (26.2) | 712 (33.0) | 412 (20.6) |
| Missing values | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Three or fewer assets [n (%)] | 87 (3.1) | 310 (15.4) | 95 (4.9) | 58 (2.9) | 432 (21.6) | 114 (5.3) | 1052 (52.5) |
| Missing values | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10/66 Dementia [n (%)] | 316 (10.8) | 235 (11.7) | 165 (8.5) | 140 (7.1) | 171 (8.5) | 137 (6.3) | 181 (9) |
| Missing values | 13 | 0 | 2 | 0 | 0 | 0 | 0 |
| Meets ESH hypertension criteria [n (%)] | 1639 (55.7) | 915 (45.5) | 224 (11.6) | 682 (44.8) | 717 (35.8) | 958 (44.3) | 730 (36.4) |
| Missing values | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Self-reported stroke [n (%)] | 230 (7.8) | 175 (8.7) | 132 (6.9) | 138 (7.1) | 141 (7.1) | 127 (5.9) | 31 (1.5) |
| Missing values | 9 | 6 | 10 | 68 | 0 | 0 | 1 |
| Self-reported diabetes [n (%)] | 543 (18.5) | 281 (14.0) | 173 (9.0) | 314 (16.1) | 435 (21.7) | 204 (9.4) | 187 (9.3) |
| Missing values | 16 | 4 | 11 | 62 | 1 | 1 | 1 |
| Self-reported CHD [n (%)] | 415 (14.1) | 60 (3.0) | 115 (6.0) | 121 (6.2) | 54 (2.7) | 127 (5.9) | 77 (3.8) |
| Missing values | 6 | 2 | 2 | 56 | 0 | 0 | 1 |
| ICD-10 depressive episode [n (%)] | 144 (4.9) | 278 (13.8) | 103 (5.3) | 108 (5.3) | 92 (4.6) | 10 (0.5) | 165 (8.2) |
| Missing values | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alcohol (units/wk)2 | 2.1 ± 13.7 | 9.7 ± 37.2 | 0.3 ± 4.9 | 2 ± 5.2 | 1 ± 5.5 | 2.2 ± 10 | 0.2 ± 1.6 |
| Missing values | 52 | 15 | 51 | 821 | 17 | 0 | 129 |
| Current or ex-smoker [n (%)] | 1319 (24.2) | 955 (17.5) | 324 (5.9) | 823 (15.1) | 624 (11.4) | 620 (11.4) | 797 (14.6) |
| Missing values | 8 | 2 | 8 | 88 | 0 | 0 | 8 |
ESH, European Society of Hypertension; CHD, coronary heart disease (including angina and myocardial infarction); ICD-10, International Classification of Disease, 10th edition.
Values are means ± SDs.
Participants' sociodemographic and health characteristics by country
The sociodemographic and health characteristics of participants are presented by country (Table 1). The age distribution across countries was fairly consistent, with Venezuelan, Chinese, and Indian participants being slightly younger than in other countries. There were more women than men in the sample in every site. Participants in Cuba and Peru were better educated than were those in other countries. Extended family living arrangements were the norm, particularly in Venezuela and Peru. Socioeconomic disadvantage, as indexed by having ≤3 household assets, was most prevalent in the Indian sample and in the Dominican Republic and Mexico. The prevalence of hypertension and cardiovascular disease was highest in the more developed Latin American centers, particularly Cuba. However, the prevalence of hypertension in Peru was strikingly low. Smoking was very uncommon among older people in Peru and considerably more common in Cuba, India, and China. The prevalence of dementia in the samples has been reported in detail elsewhere (2). In brief, there were 1340 prevalent dementia cases in the whole sample, and the prevalence varied from 6.3% to 11.7% by country, being higher in the Latin American countries than in China and India (Table 1).
Fish and meat consumption
Fish and meat consumptions varied considerably across countries (Table 2 and Table 3). Fish consumption was highest in Venezuela and China and lowest in India and the Dominican Republic. Meat consumption was lowest in Venezuela and highest in the Dominican Republic, China, Peru, and Cuba.
TABLE 2.
Weekly fish consumption and unadjusted odds ratios (and 95% CIs) for the ordered logistic regression model for fish consumption and health and sociodemographic characteristics by country1
| Country |
|||||||
| Variable | Cuba | Dominican Republic | Peru | Venezuela | Mexico | China | India |
| Sample size (n) | 2934 | 1999 | 1927 | 1939 | 1997 | 2162 | 1998 |
| Weekly fish intake [n (%)] | |||||||
| Never | 287 (9.8) | 684 (34.2) | 161 (8.4) | 88 (4.7) | 567 (28.4) | 67 (3.1) | 422 (21.1) |
| Some days | 2348 (80.0) | 1158 (57.9) | 1413 (73.3) | 850 (45.0) | 1328 (66.5) | 1467 (67.9) | 1424 (71.3) |
| Most/every day | 299 (10.2) | 157 (7.9) | 353 (18.3) | 953 (50.4) | 102 (5.1) | 628 (29.1) | 152 (7.6) |
| Missing values | 10 | 12 | 6 | 79 | 6 | 0 | 6 |
| Age2 | 0.71 (0.59, 0.86) | 0.85 (0.71, 1.01) | 1.00 (0.81, 1.23) | 1.01 (0.84, 1.22) | 0.86 (0.71, 1.03) | 0.73 (0.60, 0.90) | 0.84 (0.68, 1.04) |
| Sex (F vs M) | 1.24 (1.05, 1.46) | 1.44 (1.21, 1.71) | 1.21 (1.00, 1.47) | 1.00 (0.84, 1.18) | 0.85 (0.71, 1.02) | 1.19 (1.04, 1.37) | 1.11 (0.92, 1.34) |
| Education2 | 1.62 (1.31, 2.00) | 1.51 (1.24, 1.83) | 0.60 (0.47, 0.78) | 1.39 (1.15, 1.69) | 2.42 (1.95, 2.99) | 1.02 (0.85, 1.23) | 0.73 (0.57, 0.93) |
| Self-reported stroke | 0.87 (0.60, 1.27) | 1.00 (0.74, 1.36) | 1.02 (0.69, 1.50) | 1.06 (0.75, 1.49) | 0.80 (0.56, 1.13) | 0.47 (0.30, 0.73) | 0.87 (0.37, 2.03) |
| Self-reported CHD | 1.13 (0.87, 1.47) | 1.01 (0.59, 1.72) | 1.14 (0.75, 1.73) | 1.09 (0.74, 1.60) | 1.62 (0.83, 3.16) | 0.46 (0.31, 0.68) | 1.07 (0.66, 1.75) |
| Self-reported diabetes | 1.07 (0.85, 1.35) | 1.56 (1.23, 1.96) | 1.18 (0.87, 1.62) | 1.20 (0.95, 1.53) | 1.14 (0.91, 1.42) | 0.67 (0.48, 0.95) | 1.33 (0.92, 1.93) |
| ICD-10 depression | 0.67 (0.47, 0.97) | 0.96 (0.76, 1.23) | 0.64 (0.40, 1.03) | 0.60 (0.41, 0.88) | 0.72 (0.46, 1.14) | 0.82 (0.15, 4.55) | 1.22 (0.88, 1.69) |
| Alcohol consumption2 | 1.32 (1.04, 1.68) | 1.49 (1.19, 1.87) | 1.91 (1.21, 3.03) | 1.37 (1.08, 1.75) | 1.41 (1.12, 1.78) | 0.63 (0.47, 0.83) | 1.6 (1.24, 2.06) |
| Smoking status2 | 1.12 (0.93, 1.34) | 0.80 (0.67, 0.95) | 1.08 (0.82, 1.4) | 0.96 (0.80, 1.14) | 1.24 (1.01, 1.51) | 0.95 (0.78, 1.16) | 1.28 (1.05, 1.55) |
| Living arrangements2 | 1.12 (0.89, 1.41) | 0.87 (0.70, 1.09) | 0.74 (0.54, 1.01) | 0.76 (0.56, 1.04) | 1.09 (0.87, 1.36) | 0.90 (0.72, 1.13) | 1.20 (0.95, 1.53) |
| Assets2 | 1.19 (0.68, 2.09) | 1.76 (1.39, 2.24) | 0.48 (0.32, 0.74) | 1.07 (0.65, 1.78) | 3.96 (3.14, 4.99) | 1.13 (0.69, 1.85) | 0.99 (0.81, 1.21) |
CHD, coronary heart disease (including angina and myocardial infarction); ICD-10, International Classification of Diseases, 10th edition.
Dichotomized variables: age (>75 vs ≤75 y), sex (F vs M), education (no or some education vs completed at least primary school), alcohol consumption (0 vs ≥1 unit/wk), smoking status (never vs ex- or current smoker), living alone or with spouse only, and assets (≥3 vs <3).
TABLE 3.
Weekly meat consumption and unadjusted odds ratios (and 95% CIs) for the ordered logistic regression model for consumption and health and sociodemographic characteristics by country1
| Country |
|||||||
| Variable | Cuba | Dominican Republic | Peru | Venezuela | Mexico | China | India |
| Sample size (n) | 2934 | 1999 | 1927 | 1939 | 1997 | 2162 | 1998 |
| Weekly meat intake [n (%)] | 102 (3.5) | 114 (5.7) | 155 (8.1) | 358 (18.9) | 177 (8.9) | 60 (2.8) | 378 (18.9) |
| Never | 102 (3.5) | 114 (5.7) | 155 (8.1) | 358 (18.9) | 177 (8.9) | 60 (2.8) | 378 (18.9) |
| Some days | 1752 (59.7) | 790 (39.5) | 1021 (53) | 1251 (66.1) | 1439 (72.1) | 926 (42.8) | 1479 (74) |
| Most/every day | 1080 (36.8) | 1096 (54.8) | 750 (38.9) | 285 (15.1) | 381 (19.1) | 1176 (54.4) | 142 (7.1) |
| Missing values | 10 | 11 | 7 | 76 | 6 | 0 | 5 |
| Age2 | 1.00 (0.86, 1.16) | 0.99 (0.83, 1.18) | 1.06 (0.89, 1.27) | 0.80 (0.65, 0.98) | 0.94 (0.77, 1.15) | 0.94 (0.78–1.14) | 0.86 (0.69–1.07) |
| Sex (F vs M) | 0.86 (0.75, 0.99) | 1.21 (1.01, 1.44) | 0.81 (0.68, 0.97) | 1.36 (1.14, 1.63) | 1.03 (0.85, 1.24) | 1.37 (1.20–1.57) | 1.04 (0.85–1.26) |
| Education2 | 1.35 (1.13, 1.61) | 1.18 (0.97, 1.43) | 1.34 (1.08, 1.66) | 1.30 (1.05, 1.59) | 1.30 (1.04, 1.63) | 1.58 (1.32–1.88) | 0.51 (0.39–0.66) |
| Self-reported stroke | 1.25 (0.94, 1.64) | 1.07 (0.79, 1.47) | 1.08 (0.72, 1.62) | 0.69 (0.48, 1.00) | 1.04 (0.72, 1.49) | 1.11 (0.76–1.62) | 0.68 (0.27–1.67) |
| Self-reported CHD | 0.87 (0.70, 1.08) | 0.84 (0.52, 1.36) | 1.46 (1.04, 2.06) | 0.88 (0.57, 1.36) | 1.14 (0.58, 2.21) | 0.98 (0.67–1.42) | 0.98 (0.56–1.72) |
| Self-reported diabetes | 1.47 (1.22, 1.79) | 1.18 (0.92, 1.52) | 1.09 (0.79, 1.49) | 1.01 (0.79, 1.29) | 1.11 (0.88, 1.41) | 1.40 (1.04–1.89) | 1.53 (1.02–2.29) |
| ICD-10 depression | 0.85 (0.61, 1.18) | 0.75 (0.58, 0.97) | 1.33 (0.86, 2.06) | 1.11 (0.69, 1.78) | 0.98 (0.59, 1.65) | 1.44 (0.23–9.14) | 0.98 (0.70–1.37) |
| Alcohol consumption2 | 1.32 (1.04, 1.68) | 1.49 (1.19, 1.87) | 1.91 (1.21, 3.03) | 1.37 (1.08, 1.75) | 1.41 (1.12, 1.78) | 0.63 (0.47–0.83) | 1.6 (1.24–2.06) |
| Smoking status2 | 0.87 (0.76, 1.01) | 0.83 (0.69, 0.99) | 1.41 (1.10, 1.81) | 1.26 (1.04, 1.52) | 1.39 (1.13, 1.72) | 1.33 (1.10–1.61) | 1.43 (1.17–1.75) |
| Living arrangements2 | 1.11 (0.91, 1.35) | 0.96 (0.76, 1.20) | 1.05 (0.80, 1.37) | 0.87 (0.63, 1.22) | 1.40 (1.12, 1.74) | 0.77 (0.62–0.95) | 1.15 (0.89–1.49) |
| Assets2 | 2.01 (1.20, 3.37) | 1.76 (1.40, 2.21) | 1.44 (1.03, 2.01) | 0.34 (0.19, 0.62) | 2.14 (1.73, 2.65) | 1.93 (1.26–2.95) | 0.72 (0.58–0.89) |
CHD, coronary heart disease (including angina and myocardial infarction); ICD-10, International Classification of Diseases, 10th edition.
Dichotomized variables: age (>75 vs ≤75 y), sex (F vs M), education (no or some education vs completed at least primary school), alcohol consumption (0 vs ≥1 unit/wk), smoking status (never vs ex- or current smoker), living alone or with spouse only, and assets (≥3 vs <3).
Fish and meat consumption was modestly positively correlated in Cuba (Kendall's τ = 0.14), the Dominican Republic (0.10), Peru (0.19), and Mexico (0.14); strongly correlated in China (0.56) and India (0.77); and not correlated in Venezuela (−0.05). In all countries but Venezuela (P = 0.21), the correlations were statistically significant (P < 0.001). There was a general trend toward lower consumption of both fish and meat among older participants; however, this trend was only statistically significant in Cuba and China for fish and in Venezuela for meat. The ordered logistic regression models showed that in all countries except China, alcohol consumption was consistently higher among those with higher fish and meat intakes. Those with higher educational levels and more assets reported higher fish and meat intake across all countries. Crude associations between fish and meat intakes and history of stroke, self-reported diabetes (type 1 or 2), smoking habit, and depression were inconsistent across countries (Table 2 and Table 3).
Association between prevalent dementia and dietary fish
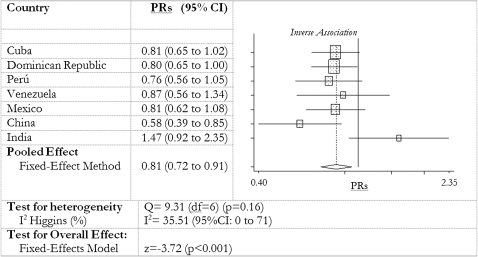
There was a consistent finding in all countries, except India, of an inverse association between reported fish consumption and dementia prevalence. In the crude model, the PRs for each increase in fish consumption category ranged from 0.40 (95% CI: 0.26, 0.60) in China to 1.13 (95% CI: 0.84, 1.50) in India (Table 4). In model 1 the inverse association of dietary fish with dementia was attenuated somewhat after age, sex, and educational level were adjusted for. PRs did not substantially change in model 2 after family history of dementia, self-reported chronic disease diagnoses, ICD-10 depression, smoking habits, living arrangements, and number of assets were adjusted for. Further adjustment for the effect of dietary meat, alcohol consumption, and daily portions of fruit and vegetables (model 3) did not substantially alter the estimates. Likelihood ratio tests showed no significant departures from linearity in any country (Table 4), although the inverse linear trend was only statistically significant in China (Table 4). To summarize the effect of fish consumption on prevalent dementia across the whole sample, the country-specific robust PRs (and 95% CI) from model 3 were combined into a meta-analysis that estimated a combined PR from the fixed-effect model of 0.81 (95% CI: 0.72, 0.91) among those who ate fish more often (Figure 1). The heterogeneity of effect between countries was not significant (P = 0.16), and the degree of inconsistency in results was very low (Higgins I2 = 36%; 95% CI: 0, 73). In no country was there a significant association between fish consumption and degree of dementia severity (according to the Clinical Dementia Rating scale).
FIGURE 1.
Meta-analysis (fixed-effect model) of country prevalence ratios (PRs) (and 95% CIs) for the association between fish consumption and 10/66 dementia. PRs are from robust Poisson regression models adjusted for household clustering as for model 3 in Table 4, ie, adjusted for age, sex, educational level, and family history of dementia and controlled for number of International Classification of Diseases, 10th edition, depressive symptoms; self-reported stroke; self-reported diabetes; self-reported coronary heart disease (including angina and myocardial infarction); smoking habit; living arrangements (live alone or only with spouse); number of assets; meat intake; and number of daily portions of fruit and vegetables.
Association between prevalent dementia and dietary meat
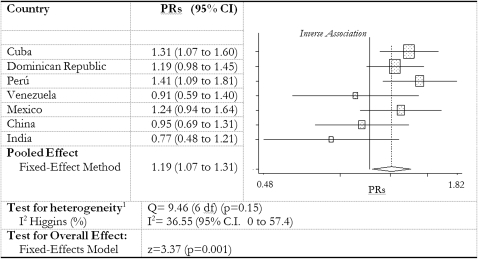
The crude association between meat intake and prevalent dementia was inconsistent across countries. In Cuba (1.28; 95% CI: 1.04, 1.58) and Peru (1.52; 95% CI: 1.16, 1.99), PRs showed a statistically significant direct association between meat consumption and dementia risk, which remained statistically significant after potential confounders were adjusted for (Table 5). In China the crude PR suggested a statistically significant inverse association between meat consumption and risk of dementia (0.67; 95% CI: 0.50, 0.90), which became statistically nonsignificant after adjustment (Table 5). The likelihood ratio tests showed no departure from linearity, whereas the hypothesis that meat consumption would be associated with a higher risk of dementia was only supported in Cuba and Peru. When we combined the country-specific robust PRs (with 95% CI) of model 3 into a fixed-effect model meta-analysis, we estimated a combined PR of 1.19 (95% CI: 1.07, 1.31) for the association between meat consumption and prevalent dementia (Figure 2). There was no statistically significant heterogeneity of estimates between countries (P = 0.15), and the degree of inconsistency in results was very low (Higgins I2 = 37%; 95% CI: 0, 73). In Cuba there was a significant association between severity of dementia and meat consumption, with higher meat consumption among those with more severe dementia according to the Clinical Dementia Rating scale (χ2 = 6.1, df = 1, P = 0.014).
FIGURE 2.
Meta-analysis (fixed-effect model) of country prevalence ratios (PRs) (and 95% CIs) for the association between meat consumption and 10/66 dementia. PRs are from robust Poisson regression models adjusted for household clustering as for model 3 in Table 5, ie, adjusted for age, sex, educational level, and family history of dementia and controlled for the number of International Classification of Diseases, 10th edition, depressive symptoms; self-reported stroke; self-reported diabetes; self-reported coronary heart disease (including angina and myocardial infarction); smoke habit; living arrangements (live alone or only with spouse); number of assets; fish intake; and number of daily portions of fruit and vegetables.
DISCUSSION
We showed for the first time that a statistically significant trend toward a lower prevalence of dementia among those with higher dietary fish intake in large population-based samples of older people living in 5 countries in Latin America, China, and India. The country-specific association of fish intake with dementia was only statistically significant in China, but meta-analysis combining data from all countries showed a statistically significant association (PR: 0.81; 95% CI: 0.72, 0.91) that remained even after adjustment for a large array of potentially relevant sociodemographic and health-related factors. The negative association was not present in India (PR: 1.47; 95% CI: 0.92, 2.35). Reported fish and meat consumption was positively correlated in most countries, and there was also evidence of a modest increased risk of dementia among those with higher meat consumption (PR: 1.19; 95% CI: 1.07, 1.31). We did not have information on type of fish and meat consumed, portion size, and method of cooking, which may all be relevant factors.
To our knowledge, this is the largest population-based study on this topic to date from either developing or developed country samples. Our findings are consistent with some (12, 15, 39) but not all (16, 17) studies on fish intake in Western countries and support previous associations of saturated fat (12, 26–28), although not meat (11), with cognitive health. We are not able to say whether the inconsistency in findings was due to the type of fish and meat reportedly consumed in our study. Our findings should be interpreted with caution given the cross-sectional study design and the limitations in our dietary assessments. Moreover, although our study's internal validity and intercountry comparisons are strong, we recognize that our findings should only be generalized to populations with similar dietary and health characteristics.
Identical study protocols were used across the 7 LAMICs. The validity of the dementia outcome has been shown in cross-cultural pilot studies in 25 countries worldwide (31). Our face-to-face questions to assess dietary habits were simple and well tolerated both by healthy participants and by those with dementia, whose answers were confirmed by an informant. The major limitations of this report arise from the cross-sectional design. We cannot entirely exclude the possibility that reverse causality, information bias, differential mortality, or residual confounding may have accounted for the observed findings (40). Whereas those with dementia might be expected to have a lower food intake, this seems unlikely to have accounted for the potential protective effect of fish intake given that those with dementia generally reported a higher intake of meat. There was no evidence among those with dementia that progression of the disease was associated with reduced fish intake. Those with a more sufficient diet of meat may have survived longer with dementia, accounting for the positive cross-sectional association. This would be consistent with the finding in Cuba that those with more advanced dementia had higher meat intakes. This association may also have arisen from information bias because those with dementia may have been selectively likely to over- or underreport their dietary exposure to meat or fish. Random errors in reporting of dietary exposures will also have occurred and may have led to an underestimate of the true associations. Attrition was avoided in our one-phase design (41), and high response rates were obtained in all countries. We cannot exclude the possibility of selection bias, which might explain our findings if those without dementia reporting higher fish intakes were more likely to participate in the study. However, in our experience, healthy persons were less available than impaired persons for interviews; moreover, older persons in LAMICs may have scant awareness of the health-related properties of fish.
Our results extend previous findings on the beneficial effects of fish consumption on dementia in LAMICS and provide preliminary evidence of the etiological significance of diet in dementia. More substantive evidence will come from the incidence phase of our project, in which we will be able to compare the incidence of dementia according to dietary exposure at baseline (30), and from randomized controlled trials of the effectiveness of n−3 LCP supplementation for the prevention of cognitive decline (19, 20).
Acknowledgments
The authors' responsibilities were as follows—MJP: leader of the 10/66 Dementia Research Group; CF and EA: research coordinators; JLdR (Cuba), DA (Dominican Republic), MG (Peru), AS (Venezuela), ALS (Mexico), KSJ (Vellore, India), JW (Chennai, India), and YH (China): principal investigators responsible for the fieldwork in their countries; EA: wrote the first draft of the manuscript and conducted the analyses; and ADD, MJP, and RU: extensively revised the manuscript at all stages. All other authors reviewed the report and provided further contributions and suggestions. There were no conflicts of interest to declare.
REFERENCES
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llibre Rodriguez JJ, Ferri CP, Acosta D, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet 2008;372:464–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prince M, Graham N, Brodaty H, et al. Alzheimer Disease International's 10/66 Dementia Research Group—one model for action research in developing countries. Int J Geriatr Psychiatry 2004;19:178–81 [DOI] [PubMed] [Google Scholar]
- 4.Prince M, Livingston G, Katona C. Mental health care for the elderly in low-income countries: a health systems approach. World Psychiatry 2007;6:5–13 [PMC free article] [PubMed] [Google Scholar]
- 5.Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev 2006;64:S24–33(discussion S72–91) [DOI] [PubMed] [Google Scholar]
- 6.Connor WE, Connor SL. The importance of fish and docosahexaenoic acid in Alzheimer disease. Am J Clin Nutr 2007;85:929–30 [DOI] [PubMed] [Google Scholar]
- 7.Friedland RP. Fish consumption and the risk of Alzheimer disease: is it time to make dietary recommendations? Arch Neurol 2003;60:923–4 [DOI] [PubMed] [Google Scholar]
- 8.Whalley LJ, Deary IJ, Starr JM, et al. n−3 Fatty acid erythrocyte membrane content, APOE e4, and cognitive variation: an observational follow-up study in late adulthood. Am J Clin Nutr 2008;87:449–54 [DOI] [PubMed] [Google Scholar]
- 9.Ortega RM, Requejo AM, Andres P, et al. Dietary intake and cognitive function in a group of elderly people. Am J Clin Nutr 1997;66:803–9 [DOI] [PubMed] [Google Scholar]
- 10.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 2004;62:275–80 [DOI] [PubMed] [Google Scholar]
- 11.Barberger-Gateau P, Letenneur L, Deschamps V, Peres K, Dartigues JF, Renaud S. Fish, meat, and risk of dementia: cohort study. BMJ 2002;325:932–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n−3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 2003;60:940–6 [DOI] [PubMed] [Google Scholar]
- 13.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol 2005;62:1849–53 [DOI] [PubMed] [Google Scholar]
- 14.Huang TL, Zandi PP, Tucker KL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology 2005;65:1409–14 [DOI] [PubMed] [Google Scholar]
- 15.van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n−3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr 2007;85:1142–7 [DOI] [PubMed] [Google Scholar]
- 16.Laurin D, Verreault R, Lindsay J, Dewailly E, Holub BJ. Omega-3 fatty acids and risk of cognitive impairment and dementia. J Alzheimers Dis 2003;5:315–22 [DOI] [PubMed] [Google Scholar]
- 17.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Diet and risk of dementia: does fat matter?: the Rotterdam Study. Neurology 2002;59:1915–21 [DOI] [PubMed] [Google Scholar]
- 18.van de Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 2008;71:430–8 [DOI] [PubMed] [Google Scholar]
- 19.Dangour AD, Clemens F, Elbourne D, et al. A randomised controlled trial investigating the effect of n−3 long-chain polyunsaturated fatty acid supplementation on cognitive and retinal function in cognitively healthy older people: the Older People And n−3 long-chain polyunsaturated fatty acids (OPAL) study protocol. Nutr J 2006;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillette-Guyonnet S, Andrieu S, Dantoine T, Dartigues JF, Touchon J, Vellas B. Commentary on “a roadmap for the prevention of dementia II. Leon Thal Symposium 2008.” The Multidomain Alzheimer Preventive Trial (MAPT): a new approach to the prevention of Alzheimer's disease. Alzheimers Dement 2009;5:114–21 [DOI] [PubMed] [Google Scholar]
- 21.Lim WS, Gammack JK, Van Niekerk J, Dangour AD. Omega 3 fatty acid for the prevention of dementia. Cochrane Database Syst Rev 2006;CD005379. [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296:1885–99 [DOI] [PubMed] [Google Scholar]
- 23.Yaffe K, Barrett-Connor E, Lin F, Grady D. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol 2002;59:378–84 [DOI] [PubMed] [Google Scholar]
- 24.Mielke MM, Zandi PP, Sjogren M, et al. High total cholesterol levels in late like associated with reduced risk of dementia. Neurology 2005;64:1689–95 [DOI] [PubMed] [Google Scholar]
- 25.Evans RM, Hui S, Perkins A, Lahiri DK, Poirier J, Farlow MR. Cholesterol and APOE genotype interact to influence Alzheimer disease progression. Neurology 2004;62:1869–71 [DOI] [PubMed] [Google Scholar]
- 26.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology 2004;62:1573–9 [DOI] [PubMed] [Google Scholar]
- 27.Morris MC, Evans DA, Tangney CC, et al. Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Arch Neurol 2006;63:1085–8 [DOI] [PubMed] [Google Scholar]
- 28.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol 1997;42:776–82 [DOI] [PubMed] [Google Scholar]
- 29.Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol 1997;145:33–41 [DOI] [PubMed] [Google Scholar]
- 30.Prince M, Ferri CP, Acosta D, et al. The protocols for the 10/66 dementia research group population-based research programme. BMC Public Health 2007;7:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince M, Acosta D, Chiu H, Scazufca M, Varghese M. Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet 2003;361:909–17 [DOI] [PubMed] [Google Scholar]
- 32.EpiData EpiData Entry, data management and basic Statistic Analysis System. Lauritsen JM, Odense, Denmark: EpiData Association, 2000–2008 [Google Scholar]
- 33.SPSS SPSS software version 15.0. Chicago, IL: SPSS Inc, 2004. Chicago, IL: SPSS Inc, 2004 [Google Scholar]
- 34.STATA Stata statistical software: release 10. College Station, TX: StataCorp, LP, 2007 [Google Scholar]
- 35.Copeland JR, Dewey ME, Griffiths-Jones HM. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol Med 1986;16:89–99 [DOI] [PubMed] [Google Scholar]
- 36.WHO Clinical descriptions and diagnostic guidelines, MNH/MEP/87.1. In: WHO, ed. Tenth revision of the International Classification of Diseases Geneva, Switzerland: WHO, 1987 [Google Scholar]
- 37.European Society of Hypertension. 2002. Available from: www.eshonline.org (cited January 2009) [Google Scholar]
- 38.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58 [DOI] [PubMed] [Google Scholar]
- 39.Nurk E, Drevon CA, Refsum H, et al. Cognitive performance among the elderly and dietary fish intake: the Hordaland Health Study. Am J Clin Nutr 2007;86:1470–8 [DOI] [PubMed] [Google Scholar]
- 40.Lawlor DA, Davey Smith G, Kundu D, Bruckdorfer KR, Ebrahim S. Those confounded vitamins: what can we learn from the differences between observational versus randomised trial evidence? Lancet 2004;363:1724–7 [DOI] [PubMed] [Google Scholar]
- 41.Prince M. Commentary: two-phase surveys. A death is announced; no flowers, please. Int J Epidemiol 2003;32:1078–80 [DOI] [PubMed] [Google Scholar]