Abstract
Focal splenic lesions (FSL) occur in Gaucher disease type I (GD1), but their clinical significance is not known. Previous studies estimated the prevalence of FSL at 4% (pediatric) to 33% (adult) of GD1 patients and reported an association with splenomegaly. We tested the hypothesis that the presence of FSL is associated with suboptimal response to macrophage-directed enzyme replacement therapy (ERT). Additionally we investigated whether FSL were associated with other phenotypic features of GD1. The splenic parenchyma was assessed by MRI performed for routine evaluation of GD1 in 239 consecutive GD1 patients with intact spleens. The prevalence of FSL was 18.4% (44/239). Following a mean of 3.5 years of ERT, platelet response was inferior among patients with FSL (80,700± 9,600 to 90,100±7,200/mm3, P=0.2) compared to patients without FSL in whom there was a robust platelet response: 108,600±5,670 to 150,200±6,710/mm3, P<0.001. Compared to patients without FSL, patients harboring FSL had worse thrombocytopenia (platelet count: 83,700±8,800 vs. 112,100±4,200/mm3, P=0.004), greater frequency of pre-ERT splenomegaly, and greater post-ERT splenomegaly (8.5±0.77 vs. 4.8±0.25× normal, P<0.001). Additionally, the prevalence of osteonecrosis was higher among patients with FSL compared to patients without FSL (38 vs. 20.7%, P=0.026). FSL appear to be a determinant of response to ERT, suggesting studies comparing relative efficacy of newly emerging therapies for GD1 should adjust for this factor. Moreover, occurrences of FSL coincide with more severe manifestations of GD1 such as avascular osteonecrosis.
Background
In Gaucher disease, defective activity of lysosomal glucocerebrosidase due to mutations in the GBA1 gene leads to accumulation of the substrate glucocerebroside in lysosomes of mononuclear phagocytes. The result is widespread accumulation of glucocerebroside-laden macrophages, systemic macrophage activation, and a complex phenotype involving the liver, spleen, bone, and bone marrow, and less commonly the lungs (Grabowski 2008). Gaucher disease type 1 (GD1) is characterized by the absence of neurodegenerative disease, present in the rare neuronopathic forms. Splenomegaly is the most common consequence of GBA1 deficiency. Focal splenic lesions (FSL) have been described on ultrasound in GD1 patients spanning a spectrum from single to multiple lesions with hyperechoic, hypoechoic, or mixed appearance. The incidental finding of FSL in a patient not yet diagnosed with GD1 may lead to misdiagnosis of malignancy and inappropriate splenectomy (Neudorfer et al. 1997). Previous studies using ultrasonography or MRI estimated the prevalence of FSL to be 4% (pediatric) (Patlas et al. 2002) to 33% (adult) (Lee 1982) of GD1 patients. Frequency of FSL increases with age: for example, 12/23 (52%) patients aged >18 years had splenic lesions compared to only 2/13 (8.9%) patients <18 years (Hill et al. 1992). The cause of FSL in GD1 is not known but is likely multifactorial, including focal Gaucher cell deposits, infarction, extramedullary hematopoiesis, fibrosis, and/or neovascularization.
Macrophage-directed enzyme replacement therapy (ERT) is highly effective in reversing and preventing numerous manifestations of GD1 (Grabowski 2008). However, the response is variable and the basis for this variability is not understood (Weinreb et al. 2002; Zimran et al. 1994). Potential determinants of response to ERT include age, sex, severity score index (SSI), genotype, and spleen status. In a study by Zimran et al. in 1994, there was no correlation between the above variables and response to treatment. In a study based on ICGG registry data, patients that had undergone splenectomy had a superior platelet count response (Weinreb et al. 2002). Our anecdotal observations indicate that patients with FSL demonstrate a weaker platelet response to ERT. However it is not known whether FSL determine splenic and platelet response to ERT or if FSL can be reversed consistently by ERT. A recent report suggested that in pediatric patients these lesions can be reversed by ERT (Chippington et al. 2008), but in adults such reversal has not been reported (Neudorfer et al. 1997).
The goal of our study was to test the hypothesis that patients harboring FSL have a suboptimal platelet response to ERT. We estimated the frequency of FSL in GD1 in a large cohort of well-characterized patients, and in addition determined whether these lesions were associated with other genotypic or phenotypic features of GD1.
Methods
We performed a retrospective chart review of consecutively evaluated patients with GD1 followed for up to 12 years at the Gaucher Disease Treatment Center of Yale School of Medicine and NYU School of Medicine. For this study, the dataset was limited to consecutively evaluated patients who had intact spleens. MRI scans of 239 consecutive patients with intact spleens were performed for routine evaluation of GD1. Axial T1 dual in-phase and out-of-phase sequences were acquired at 1 cm intervals of the spleen and liver parenchyma. A chart review examining these reports ascertained presence or absence of FSL. FSLs were defined as discrete round lesions on MRI or wedge-shaped areas of signal abnormality extending to the periphery of the parenchyma. Figure 1 depicts the range of parenchymal abnormalities that are encountered and that constitute FSL. Streaky heterogeneity of spleen parenchyma depicted in Fig. 1 by a black arrow, present in isolation, was not classified as FSL; only discrete round abnormalities were classified as FSL. Osteonecrosis was ascertained from MRI appearances of bilateral femora, tibia, and of thoracolumbar spine. Method of determination of osteonecrosis is as previously described by our center (DeMayo et al. 2008). The designation of FSL was made by consensus in chart review of the radiology reports.
Fig. 1.
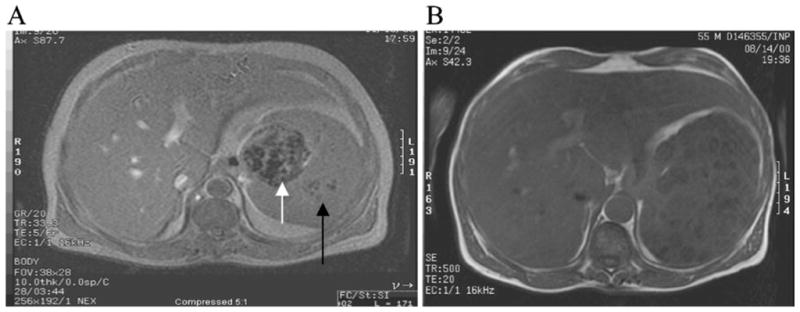
a, b MRI of two patients with splenic lesions. a Axial T1-weighted gradient-echo image through the liver and spleen demonstrates a heterogenous mass (white arrow) in the splenic hilum with focal areas of decreased signal within the lesion and within the splenic parenchyma. The black arrow represents streaky heterogeneity in isolation, which would not be regarded as an FSL. Patient presented as 46-year-old male with N370S/84GG genotype. Liver size was 2.7× normal and spleen size was 29.97× normal. Hemoglobin was 9.8 gm/dL, and platelet count was 44×109/L. He had a Hermann score of 4 and severity score index (SSI) of 14. After 8 years of ERT, spleen size was 11.8× normal with a platelet count of 78×109/L. b Axial T1-weighted spin-echo image through the liver and spleen demonstrates multiple low-signal masses diffusely through the splenic parenchyma. Patient presented as a 55-year-old male with N370S/84GG genotype. Liver size was 2.7× normal, and spleen size was 19× normal. Hemoglobin was 11.8 gm/dL and platelet count was 64×109/L. He had a Hermann score of 5 and SSI of 19
Patient demographics are summarized in Table 1. Data are described as mean ± standard error of the mean (SEM). Patients had confirmed diagnosis of GD1 based on low (i.e., <10% of normal) acid β-glucosidase activity in peripheral blood leucocytes. GBA1 genotyping was performed as described previously (Taddei et al. 2009). The frequency of numerous GD1 characteristics was examined. These findings were compared before and after ERT and stratified according to whether patients had FSL. We considered the possibility that since ischemia/infarction is the most commonly postulated mechanism underlying FSL, these lesions are likely associated with avascular osteonecrosis (AVN). Therefore, we compared the prevalence of AVN in patients with and without FSL by chi-squared test. Liver and spleen volumes were determined by volumetric MRI and converted to fold-enlargement compared to normal (× N) as previously described (Charrow et al. 1998). For these calculations, we used a normal liver volume of 2.5% of the total body weight and a normal spleen volume of 0.2% body weight (Barton et al. 1991). Baseline CBC, MRI, SSI (Zimran et al. 1992), and Hermann score (Taddei et al. 2009) were determined before ERT and after ERT. Pretreatment and posttreatment data on the appearance of spleen parenchyma were available in a total of 88 patients (54 patients without FSL and 34 patients with FSL). Mean duration of ERT was 3.57±0.34 years.
Table 1.
Baseline characteristics of all patients
| Category | FSL negative | FSL positive | P value |
|---|---|---|---|
| Age (years) | 33.0±1.5 | 41.2±2.0 | 0.002 |
| Gender (male, %) | 51.4 | 65.9 | 0.1 |
| Genotype (N370S, %) | 60.3 | 59.1 | 0.88 |
| Liver volume (× N) | 1.4±0.04 | 1.4±0.09 | 0.7 |
| Spleen volume (× N) | 10.7±0.82 | 13.6±1.94 | 0.14 |
| Hermann score | 2.0±0.1 | 2.6±0.2 | 0.013 |
| SSI | 6.0±0.24 | 7.5±0.57 | 0.019 |
| WBC (× 109/L) | 5.2±0.15 | 4.5±0.3 | 0.06 |
| Hemoglobin (gm/dL) | 12.1±0.13 | 11.9±0.314 | 0.6 |
| Platelet (× 109/L) | 112.1±4.2 | 83.7±7.4 | 0.004 |
FSL Focal splenic lesions, x N times normal, SSI severity score index, WBC white blood cells
Unless otherwise stated, values are mean ± standard error of mean. Patient age at baseline evaluation ranged from 2 to 81 years. Differences in means were assessed by the two-tailed t-test. Categorical data were analyzed by chi-squared test. If one were to use a Bonferroni correction for the multiple comparisons in this dataset, the P would need be less than 0.005 to be significant
To determine whether there was an association between extent of splenomegaly and FSL, patients were placed in categories of mild to severe splenomegaly defined as follows: 5×N, 5–10×N, 10–15×N, and >15×N. The percentage of patients with FSL in each category of severity of splenomegaly was determined.
Statistical analysis
Differences in means among the subgroups of patients with and without FSL were determined by an independent samples two-tailed t-test for equality of means for continuous data, and chi squared test was used for categorical data. The F-test (Levine’s test) for equality of variances was run first. Based on the results of the F-test, equal variances were either assumed or not assumed in the determination of whether a nonparametric test was needed. In patients who received ERT, a paired t-test was performed to determine differences in indicators of disease severity pre- and post-ERT. Our central hypothesis based on previous observations was that the presence of FSL is associated with suboptimal platelet response to ERT. We used this as our primary endpoint, and secondary aims were to define associations of FSL with other phenotypic characteristics of Gaucher disease. In these analyses, to address multiple hypothesis testing, we used the Bonferroni correction in order to decrease the chance of falsely finding significance by chance. This however may come at the cost of increasing the probability of a type II error. To be conservative, the P value of significance using a Bonferroni correction is also provided in each table.
The study was approved by the Human Investigation Committee of the Yale University of Medicine and IRB of NYU School of Medicine.
Results
The prevalence of FSL in our cohort was 18.4% (44/239). The lesions were either solitary or multiple as depicted in Fig. 1. The gender distribution and GBA1 genotype distribution of patients with and without FSL were similar (Table 1). Compared to patients without FSL, patients with FSL were older (41.2±2 vs. 33±1.5 years, P=0.002). Patient age at baseline evaluation ranged from 2 to 81 years; in patients younger than 10 years, ascertainment of FSL was performed by ultrasonography. On univariate analysis, patients with FSL had worse SSI (7.5±0.6 vs. 6±0.2, P= 0.019) and higher Hermann score (2.6±0.2 vs. 2±0.1, P= 0.013); the P value of significance after the Bonferonni correction was 0.005. There was no significant difference in baseline mean liver volume, spleen volume, white blood cell count, and hemoglobin in the two groups of patients.
Interestingly, compared to patients with no evident spleen parenchymal changes detected by MRI, those with FSL had a higher incidence of AVN (38 vs. 20.7%, P= 0.026). The most common area of osteonecrosis was at the hip in 85% of the patients.
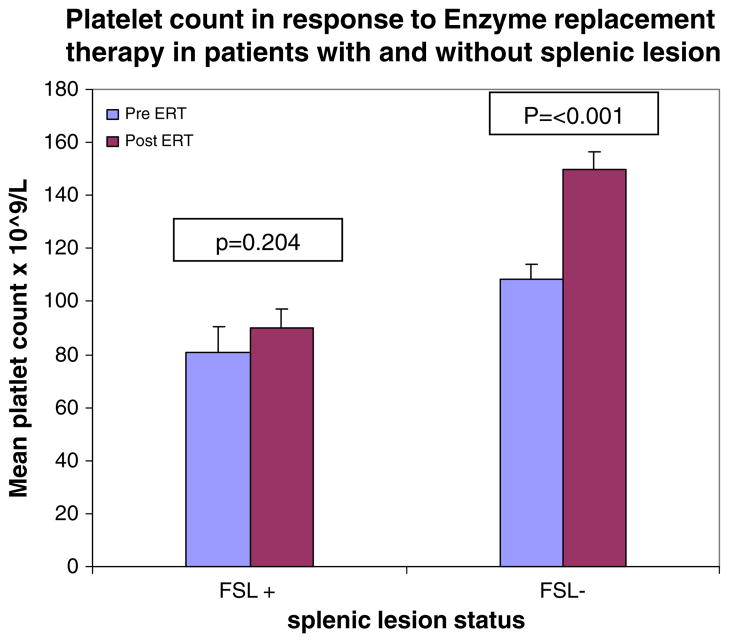
Although hemoglobin and WBC counts were normal in the two groups of patients, thrombocytopenia was worse in patients with FSL (platelet count 83,700±7,400 vs. 112,100±4,200/mm3, P=0.004). Moreover, as shown in Table 2, patients with FSL had a weak platelet response to ERT compared to patients with normal spleen parenchyma: mean pre-ERT platelet count showed a minimal nonsignificant rise from 80,700±9,600 to 90,100±7,200/mm3 (P= 0.2 by paired t-test). In contrast, patients without FSL had the expected robust response to ERT with a rise in platelets from 108,600±5,670 to 150,200±6,710/mm3 (P<0.001). ERT resulted in significant amelioration of anemia, hepatomegaly, and splenomegaly in both groups of patients. White blood cell count rose with ERT in patients with FSL, but the increase in WBC in patients without FSL was not significant.
Table 2.
Pre- and post-ERT data
| FSL positive, pre-ERT | FSL negative, post-ERT | Paired t-test, P value (pre vs post) | FSL negative, pre-ERT | FSL negative, post-ERT | Paired t-test, P value (pre vs. post) | |
|---|---|---|---|---|---|---|
| Liver volume (× N) | 1.6±0.1 | 1.2±0.05 | 0.002 | 1.6±0.07 | 1.2±0.04 | <0.001 |
| Spleen volume (× N) | 16.7±2.45 | 8.5±0.77 | 0.003 | 13.3±2.0 | 4.8±0.39 | <0.001 |
| WBC (× 109/L) | 4.3±2.4 | 8.2±0.8 | 0.004 | 4.8±0.25 | 5.2±0.21 | 0.104 |
| HGB (gm/dL) | 11.3±0.41 | 13.1±0.3 | <0.001 | 11.9±0.26 | 13.2±0.21 | <0.001 |
| Platelet (× 109/L) | 80.7±9.6 | 90.1±7.2 | 0.204 | 108.6±5.67 | 150±6.71 | <0.001 |
FSL Focal splenic lesions, ERT enzyme replacement therapy, x N times normal, WBC white blood cells, HGB hemoglobin
Pretreatment and posttreatment data are available for a total of 88 patients (54 patients without FSL and 34 patients with FSL). Mean duration of ERT was 3.57±0.34 years. P values above represent paired t-test comparing pre- and post-ERT data for each group. Upon comparing post-ERT data in patients with and without FSL, the P values are as follows: liver volume 0.368, spleen volume <0.001, white blood cell count <0.001, hemoglobin 0.899, platelet count <0.001. The P value using a Bonferroni correction would be less than 0.01
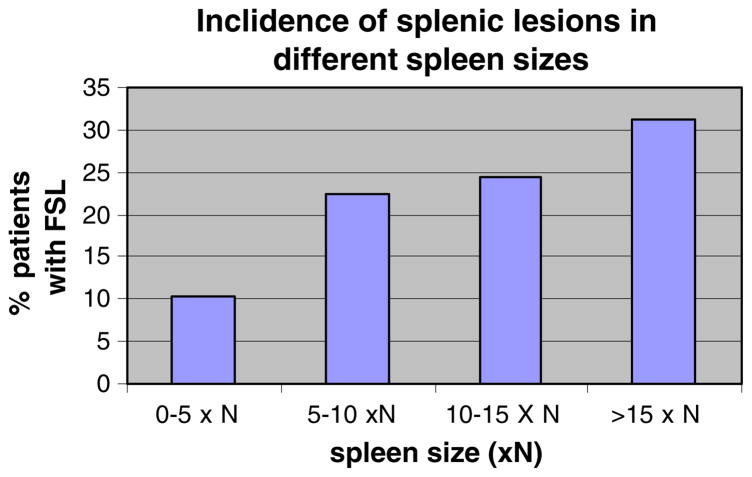
There was a trend toward an increasing percentage of patients with FSL at more severe levels of splenomegaly (Fig. 2). ERT resulted in significant reversal of splenomegaly in both groups of patients. Patients with FSL showed a 49.2% reduction in spleen size while those without FSL had a 63.9% decrease in spleen volume from baseline. Residual splenomegaly post-ERT was significantly greater in patients with FSL compared to patients without FSL.
Fig. 2.
Frequency of FSL at different levels of splenomegaly. Patients were placed in four categories of spleen size based on normal spleen size (× N). The percentage of patients with FSL in each spleen size category was determined
Extent of splenomegaly correlated negatively with platelet count in all patients (Pearson correlation r=−0.355, P<0.001) and among patients with FSL (Pearson correlation r=0.387, P=0.009). WBC count correlated negatively with splenomegaly (Pearson correlation of r=−0.344, P<0.001) in all patients, and among patients with FSL, it was r=−0.398, P= 0.008. Hemoglobin was also correlated negatively with splenomegaly (Pearson coefficient r=−0.529, P<0.001 in all patients, and among patients with FSL, it was r=−0.497, P<0.001).
Discussion
There is extreme heterogeneity of disease manifestations in GD1 that occurs among patients harboring identical GBA1 genotypes (Taddei et al. 2009; Sidransky 2004) and even among affected sib-pairs (Elstein et al. 2010). Likewise, response to ERT is highly variable, but the determinants of response are not known. The environmental, genetic, and epigenetic modifiers of the phenotype are also not known.
We found approximately 20% of patients in our cohort harbored FSL. We tested the hypothesis that presence of FSL is associated with suboptimal response to ERT. In addition we searched for associations of FSL with other phenotypic features of GD. Our study clearly reveals that the presence of FSL was associated with relatively poor platelet (Fig. 3) and splenic response to ERT, thus identifying FSL as an important determinant of responsiveness to ERT. In keeping with previous studies, we found these splenic lesions were associated with older age and extent of splenomegaly (Neudorfer et al. 1997; Patlas et al. 2002; Lee 1982; Hill et al. 1986; Chippington et al. 2008). A new finding in our study was that FSL were associated with a higher incidence of osteonecrosis.
Fig. 3.
Platelet response to enzyme replacement therapy (ERT). Patients had platelet counts determined at their most recent visit prior to ERT in both groups. Patients had post-ERT platelet results at a mean of 3.5 years after initiation of therapy. Error bars are provided and represent SEM. P values represent paired t-test comparing each group before and after therapy
In a previous study of GD1 patients with FSL, discrete hypoechoic lesions were found to correspond pathologically with focal homogeneous clusters of Gaucher cells. Several patients had splenic lesions that were hyperechoic and found to be composed of Gaucher cells and fibrosis or infarction (Hill et al. 1986). Radiological/pathological correlations in a single patient with FSL who underwent splenectomy revealed translucent nodules correlated with focal collections of Gaucher cells. Red nodules contained congested sinusoids with foci of extramedullary hematopoiesis while white nodules were mostly fibrous areas with Gaucher cells and hemosiderin deposits associated with calcification. It was suggested that these lesions arose from microcirculatory disturbances resulting in ischemia, infarction, and hemorrhage (Chatelain et al. 2002). It is not known whether FSL are associated with the classic ischemic lesion of GD1, osteonecrosis. This topic has not been previously studied.
A study in the pre-ERT era of GD1 patients reported high prevalence of FSL, i.e., 33% (16/49 patients) (Hill et al. 1986). Presence of FSL indicated a risk for severe splenic disease and the need for future splenectomy: of the 16 patients with FSL, 7 subsequently underwent splenectomy. Pathological investigations revealed that the lesions reflected variable processes: focal Gaucher cell deposits corresponded with hypoechoic lesions (in four patients), focal areas of fibrosis, and infarction; and Gaucher cells corresponded with hyperechoic areas (in two patients), extramedullary hematopoiesis (one patient), and lymphoma (one patient) (Hill et al. 1986). A more recent study reported 20% prevalence of FSL, which increased with age and the extent of splenomegaly. However, in this study there was no relationship with SSI (Neudorfer et al. 1997).
A recent study that focused on 28 pediatric patients reported a 21% incidence of FSL; interestingly the lesions resolved upon ERT (Chippington et al. 2008). These findings are at variance with those in adult patients with FSL, which appear to be irreversible with ERT (Neudorfer et al. 1997).
In keeping with previous reports, we found patients with FSL were older than those with normal splenic parenchyma (Neudorfer et al. 1997; Patlas et al 2002; Lee 1982; Hill et al. 1986; Chippington et al. 2008). This finding is consistent with development of these splenic lesions with disease progression, an aspect of the natural history which may reflect fibrotic changes resulting from chronic local inflammation triggered by lipid-laden macrophages.
We found patients harboring FSL had more severe thrombocytopenia compared with patients with normal spleen parenchyma. Moreover, after a mean of 3.5 years of ERT, platelet count was restored to normal among patients without FSL, but patients harboring FSL remained thrombocytopenic; in fact ERT did not result in a significant increase in platelet count. Based on data in the ICGG registry, by 3 years of ERT, the platelet response reaches a plateau in most patients (Weinreb et al. 2002). Presence of FSL was associated with decreased splenic and platelet response, however FSL did not affect response in other disease compartments. For example, hepatic response and response of hemoglobin were similar in the two groups of patients. Weaker platelet response and reduction of splenomegaly among patients harboring FSL are probably interrelated; it is notable however that while ERT reduced splenomegaly in patients harboring FSL, there was no change in platelet count.
In an ICGG Registry study of the response to ERT in 1,068 GD1 patients, temporal response was most rapid for hemoglobin compared to platelet response, which in turn seemed to be related to severity of thrombocytopenia at baseline (Weinreb et al. 2002). The latter finding was relevant in cases with the most severe thrombocytopenia, i.e., only 16% of patients with platelet count <60,000 ×109/L achieved normal platelet count after 24 months of ERT compared to >50% of patients with less severe thrombocytopenia. In this most severe group, although only 16% had resolution of thrombocytopenia, the mean platelet count doubled after 24 months of ERT. It should be noted that in our cohort, patients with a less severe thrombocytopenia nevertheless had a decreased platelet response. These findings further underscore the importance of FSL as a determinant of response to ERT.
Based on MRI, one study of 26 GD1 patients found half had FSL, and of these one-third represented areas of focal infarction (Hill et al. 1992), which suggests an underlying vascular etiology at least in a proportion of patients harboring FSL. In keeping with this concept, we found a higher incidence of osteonecrosis in patients harboring FSL compared to those with no such lesions.
Our study was retrospective. We did not consider ERT dose as a determinant of response. However it should be noted that the mean dose of ERT was similar in the two groups of patients. As many outcomes of ERT may be interrelated, such as FSL and increased spleen size, it is unknown whether the refractory thrombocytopenia in GD1 patients with FSL is due to persistent residual splenomegaly or due to decreased bone marrow production or both. In future prospective studies, logistic regression would enable further clarification. Reticulated platelet count could also be examined to see if platelet count was decreased due to bone marrow failure vs. splenic sequestration.
In conclusion, our study is the first to demonstrate that compared to patients with normal spleen parenchyma, patients with FSL had poor platelet and splenic response to ERT. The decreased effectiveness of ERT in FSL should be taken into consideration by physicians, especially when platelet counts and splenomegaly do not improve as rapidly as expected. Moreover, this factor should be considered when comparing relative efficacies of new emerging therapies for GD1.
Acknowledgments
Contract grant sponsor An NIDDK K24DK066306 Mid-career Clinical Investigator Award supported P.K.M. P.K.M. and G.M.P. receive research support from Genzyme Corporation for participation in the International Gaucher Registry (ICGG). P.S. is supported by a Lysosomal Storage Disease Fellowship Award from Genzyme Corporation.
We are grateful to our patients for their participation in these studies. P.K.M. was supported by an NIDDK K24DK066306 Mid-Career Clinical Investigator award. P.S. was supported by a Lysosomal Storage Fellowship Award from Genzyme Corporation.
Footnotes
Competing interests: None declared
Presented at LDN WORLD symposium Feb 2010. Abstract published as Stein P, Mistry PK. The significance of focal splenic lesions (FSL) in Gaucher disease. Molecular Genetics and Metabolism Feb 2010; 99:S35.
Contributor Information
Philip Stein, Email: philip.stein@yale.edu, Department of Pediatrics, National Gaucher Disease Treatment Center, Yale University School of Medicine, 333 Cedar Street, LMP 4093, New Haven, CT 06562, USA.
Advitya Malhotra, Email: admalhot@utmb.edu, Department of Medicine, The University of Texas Medical Branch, Galveston, TX, USA.
Andrew Haims, Email: andrew.haims@yale.edu, Department of Radiology, National Gaucher Disease Treatment Center, Yale University School of Medicine, 333 Cedar Street, New Haven, CT 06562, USA.
Gregory M. Pastores, Email: gregory.pastores@nyumc.org, Department of Neurology, NYU School of Medicine, New York, NY, USA
Pramod K. Mistry, Email: pramod.mistry@yale.edu, Pediatric Gastroenterology and Hepatology, National Gaucher Disease Treatment Center, Yale University School of Medicine, P.O. Box 208064, 333 Cedar Street, LMP 4093, New Haven, CT 06520, USA
References
- Barton NW, Brady RO, Dambrosia JM, et al. Replacement therapy for inherited enzyme deficiency—macrophage-targeted glucocerebrosidase for Gaucher’s disease. N Engl J Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Charrow J, Esplin JA, Gribble TJ, et al. Gaucher disease: recommendations on diagnosis, evaluation, and monitoring. Arch Intern Med. 1998;158:1754–60. doi: 10.1001/archinte.158.16.1754. [DOI] [PubMed] [Google Scholar]
- Chatelain D, Bralet MP, Brière J, et al. Multiple splenic nodules revealing Gaucher’s disease. Histopathology. 2002;40:203–4. doi: 10.1046/j.1365-2559.2002.1179c.x. [DOI] [PubMed] [Google Scholar]
- Chippington S, McHugh K, Vellodi A. Splenic nodules in paediatric Gaucher disease treated by enzyme replacement therapy. Pediatr Radiol. 2008;38:657–60. doi: 10.1007/s00247-008-0811-3. [DOI] [PubMed] [Google Scholar]
- DeMayo RF, Haims AH, McRae MC, Yang R, Mistry PK. A correlation of MRI-based bone marrow burden score with genotype and spleen status in Gaucher’s disease. Am J Roentgenol. 2008;191:115–23. doi: 10.2214/AJR.07.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstein D, Gellman A, Altarescu G, et al. Disease severity in sibling pairs with type I Gaucher disease. J Inherit Metab Dis. 2010;33:79–83. doi: 10.1007/s10545-009-9024-7. [DOI] [PubMed] [Google Scholar]
- Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372:1263–71. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- Hill SC, Damaska BM, Ling AL, et al. Gaucher disease: abdominal MR imaging findings in 46 patients. Radiology. 1992;184:561–566. doi: 10.1148/radiology.184.2.1620865. [DOI] [PubMed] [Google Scholar]
- Hill SC, Reinig JW, Barranger JA, Fink J, Shawker TH. Gaucher disease: sonographic appearance of the spleen. Radiology. 1986;160:631–634. doi: 10.1148/radiology.160.3.3526400. [DOI] [PubMed] [Google Scholar]
- Lee RE. The pathology of Gaucher disease. Prog Clin Biol Reg. 1982;95:177–21. [PubMed] [Google Scholar]
- Neudorfer O, Hadas-Halpern I, Elstein D, Abrahamov A, Zimran A. Abdominal ultrasound findings mimicking hematological malignancies in a study of 218 Gaucher patients. Am J Hematol. 1997;55:28–34. doi: 10.1002/(sici)1096-8652(199705)55:1<28::aid-ajh5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Patlas M, Hadas-Halpern I, Abrahamov A, Elstein D, Zimran A. Spectrum of abdominal sonographic findings in 103 pediatric patients with Gaucher disease. Eur Radiol. 2002;12:397–400. doi: 10.1007/s003300101031. [DOI] [PubMed] [Google Scholar]
- Sidransky E. Gaucher disease: complexity in a simple disorder. Mol Genet Med. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Taddei TH, Kacena KA, Yang M, et al. The underrecognized progressive nature of N370S Gaucher disease and assessment of cancer risk in 403 patients. Am J Hematol. 2009;84:208–14. doi: 10.1002/ajh.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb NJ, Charrow J, Andersson HC, et al. Effectiveness of enzyme replacement therapy in 1028 patients with type 1 Gaucher disease after 2 to 5 years of treatment: a report from the Gaucher Registry. Am J Med. 2002;113:112–119. doi: 10.1016/s0002-9343(02)01150-6. [DOI] [PubMed] [Google Scholar]
- Zimran A, Kay A, Gelbart T, et al. Gaucher disease. Clinical, laboratory, radiologic, and genetic features of 53 patients. Medicine (Baltimore) 1992;71(6):337–53. [PubMed] [Google Scholar]
- Zimran A, Elstein D, Kannai R, et al. Low-dose enzyme replacement therapy for Gaucher’s disease: effects of age, sex, genotype, and clinical features on response to treatment. Am J Med. 1994;97:3–13. doi: 10.1016/0002-9343(94)90042-6. [DOI] [PubMed] [Google Scholar]