Abstract
Background
Metastasis to the lung may be the final step in the breast cancer-related morbidity. Conventional therapies such as chemotherapy and surgery are somewhat successful, however, metastasis-related breast cancer morbidity remains high. Thus, a novel approach to prevent breast tumor metastasis is needed.
Methodology/Principal Finding
Aerosol of lentivirus-based small hairpin osteopontin was delivered into mice with breast cancer twice a week for 1 or 2 months using a nose-only inhalation system. The effects of small hairpin osteopontin on breast cancer metastasis to the lung were evaluated using near infrared imaging as well as diverse molecular techniques. Aerosol-delivered small hairpin osteopontin significantly decreased the expression level of osteopontin and altered the expression of several important metastasis-related proteins in our murine breast cancer model.
Conclusion/Significance
Aerosol-delivered small hairpin osteopontin blocked breast cancer metastasis. Our results showed that noninvasive targeting of pulmonary osteopontin or other specific genes responsible for cancer metastasis could be used as an effective therapeutic regimen for the treatment of metastatic epithelial tumors.
Introduction
Osteopontin (OPN) is a secreted glycophosphoprotein that is believed to play a role in several apparently distinct cellular processes [1]. High-level OPN expression is one of the characteristics often associated with metastatic cancer cells [2]–[4]. As such, the metastatic activity of various cancer cells can be significantly inhibited by downregulation of OPN expression [5]–[8]. Many studies have shown a correlation between OPN and the progression and severity of many cancers, including breast, colon, lung, and prostate cancers. Particularly, elevated OPN levels and poor prognoses are highly associated with metastatic breast cancer [9], [10]. Moreover, the lungs are one of the most susceptible organs to breast cancer metastasis, which can differ in terms of evolution, treatment, morbidity, and mortality [11].
Recent studies have shown that RNA interference (RNAi)-mediated reduction of OPN expression may have therapeutic efficiency for many types of cancers [12.13]. RNAi, which can be induced in mammalian cells by small hairpin RNAs (shRNAs), is an evolutionarily conserved surveillance mechanism that targets double-stranded RNAs (dsRNAs) by sequence-specific silencing of homologous genes [14]. Gene therapy involving RNAi is an attractive strategy for the development of effective anticancer therapies due to its low level of toxicity. However, low gene transfection efficiency as well as technical difficulties associated with delivery regimen hinder the practical application of in vivo gene delivery. Especially, such problems clearly manisfest with regards to gene therapy to the lung [15].
To solve this, aerosol delivery distributes material uniformly and represents a noninvasive alternative for targeting genes to the lung. In fact, our group has demonstrated that viral as well as nonviral carrier-mediated gene delivery via inhalation may provide a means of treatment for a wide range of pulmonary disorders and offer numerous advantages over invasive modes of delivery [16], [17], [18].
Our successful noninvasive aerosol gene delivery system featuring shRNA-mediated RNAi has prompted us to develop a novel approach for the prevention of lung metastasis of breast cancer. In this study, shOPN was delivered into the lungs of mice having breast cancer via a nose-only aerosol delivery system in order to determine the preventive effects of suppressed OPN in breast cancer metastasis to the lung. Here, we report that aerosol-delivered shOPN blocked the metastasis of breast cancer to the lung. Our results envision that noninvasive targeting of pulmonary OPN or other specific genes responsible for cancer metastasis may constitute an effective therapeutic regimen for the treatment of metastatic epithelial tumors.
Materials and Methods
Lentivirus Construct for shOPN
The shRNA sequence targeting mouse OPN mRNA was designed. The sequence for knockdown of murine OPN expression was 5′-CGAGGTGATAGCTTGGCTTAT-3′. The scrambled sequence 5′-AAUCGCAUAGCGUAUGCCG-3′ was used as a control. shRNA was generated based on the above siRNA sequence and cloned into the pENTR/U6™ entry vector (Invitrogen, Carlsbad, CA, USA). Cassettes containing a U6 promoter and the shRNA target sequences were transferred into a lentivirus vector (pLenti6/BLOCK-iT™-DEST vector) by following the manufacturer's instructions. Recombinant lentiviral vectors were packaged using the ViraPower™ Lentiviral Packaging Mix (Invitrogen), after which the virus titer was determined using a HIV-1 p24 ELISA KIT (PerkinElmer Life Sciences, Boston, MA, USA).
In Vivo Aerosol Delivery of Lentiviral shOPN
Five-week-old female Balb/c nude mice were purchased from Joongang Laboratory Animal Inc (Seoul, Korea). Animals were kept in the laboratory animal facility at a constant temperature and relative humidity of 23±2°C and 50±20%, respectively, under a 12 h light/dark cycle. Balb/c nude mice were injected with MDA-MB 231 cells (1×106 cells/each in PBS) (Korean Cell Line Bank, Seoul, Korea) in the mammary fat pad. After 2 weeks [19], [20], the mice were divided into 3 groups (the control group was left untreated while the other two groups were exposed to aerosol containing lentivirus-shOPN or lentivirus-scrambled) [16], [17], [18]. The mice were then placed in a nose-only inhalation chamber and exposed to aerosol containing lentivirus solution (50 ml) containing 40 ng/ml of lentivirus-shOPN or scrambled control twice a week for 1 or 2 months, respectively. At the end of each time period, the mice were sacrificed and lungs were collected. During necropsy, neoplastic lesions on the lung surfaces were carefully counted under a microscope, as described by Singh et al [21]. All methods used in this study were approved by the Animal Care and Use Committee at Seoul National University (SNU-100303-1).
Western Blot Analysis
Protein concentration of the homogenized lysates was measured using a Bradford kit (Bio-Rad, Hercules, CA, USA). Equal amounts (50 µm) of protein were separated by SDS–PAGE (10–15%) and transferred onto nitrocellulose membranes (Amersham Pharmacia, Cambridge, UK). After membranes were blocked in T-TBS containing 5% skim milk for 1 h, immunoblotting was performed by incubation overnight at 4°C with primary antibodies corresponding to OPN (R&D system, Minneapolis, MN, USA), CD44v6 (Millipore, Billerica, MA, USA), MMP-9 (Abcam, Billerica, MA, USA), MMP-2 (Novus, Littleton, CO, USA), VEGF (Santa Cruz, CA, USA), and PCNA (Santa Cruz, CA, USA) diluted 1∶1000 v/v in 5% skim milk, followed by incubation with second antibodies conjugated to horseradish peroxidase (HRP) for 3 h at room temperature or overnight at 4°C. After washing, the bands-of-interests were analyzed using a luminescent image analyzer LAS-3000 (Fujifilm, Tokyo, Japan).
Histopathological Examinationand Immunohistochemistry (IHC)
After formalin-fixation, paraffin-embedded tissue sections were cut to 5 mm and transferred to Plus slides (Fisher Scientific, Pittsburgh, PA, USA). For histological analysis, tissue sections were stained with hematoxylin and eosin (H&E). For IHC, the tissues were deparaffinized in xylene (10 min ×2 times) and rehydrated through an alcohol gradient (each 5 min). The slides were then boiled in TE buffer (pH 9.0) for retrieval process, washed in tap water, and incubated in 3% hydrogen peroxide (AppliChem, Darmstadt, Germany) for 30 min to quench endogenous peroxidase activity. After washing in phosphate-buffered saline (PBS), the tissue sections were incubated with 3% bovine serum albumin in PBS for 1 h at room temperature in order to block unspecific binding sites. Primary antibodies were applied to tissue sections overnight at 4°C. On the second day, tissue slides were washed and incubated with secondary HRP-conjugated antibodies for 1 h at room temperature. After washing, tissue sections were counterstained with Mayer's Hematoxylin (DAKO, Carpinteria, CA, USA) and washed with xylene. Cover slips were then mounted using Permount (Fisher Scientific), and the slides were reviewed using a light microscope (Carl Zeiss, Thornwood, NY, USA). Staining of PCNA and OPN for quantification by IHC analysis was performed using the In Studio version 3.01 program (Pixera, San Jose, CA, USA). Staining intensity was assessed by counting the number of positive cells in randomly selected fields viewed at appropriate magnification through the objective lens.
Wound Healing Assay
MDA-MB-231 cells were cultured to sub-confluence in 6-well plates. Streaks were made in the monolayer culture using 10 µl pipette tips. After washing away suspended cells, the remaining cells were treated with medium containing lentivirus-shOPN and lentivirus-scrambled. The progress of migration was photographed immediately 24 h after wounding.
Statistical Analysis
The results of the Western blot analysis, IHC, and wound healing assay are expressed as the mean ± S.E.M of 3 independent experiments. Statistical analyses were performed following analysis of Student's t-test whenever the data consisted of only two groups. Quantification of Western blot analysis was performed using the Multi Gauge version 3.0 program (Fujifilm).
Results
Aerosol Delivery of Lentivirus-shOPN Decreases Breast Cancer Metastasis to Lung
To determine whether or not aerosol delivery of shOPN affects breast cancer metastasis to the lung, lentivirus-shOPN was delivered twice a week for 1 or 2 months to breast cancer model mice. Gross morphological examination demonstrated that shOPN decreased lung tumor mass in the shOPN group compared to the control and scrambled group (Fig. 1A). Suppression of lung tumor metastasis was further confirmed by histolopathological analysis (Fig. 1B). Nodules formed in the lungs of the control and scrambled group were typical adenocarcinomas. However, the shOPN group demonstrated only a tumor mass, which was mostly likely an adenoma or hyperplasia. Such preventive effects against lung metastasis of breast cancer are clearly summarized in Table 1.
Figure 1. Lentivirus-shOPN decreases metastasis to the lung.
(A) Gross morphological examination of the lung after aerosol delivery of shOPN for 1 or 2 months. White circles indicate neoplastic nodules observed in the lung. (B) Histopathological examination of lung after aerosol delivery of shOPN for 1 or 2 months. Magnification (×200) scale bar: 50 µm.
Table 1. Summary of tumor incidence in mouse model of metastatic breast cancer.
| Group | Number of mouse | Tumor numbers/mouse | Adenocarcinomaincidence | Adenoma | ||||
| Total | >1.5 mm¶ | <1.5 mm£ | ++$ | +¥ | ||||
| Control | 4 | 6.25±0.87 | 1.5±1.73 | 4.75±1.94 | 2 | 1 | 1 | |
| 1month | scrambled | 4 | 6±2.61 | 2±1.83 | 4±1.08 | 1 | 1 | 2 |
| shOPN | 4 | 2.13±1.03** | N.D. | 2.13±1.03## | N.D. | 2 | ||
| 2months | scrambled | 4 | 9.25±0.87 | 2.5±1.29 | 6.75±0.87 | 1 | 1 | 2 |
| shOPN | 4 | 2.38±0.63## ** | N.D. | 2.38±0.63## | N.D. | 2 | ||
Difinition of abbreviations: shOPN = small hairpin Osteopontin. N.D. = Not Detected.
Breast cancer model mice were exposed to lentivirus-shOPN twice a week for 1 or 2 months. After 1 or 2 months, the mice were sacrificed, lungs were collected, and the lesions on the lung surfaces were counted. Incidence and tumor number of lung mass were compared. Data were expressed as means ± S.E.M.
: Number of tumors of smaller than 1.5 mm in diameter.
: Number of tumors of greater than 1.5 mm in diameter.
: Adenocarcinoma grade was mild.
: Adenocarcinoma grade was moderate.
: p<0.01 compared to scrambled group.
**: p<0.01 compared to control group.
Aerosol Delivery of shOPN Decreases OPN Expression in the Lung
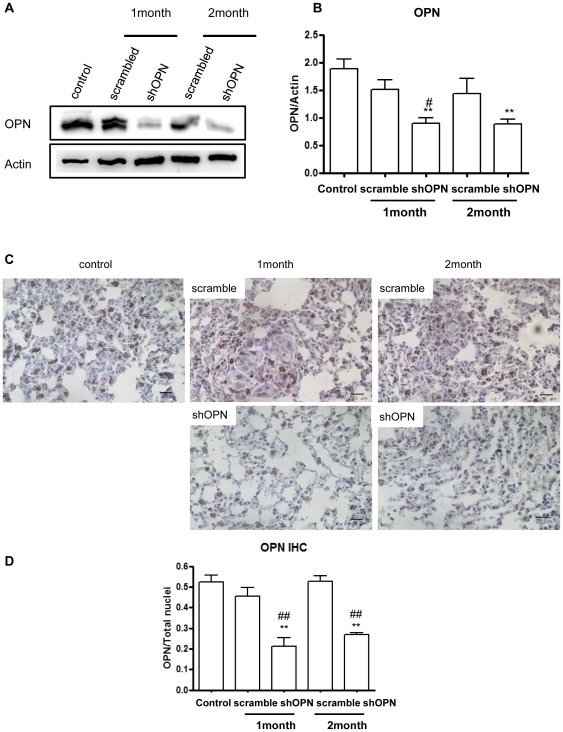
To investigate the expression level of OPN in the lungs of breast cancer model mice after shOPN aerosol delivery, Western blot analysis was carried out. Lentiviral shOPN significantly decreased expression of OPN in the lungs of breast cancer model mice (Fig. 2A). Quantification of the bands-of-interest reconfirmed the Western blot results (Fig. 2B). IHC analysis also demonstrated that aerosol-delivered shOPN decreased expression of OPN compared to the control and scrambled group (Fig. 2C). Relative staining intensity for OPN clearly confirmed this finding (Fig. 2D).
Figure 2. Effect of Aerosol-delivered shOPN on expression level of OPN.
(A) Western blot analysis of OPN. (B) Densitometric analysis of OPN. (C) Immunohistochemical analysis of OPN. Magnification: ×400. Scale bar: 20 µm. (D) Comparison of OPN labeling index. Each bar represents the mean± S.E.M (n = 3). *Statistically significant different (P<0.05) compared to control group. **Statistically different (P<0.01) compared to control group. #Statistically different (P<0.05) compared to scrambled control. ##Statistically different (P<0.01) compared to scrambled control.
Aerosol Delivery of shOPN Suppresses Tumor Invasion and Angiogenesis
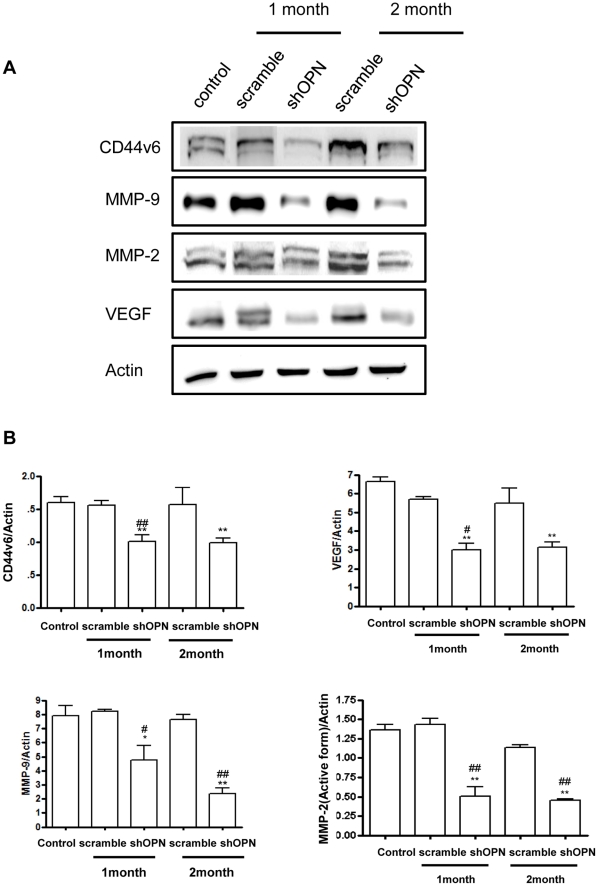
Angiogenesis, the growth of new blood vessels, is known to promote tumor progression and metastasis [22]. Tumor invasion involves tumor cell penetration or infiltration into adjacent tissue and is related to the onset of metastasis [23]. In this study, we examined changes in the expression of angiogenesis- and invasion-related proteins by Western blotting. Our results clearly showed that aerosol delivery of shOPN significantly decreased the expression levels of CD44v6, VEGF, MMP-2, and MMP-9, as observed by densitometric analysis (Fig. 3A and 3B).
Figure 3. Western Blot Analysis of CD44v6, VEGF, MMP-9, and MMP-2.
(A) Expression of CD44v6, VEGF, MMP-2, and MMP-9 in the lung. (B) Bands-of-interest were further analyzed by densitometry. Each bar represents the mean± S.E.M (n = 3). *Statistically different (P<0.05) compared to control group. **Statistically different (P<0.01) compared to control group. #Statistically different (P<0.05) compared to scrambled control. ##Statistically different (P<0.01) compared to scrambled control.
Aerosol Delivery of shOPN Suppresses Cell Proliferation in the Lung
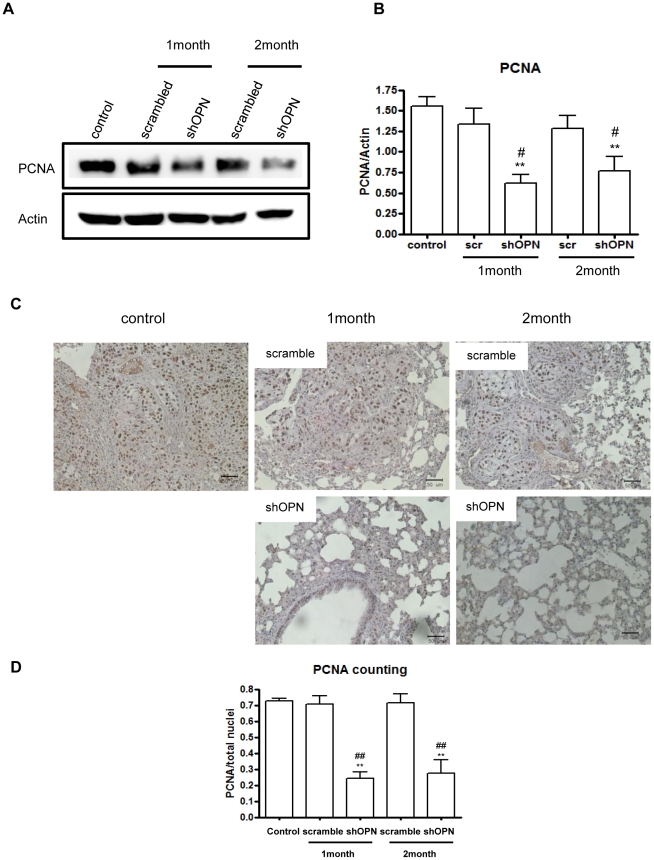
Expression of proliferating cell nuclear antigen (PCNA) is increased in several types of cancers. Moreover, it has been postulated as a useful prognostic parameter for certain cancers [24]. Therefore, we determined the expression level of PCNA by Western blot analysis, which revealed that aerosol-delivered shOPN decreased PCNA expression in the lung of breast cancer model mice (Fig. 4A). Densitometric analysis further confirmed the Western blot results (Fig. 4B). Furthermore, IHC analysis showed that aerosol-delivered shOPN significantly decreased the expression level of PCNA compared to the control and scrambled group (Fig. 4C). The relative staining intensity also clearly confirmed this (Fig. 4D).
Figure 4. Effect of Aerosol-delivered shOPN on expression level of PCNA.
(A) Western blot of PCNA. (B) Densitometric analysis of PCNA. (C) Immunohistochemical analysis of PCNA. Magnification: ×400. Scale bar: 20 µm. (D) Comparison of PCNA labeling index. Each bar represents the mean± S.E.M (n = 3). *Statistically different (P<0.05) compared to control group. **Statistically different (P<0.01) compared to control group. #Statistically different (P<0.05) compared to scrambled control. ##Statistically different (P<0.01) compared to scrambled control.
shOPN Inhibits Cell Migration
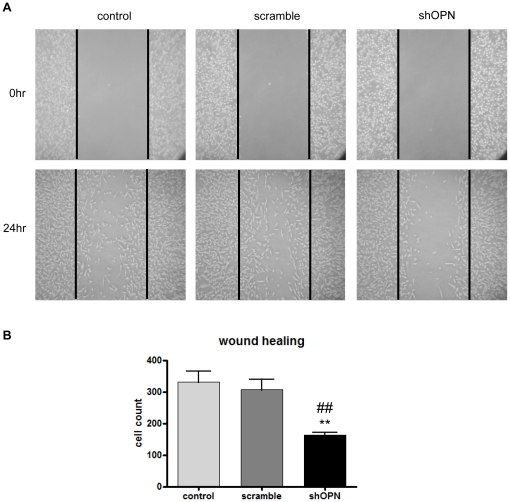
To investigate whether or not inhibition of OPN expression would reduce the migration of breast adenocarcinoma cells (MDA-MB231), wound healing assay was carried out. Our results showed that shOPN reduced the number of migrated cells after 24 h. Specifically, lentivirus-shOPN significantly inhibited motility of MDA-MB231 cells compared to the control and scrambled group (Fig. 5B).
Figure 5. Wound Healing Assay.
(A) Cell migration was inhibited by lentivirus-shOPN. MDA-MB 231 cells were grown up to 90% confluence, a single wound was made in the center of the cell monolayer and cell debris was removed by washing. After 24 h of incubation, the wound closure areas were visualized under an inverted microscope with a magnification ×100, (B) and the migrated cells were counted. **Statistically different (P<0.01) compared to control group. ##Statistically different (P<0.01) compared to scrambled control.
Discussion
Tumor metastasis may be divided into three different steps; separation of cancer cells from the primary tumor, circulation and migration of the cells through blood vessels to other organs, and finally, inhabitation of the cells in the target organs [25]. Many lines of evidence have reported that metastasis of breast cancer cells to the lungs probably occurs through the sentinel lymph node [26]. Therefore, metastasis to the lymph nodes can be a key prognostic factor of advanced disease status with the possibility of cancer cell migration from the original site to other more distant sites. [26], [27], [28]. In this study, with the aid of NIR imaging technology, we were able to detect the migration of cancer cells to the sentinel lymph node 2 weeks after injection of MDA-MB 231 cells into the mammary fat pad (Fig. S1). This finding is what caused us to perform aerosol gene delivery 2 weeks after cancer cell injection in order to focus on the 3rd step of metastasis for preventing development of breast cancer cells to the lung.
Different approaches to gene delivery to the lung, such as intravenous injection and intratracheal instillation, have been reported in animal models [29.30]. These strategies are invasive or may not be appropriate for targeting pulmonary tissues. However, aerosol delivery is known to be an efficient method for delivering genes directly to the lungs [16], [17], [18]. Viral vectors have been demonstrated to be highly effective for gene delivery, and among these, lentiviral vectors provide for efficient gene transfer in cells and mediate stable, high-level transgene expression both in vitro and in vivo [17], [31], [32]. Therefore, in this study, we downregulated OPN expression in breast cancer model mice via aerosol delivery of lentiviral hOPN in order to investigate whether or not aerosol-delivered shOPN decreases metastatic spread of breast cancer cells to the lung.
OPN is a matricellular protein that is expressed in different tissues in the body, but mostly in bone. It is also produced by cancer cells and is known to play a crucial role in the growth, progression, and metastasis of cancer [33]. Actually, in breast cancer progression and metastasis, OPN is known as an important mediator, and it has been investigated as a potential therapeutic target in the treatment of breast cancer [34]. Patients of breast cancer suffering metastasis to the lung are faced with limited treatment options [35]. Since secreted phosphoproteins are readily accessible in the extracellular environment, OPN has gained much attention as an attractive therapeutic target for the blockade of tumor growth and metastasis. OPN binds to certain CD44 variants and integrin receptors [36], [37]. Expression of CD44 and its variant isoforms is associated with aggressive behavior of various tumors [38], [39]. Actually, CD44 variant 6 (CD44v6) is known to be overexpressed in lung and breast cancers [40], [41]. In this regard, our results showing significant suppression of CD44v6 by shOPN (Fig. 3) holds potential that shOPN delivery may be used in the treatment of breast cancer metastasis.
VEGF is a potent angiogenic cytokine with critical roles in tumor angiogenesis. VEGF produces may biological effects such as endothelial cell mitogenesis, migration, induction of proteinases leading to remodeling of the extracellular matrix, increased vascular permeability, and maintenance of the survival of newly formed blood vessels [42]. Overexpression of VEGF has been observed in a variety of human tumors; thus, increased VEGF expression in tumors has been established as an accurate biomarker of invasiveness, vascular density, metastasis, and recurrence [42], [43], [44]. Many researchers have demonstrated that co-expression of VEGF and OPN is well correlated with angiogenesis in patients with stage I lung adenocarcinoma [45]. More importantly, patients with VEGF-positive and OPN-positive lung adenocarcinoma suffer poor results due to increased postoperative metastasis and poor prognoses [46]. MMP-2 and MMP-9 are among the known factors that promote tumor cell motility, invasiveness, and epithelial-mesenchymal transition. MMPs are known to degrade dense extracellular matrix molecules (ECM) such as laminin, collagens, fibronectin, and proteoglycans in order to create space for tumor cell migration. Moreover, MMPs are known to activate certain growth factors associated with the ECM or cell surface [47]. Our results showed that aerosol delivery of shOPN significantly decreased the expression levels of CD44v6, VEGF, MMP-2, and MMP-9, strongly suggesting that suppression of OPN by lentiviral shOPN via aerosol can effectively inhibit migration, angiogenesis, and invasion of breast cancer cells into the lung. Epithelial cell proliferation in normal and cancerous lungs can be measured efficiently by quantifying the percentage of tumor cells positive for PCNA [48]. Our results showed that aerosol-delivered shOPN significantly decreased expression of PCNA. Therefore, combined with the effective suppression of several important angiogenic factors (Fig. 3), suppression of OPN by aerosol-delivered shOPN could probably prevent breast cancer cell metastasis into the lung.
In summary, our results demonstrated that aerosol-delivered shOPN suppressed the migration and invasion of breast cancer cells, leading to reduction of metastasis from the breast to the lung. Therefore, we suggest that aerosol gene delivery may prove to be a useful noninvasive model of gene delivery and that noninvasive targeting of pulmonary OPN or other genes responsible for cancer metastasis may be an effective therapeutic regimen for the treatment of metastatic epithelial tumors.
Supporting Information
Image of mouse model of metastatic breast cancer. (A) Tagged MDA-MB 231 with NEO-LIVETM 797 (Biterials, Seoul, Korea) was injected into mouse mammary fat pad (red signals) and NIR signal detected using an image analyzer (MaestroTM, CRi, MA, USA). (B) In the mouse foot pad, NEO-LIVETM 675 (Biterials) was injected (green signals) and NIR signal detected using the same image analyzer. (C) Merged imaging picture. (D) Magnification image of indicated area (white square). (E) After NIR detection, sentinel lymph node was removed and stained with H&E. Red square indicates metastasis area of breast cancer cells (MDA-MB 231). Black dotted line indicates the boundary between invasion area and lymph node. C; Cortex, PF; Primary follicle. Scale bar: 50 μm (×200) (F) Magnified image of indicated area (red box of E). Scale bar: 20 μm (×400)
(TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Research Foundation (NRF-2010-0000784), Ministry of Education, Science and Technology (MEST), Korea. Kee-Ho Lee was supported by the 21C Frontier Functional Human Genome Project (FG03-0601-003-1-0-0) and by the National Nuclear R&D Program of the Ministry of Science and Technology. George R. Beck Jr was supported in part by grants from NIH/NIAMS (AR056090) and NIH/NCI (CA136059). Myung-Haing Cho was also partially supported by the Research Institute for Veterinary Science, Seoul National University. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Oates AJ, Barraclough R, Rudland PS. The role of osteopontin in tumorigenesis and metastasis. Invasion Metastasis. 1997;17:1–15. [PubMed] [Google Scholar]
- 2.Senger DR, Perruzzi CA, Papadopoulos A. Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res. 1989;9:1291–1299. [PubMed] [Google Scholar]
- 3.Brown LF, Papadopoulos-Sergiou A, Berse B, Manseau EJ, Tognazzi K, et al. Osteopontin expression and distribution in human carcinomas. Am J Pathol. 1994;145:610–623. [PMC free article] [PubMed] [Google Scholar]
- 4.Denhardt DT. Oncogene-initiated aberrant signaling engenders the metastatic phenotype: synergistic transcription factor interactions are targets for cancer therapy. Crit Rev Oncog. 1996;7:261–291. doi: 10.1615/critrevoncog.v7.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty G, Jain S, Patil TV, Kundu GC. Down-regulation of osteopontin attenuates breast tumour progression in vivo. Cell Mol Med. 2008;12:2305–2318. doi: 10.1111/j.1582-4934.2008.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrend EI, Craig AM, Wilson SM, Denhardt DT, Chambers AF. Expression of antisense osteopontin RNA in metastatic mouse fibroblasts is associated with reduced malignancy. Ann N Y Acad Sci. 1995;760:299–301. doi: 10.1111/j.1749-6632.1995.tb44640.x. [DOI] [PubMed] [Google Scholar]
- 7.Behrend EI, Craig AM, Wilson SM, Denhardt DT, Chambers AF. Reduced malignancy of ras-transformed NIH 3T3 cells expressing antisense osteopontin RNA. Cancer Res. 1994;54:832–837. [PubMed] [Google Scholar]
- 8.Adwan H, Bäuerle T, Najajreh Y, Elazer V, Golomb G, et al. Decreased levels of osteopontin and bone sialoprotein II are correlated with reduced proliferation, colony formation, and migration of GFP-MDA-MB-231 cells. Int J Oncol. 2004;24:1235–1244. [PubMed] [Google Scholar]
- 9.Singhal H, Bautista DS, Tonkin KS, O'Malley FP, Tuck AB, et al. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res. 1997;3:605–611. [PubMed] [Google Scholar]
- 10.Weber GF, Lett GS, Haubein NC. Osteopontin is a marker for cancer aggressiveness and patient survival. Br J Cancer. 2010;103:861–869. doi: 10.1038/sj.bjc.6605834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomayer EF, Diel IJ, Meyberg GC, Gollan C, Bastert G. Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat. 2000;59:271–278. doi: 10.1023/a:1006308619659. [DOI] [PubMed] [Google Scholar]
- 12.Deng Lei, Li Guoqiang, Xi Lisen, Yin Aihong, Gao Yun, et al. Hepatitis B virus inhibition in mice by lentiviral vector mediated short hairpin RNA. BMC Gastroenterol. 2009;9:73–82. doi: 10.1186/1471-230X-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohno K, Nishimori H, Yasoshima T, Kamiguchi K, Hata F, et al. Inhibition of osteopontin reduces liver metastasis of human pancreatic cancer xenografts injected into the spleen in a mouse model. Surg Today. 2010;40:347–356. doi: 10.1007/s00595-009-4082-x. [DOI] [PubMed] [Google Scholar]
- 14.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamora-Avila DE, Zapata-Benavides P, Franco-Molina MA, Saavedra-Alonso S, Trejo-Avila LM, et al. WT1 gene silencing by aerosol delivery of PEI–RNAi complexes inhibits B16-F10 lung metastases growth. Cancer Gene Ther. 2009;16:892–899. doi: 10.1038/cgt.2009.35. [DOI] [PubMed] [Google Scholar]
- 16.Kim HW, Park IK, Cho CS, Lee KH, Beck GR, Jr, et al. Aerosol delivery of glucosylated polyethylenimine/phosphatase and tensin homologue deleted on chromosome 10 complex suppresses Akt downstream pathways in the lung of K-ras null mice. Cancer Res. 2004;64:7971–7976. doi: 10.1158/0008-5472.CAN-04-1231. [DOI] [PubMed] [Google Scholar]
- 17.Hwang SK, Kwon JT, Park SJ, Chang SH, Lee ES, et al. Lentivirus-mediated carboxyl-terminal modulator protein gene transfection via aerosol in lungs of K-ras null mice. Gene Ther. 2007;14:1721–1730. doi: 10.1038/sj.gt.3303042. [DOI] [PubMed] [Google Scholar]
- 18.Hwang SK, Lim HT, Minai-Tehrani A, Lee ES, Park J, et al. Repeated aerosol delivery of carboxyl-terminal modulator protein suppresses tumor in the lungs of K-rasLA1 mice. Am J Respir Crit Care Med. 2009;179:1131–1140. doi: 10.1164/rccm.200810-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizzo P, Miao H, D'Souza G, Osipo C, Song LL, et al. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res. 2008;68:5226–5235. doi: 10.1158/0008-5472.CAN-07-5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leland P, Taguchi J, Husain SR, Kreitman RJ, Pastan I, et al. Human breast carcinoma cells express type II IL-4 receptors and are sensitive to antitumor activity of a chimeric IL-4-Pseudomonas exotoxin fusion protein in vitro and in vivo. Mol Med. 2000;6:165–178. [PMC free article] [PubMed] [Google Scholar]
- 21.Ravi D, Ramadas K, Mathew BS, Nalinakumari KR, Nair MK, et al. Angiogenesis during tumor progression in the oral cavity is related to reduced apoptosis and high tumor cell proliferation. Oral Oncol. 1998;34:543–548. doi: 10.1016/s1368-8375(98)00054-2. [DOI] [PubMed] [Google Scholar]
- 22.Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, et al. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006;98:846–855. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- 23.Keleg S, Büchler P, Ludwig R, Büchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Mol Cancer. 2003;22:14–20. doi: 10.1186/1476-4598-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu P, Huang B, Shen M, Lau C, Chan E, et al. p15(PAF), a novel PCNA associated factor with increased expression in tumor tissues. Oncogene. 2001;20:484–489. doi: 10.1038/sj.onc.1204113. [DOI] [PubMed] [Google Scholar]
- 25.Trono D. Lentiviral vectors: turning a deadly foe into a therapeutic agent. Gene Ther. 2000;7:20–23. doi: 10.1038/sj.gt.3301105. [DOI] [PubMed] [Google Scholar]
- 26.Jatoi I, Hilsenbeck SG, Clark GM, Osborne CK. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol. 1999;17:2334–2340. doi: 10.1200/JCO.1999.17.8.2334. [DOI] [PubMed] [Google Scholar]
- 27.Vinh-Hung V, Verschraegen C, Promish DI, Cserni G, Van de Steene J, et al. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004;6:680–688. doi: 10.1186/bcr934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veronesi U, Paganelli G, Viale G, Galimberti V, Luini A, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91:368–373. doi: 10.1093/jnci/91.4.368. [DOI] [PubMed] [Google Scholar]
- 29.Gautam A, Densmore CL, Golunski E, Xu B, Waldrep JC. Transgene expression in mouse airway epithelium by aerosol gene therapy with PEI-DNA complexes. Mol Ther. 2001;3:551–556. doi: 10.1006/mthe.2001.0300. [DOI] [PubMed] [Google Scholar]
- 30.Goula D, Becker N, Lemkine GF, Normandie P, Rodrigues J, et al. Rapid crossing of the pulmonary endothelial barrier by polyethylenimine/DNA complexes. Gene Ther. 2000;7:499–504. doi: 10.1038/sj.gt.3301113. [DOI] [PubMed] [Google Scholar]
- 31.Lotze MT, Kost TA. Viruses as gene delivery vectors: application to gene function, target validation, and assay development. Cancer Gene Ther. 2002;9:692–699. doi: 10.1038/sj.cgt.7700493. [DOI] [PubMed] [Google Scholar]
- 32.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 33.Shevde LA, Das S, Clark DW, Samant RS. Osteopontin: an effector and an effect of tumor metastasis. Curr Mol Med. 2010;10:71–81. doi: 10.2174/156652410791065381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai J, Li B, Shi J, Peng L, Zhang D, et al. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol Immunother. 2010;59:355–366. doi: 10.1007/s00262-009-0754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toloza EM, Morse MA, Lyerly HK. Gene therapy for lung cancer. J Cell Biochem. 2006;99:1–22. doi: 10.1002/jcb.20851. [DOI] [PubMed] [Google Scholar]
- 36.Denhardt DT, Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993;7:1475–1482. [PubMed] [Google Scholar]
- 37.Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paradis V, Eschwege P, Loric S, Dumas F, Ba N, et al. De novo expression of CD44 in prostate carcinoma is correlated with systemic dissemination of prostate cancer. J Clin Pathol. 1998;51:798–802. doi: 10.1136/jcp.51.11.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao YF, Li X, Xie QX, Gui SY, Wang Y, et al. Expressions of osteopontin and CD44v6 in hepatocellular carcinoma and their clinical significance. Zhonghua Gan Zang Bing Za Zhi. 2005;13:227–228. [PubMed] [Google Scholar]
- 40.Suzuki H, Yamashiro K. Reduced expression of CD44 v3 and v6 is related to invasion in lung adenocarcinoma. Lung Cancer. 2002;38:137–141. doi: 10.1016/s0169-5002(02)00176-9. [DOI] [PubMed] [Google Scholar]
- 41.Yu P, Zhou L, Ke W, Li K. Clinical significance of pAKT and CD44v6 overexpression with breast cancer. J Cancer Res Clin Oncol. 2010;136:1283–1292. doi: 10.1007/s00432-010-0779-x. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 43.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 44.Tokunaga T, Oshika Y, Abe Y, Ozeki Y, Sadahiro S, et al. Vascular endothelial growth factor (VEGF) mRNA isoform expression pattern is correlated with liver metastasis and poor prognosis in colon cancer. Br J Cancer. 1998;77:998–1002. doi: 10.1038/bjc.1998.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shijubo N, Uede T, Kon S, Nagata M, Abe S. Vascular endothelial growth factor and osteopontin in tumor biology. Crit Rev Oncog. 2000;11:135–146. [PubMed] [Google Scholar]
- 46.Akar U, Ozpolat B, Mehta K, Lopez-Berestein G, Zhang D, et al. Targeting p70S6K prevented lung metastasis in a breast cancer xenograft model. Mol Cancer Ther. 2010;9:1180–1187. doi: 10.1158/1535-7163.MCT-09-1025. [DOI] [PubMed] [Google Scholar]
- 47.Roy R, Zhang B, Moses MA. Making the cut: protease-mediated regulation of angiogenesis. Exp Cell Res. 2006;312:608–622. doi: 10.1016/j.yexcr.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 48.Thaete LG, Ahnen DJ, Malkinson AM. Proliferating cell nuclear antigen (PCNA/cyclin) immunocytochemistry as a labeling index in mouse lung tissues. Cell Tissue Res. 1989;256:167–173. doi: 10.1007/BF00224731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Image of mouse model of metastatic breast cancer. (A) Tagged MDA-MB 231 with NEO-LIVETM 797 (Biterials, Seoul, Korea) was injected into mouse mammary fat pad (red signals) and NIR signal detected using an image analyzer (MaestroTM, CRi, MA, USA). (B) In the mouse foot pad, NEO-LIVETM 675 (Biterials) was injected (green signals) and NIR signal detected using the same image analyzer. (C) Merged imaging picture. (D) Magnification image of indicated area (white square). (E) After NIR detection, sentinel lymph node was removed and stained with H&E. Red square indicates metastasis area of breast cancer cells (MDA-MB 231). Black dotted line indicates the boundary between invasion area and lymph node. C; Cortex, PF; Primary follicle. Scale bar: 50 μm (×200) (F) Magnified image of indicated area (red box of E). Scale bar: 20 μm (×400)
(TIF)