Abstract
Attention is increasingly being given to understanding sex difference in psychopathology to better understand the etiology of disorders. This study tests the hypothesis that sex differences in ventral and middle frontal gray volume contribute to sex differences in antisocial personality disorder and crime. Participants were recruited from temporary employment agencies, consisting of normal controls, substance / alcohol dependent controls, Axis I/II psychiatric controls, and individuals with antisocial personality disorder (APD). An independent sample of female volunteers was also recruited. MRI volumes of superior frontal, middle frontal, inferior frontal, orbital frontal, and rectal gyral frontal gray matter, and dimensional scores of APD and criminal behavior. APD males compared to male controls showed an 8.7 % reduction in orbitofrontal gray volume, a 17.3% reduction in middle frontal gray, and a 16.1% reduction in right rectal gray. Reduced middle and orbito-frontal volumes were significantly associated with increased APD symptoms and criminal offending in both males and females. Males as a whole had reduced orbitofrontal and middle frontal gray volume compared to females, and controlling for these brain differences reduced the gender difference in antisocial personality/behavior by 77.3%. Findings were not a function of psychiatric comorbidity, psychosocial risk factors, head injury, or trauma exposure. Findings implicate structural differences in the ventral and middle frontal gray as both a risk factor for antisocial personality disorder and as a partial explanation for sex differences in antisocial personality disorder.
Keywords: Orbitofrontal, antisocial personality, middle frontal, sex differences
Introduction
The sex difference in violence and crime is well-replicated throughout the world 1. Classic socio-cultural explanations of this sex difference have focused on the process of differential socialization, with both parent and peer group influences reinforcing aggressive and rule-breaking behavior in boys and more prosocial behavior in girls 2. While superficially attractive, reviews and empirical analyses have failed to document any convincing support for this theoretical perspective 3 4. Alternatively, it is conceivable that neurobiological processes in part account for the sex difference in antisocial behavior. One such candidate is volume of prefrontal gray matter. Reduced orbitofrontal volumes in men compared to women have been reported 5 6 7 8. Furthermore, patients who have suffered demonstrable damage to the ventral, orbitofrontal regions of the prefrontal cortex proceed to acquire an antisocial, psychopathic-like personality 9, while volume reductions in prefrontal gray matter (including orbitofrontal cortex) have been reported in several antisocial population 10 11 12 13 14.
Despite a resurgence of interest in sex differences and how they can provide critically important leads for explain the etiology of psychopathology, it has been argued that there is a surprising dearth of good research 15. More specifically, three evidential criteria are required for causation: (1) males and females differ on the risk factor, (2) this risk factor increases the risk for psychopathology within each sex, (3) when entered into a causal model, the risk factor reduces or eliminate the sex difference in psychopathology 15. They further argued that no risk variable to date has met all criteria, although one prior study found that sex differences in a composite neurocognitive measure accounted for 18% of the sex difference in antisocial behavior, providing suggestive evidence for the possibility of brain processes as an explanatory factor for the gender difference in antisocial behavior 4.
We previously demonstrated an 11% reduction in the volume of gray matter in the prefrontal cortex in males with antisocial personality disorder (APD - 10), but important questions remain. Specifically, it is not known whether: (1) the structural prefrontal impairment is localized to the orbito-frontal region or involves dorsolateral and other prefrontal regions, (2) the same ventral prefrontal impairments are found in relation to female antisocial behavior, (3) reduced orbitofrontal volume in part explains why many more men than women have antisocial personality disorder. Conceptually, if brain structural impairments both relate to antisocial behavior in both males and females and also partly account for the gender difference in antisocial personality, this would raise the status of orbitofrontal deformation as a putative etiological agent. We addressed these three primary questions by conducting volumetric assessments of subregions of the prefrontal cortex, in both male volunteers from the community with and without antisocial personality disorder, and also an unselected female group. All three evidential criteria 15 were examined to assess whether prefrontal gray volume is an etiological candidate for antisocial behavior.
Materials and Method
Subjects
A total of 90 subjects were drawn from five temporary employment agencies in Los Angeles 10. This recruitment strategy was used because pilot data had shown that this community group had relatively high rates of violence perpetration. Subject groups consisted of 18 males with a DSM-IV diagnosis of Antisocial Personality Disorder (APD), 30 male controls who had neither antisocial personality disorder nor drug / alcohol dependence, and 24 male substance dependent controls who had a lifetime diagnosis of drug or alcohol dependence, but not APD. Subjects were unselected with the exception of the following exclusion criteria: age under 21 or over 45, non-fluency in English, history of epilepsy, claustrophobia, pacemaker, and metal implants.
Normal controls, substance dependent, and APD groups did not differ on age (31.3, 30.2, 32.9 – p = .42), IQ (100.4, 98.8, 98.8 – p = .91), or ethnicity (53.3%, 58.3%, 33.3% white - p = .25) respectively. After complete description of the study to the subjects, written informed consent was obtained.
Because the APD group had comorbid clinical conditions other than alcohol and substance abuse, a Psychiatric Control group was formed by matching the 18 APDs with 18 subjects (drawn from the remained of the larger sample lacking APD – N = 78) on Axis I and II disorders. Percentages in each group (APD and Psychiatric Control groups respectively) with a lifetime history of each class of disorders were as follows: schizophrenia spectrum disorders (schizotypal, paranoid, or schizoid personality, psychosis, schizophrenia) 46.7% vs. 53.3%, affective disorders (major depression, bipolar depression) 41.2% vs 58.8%, anxiety disorders (phobia, panic, generalized anxiety) 33.3% vs 66.7.0%, other personality disorders (borderline, histrionic, narcissistic, avoidant, dependent, depressive, obsessive-compulsive) 42.9% vs 57.1%. All differences were non-significant (p > .33).
In addition to the male sample, a small sample of 12 females was also recruited from the temporary employment agencies to make sex difference comparisons and assess generalizability of findings from males to females. Females did not differ to males on age (33.9, 31.3, – p = .22), IQ (99.7, 99.5, – p = .97), social class IQ (41.0, 39.3, – p = .66), ethnicity (58.3% vs 50.0% white respectively - p = .59), but as would be anticipated from higher externalizing behavior problems in males, females had significantly lower rates of substance / alcohol dependence (16.7% vs 53.9% respectively, chi2 = 5.76, p = .016),
Diagnostic, criminal cognitive, and psychosocial assessments
All diagnoses made using DSM-IV criteria and ascertained using the Structured Clinical Interview for Axis I DSM-IV Disorders 16 and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders 17. Diagnoses were made by research assistants who had undergone a standardized training and quality assurance program for diagnostic assessment 18.
Perpetration of criminal offending was measured using the Self-Report Crime Checklist (SRCC – 10 an adult extension and update of the self-report delinquency measure used in the National Youth Survey 19. This instrument assesses 44 types of criminal offences over the life-span (e.g. burglary, theft, fraud, robbery, rape, assault) with each item rated on a three-point scale (no commission, 1-2 occasions, 3 or more occasions). It has high internal reliability (Chronbach's alpha = 0.92), good external validity in relation to APD symptom count (r = 0.69, p < .0001), and has been used in our prior studies on self-report criminal offending 20 21 10 22. To help minimize false negatives (denial of violence by truly violent offenders), a certificate of confidentiality was obtained from the Secretary of Health which protected the research investigators under section 303 (a) of the Public Health Act 42 from being subpoenaed by any Federal, State, or local court in the U.S. to release the self-report crime data. Consequently, subjects were protected from the possible legal action that could be taken against hem for crimes they committed and admitted in interview, but which were not detected and punished by the criminal justice system.
Estimated intelligence was based on five sub-tests (vocabulary, arithmetic, digit span, digit symbol, block design) of the WAIS-R (Wechsler 1981). The 10 demographic and psychosocial measures were derived from a structured psychosocial interview with the participant 10, with social class measured using the Hollingshead classification system 23.
MRI
Imaging Protocol
Structural MRIs were conducted on a Philips S15/ACS (Selton/Conn) scanner with a magnet of 1.5 Tesla field strength. Following an initial alignment sequence of one-midsagittal and four parasagittal scans (spin-echo T1-weighted image acquisition, TR = 600 ms, TE =20 ms) to identify the AC-PC plane, 128 3D T1-weighted gradient-echo coronal images (TR 34 ms, TE 12.4 ms, flip angle 35°, 1.7 mm over-contiguous slices, 256×256 matrix, FOV = 23 cm) were taken in the plane directly orthogonal to the AC-PC line.
Image Preprocessing
All image data sets were processed with a series of preparatory steps before manual delineation of prefrontal sub-regions using the LONI Pipeline Processing Environment 24. First, non-brain tissue and the cerebellum were removed from the brain images using BrainSuite 25 and small errors were corrected manually. Second, brain volumes were subjected to signal intensity inhomogeneity corrections.26.The images were then aligned and placed into a stereotaxic coordinate system of the International Consortium for Brain Mapping (ICBM; 27,28) using a rigid body transformation without scaling the brain 29, 30 Third, fully automated tissue segmentation algorithm was applied and brain voxels were automatically classified as most representative of gray matter, white matter, or cerebrospinal fluid using a validated partial volume correction method 31. Finally, for each subject, a high-resolution shape representation of the cortex was extracted using a three-dimensional active surface algorithm to aid the identification of anatomic boundaries for delineating the prefrontal sub-regions 32.
Prefrontal Region-of-Interests Delineation
The parcellation of the prefrontal cortex into five sub-regions (superior frontal, middle frontal, inferior frontal, orbito-frontal, and rectal gyri) was conducted for each hemisphere using MNI-Display, a visualization tool developed by McConnel Brain Imaging Center (http://www.bic.mni.mcgill.ca/software) following the methods detailed elsewhere 33. All anatomical delineations were traced using each individual's three-dimensional cortical surface object and all three planes of the brain images to identify sulcal line markers for each sub-region. Delineations were also verified by using three human brain atlases 34 35 36. All raters were blind to group membership and all other information on the participants. Raters conducted segmentation on participants irrespective of group membership. For inter-rater reliability, all anatomical regions were delineated on 10 randomly chosen image data sets; intra-class correlation coefficients for gray matter and white matter ranged from .90 to .97 in all five frontal sub-regions. Total brain volumes (including gray and white matter) were also extracted.
Statistical analyses
All analyses were conducted using SPSS. Primary analyses were conducted on the larger sample of males, with the small female sample employed for testing hypotheses on gender differences and independent replication. Regional specificity was examined using a 3 × 5 × 2 repeated measures multivariate analysis of variance design (three groups: normal controls, psychiatric controls, APD; five regions: superior, middle, inferior, orbital, and rectal; two hemisphere: left and right). Regional volumes were expressed as a function of whole-brain volume. Separate analyses were conducted on gray and white matter volumes. Group x region interactions were broken down using one-way ANOVAs on each region separately followed by independent t-tests. All tests of significance are two-tailed. Effect sizes were calculated using eta2 and Cohen's d. Pearson correlation coefficients were used to assess degree of relationship between brain volumes and antisocial measures separately for males and females. The ability of measures to predict group membership was assessed using logistic regression and the Wald χ2 statistic, with the Nagelkerke statistic used for variance estimation.
Results
PFC volumes in antisocial groups
Regional specificity
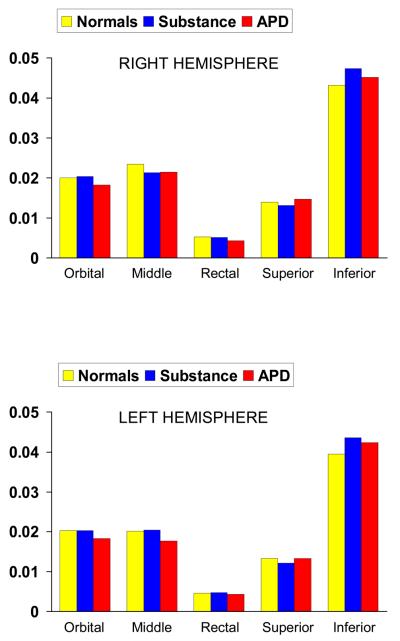
Gray volumes for all three groups across regions and hemispheres are illustrated in Figure 1. Males with APD showed significant reductions in orbito-frontal, middle frontal gray, and rectal gyral gray, but not white, volumes (see Figure 2). A repeated measures MANOVA on gray matter showed a significant group x region interaction (F (8,134) = 4.63, p < .0001, eta2 = .21). A breakdown of this interaction revealed significant main group effects for orbito-frontal gray, F (2,69) = 4.36, p = .015, eta2 = .115, middle frontal gray, F (2,69) = 6.92, p = .002, eta2 = .167, but not for superior (p = .186), inferior (p = .173), or rectal gyral (p = .131) frontal sites. APDs had significantly reduced orbitofrontal volumes compared to both Normal Controls (t = 3.0, df = 46, p = .004) and Substance Use Controls (t = 2.48, df = 40, p = .017), significantly reduced middle frontal volumes compared to both Normal Controls (t = 3.62, df = 46, p = .001) and Substance Use Controls (t = 2.81, df = 40, p = .008), and marginally significantly reduced rectal gyral volumes compared to Normal Controls (t = 1.93, df = 40, p = .06) and Substance Use Controls (t = 1.82, df = 40, p = .08). Groups did not differ on either whole brain volume, F (2,69) = 0.91, p = .41, or prefrontal white matter, F(2,69) = 0.64, p = .53.
Figure 1.
Frontal gray matter volumes (whole-brain corrected – Y axis) for left and right hemispheres in normal controls, substance dependent controls (substance), and those with antisocial personality disorder (APD).
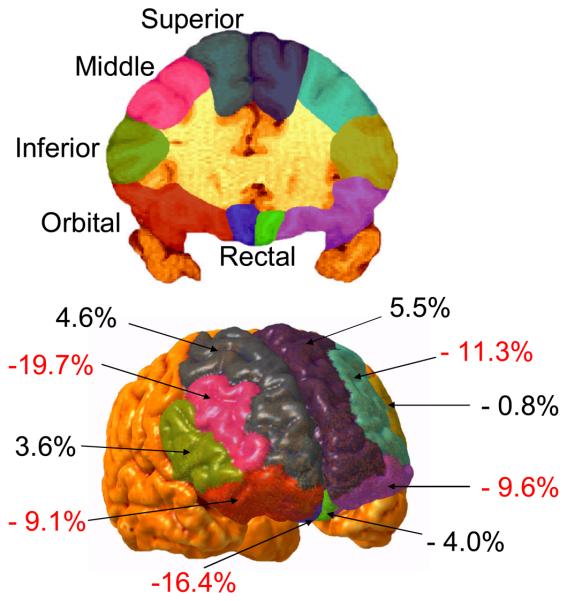
Figure 2.
Coronal view (upper figure) of the frontal cortex illustrating segmentation into superior, middle, inferior, orbital, and rectal sub-regions, and a three-dimensional view (lower figure) illustrating the percentage volume reduction (or increase) in those with Antisocial Personality Disorder compared to normal controls. Significant volume reductions in APDs are coded in red.
Influence of hemisphere
A significant group x hemisphere x region interaction was observed, F (8,134) = 2.56, p < .013, eta2 = .13. Hemisphere influences were observed for middle and rectal gyral volumes, with group effects being stronger for the right hemisphere. A group x hemisphere interaction for the middle frontal gyrus, F = 3.61, (2,69), p = .032, eta2 = .095, indicated that groups differences were significantly stronger in the right than the left hemisphere. APDs had significantly reduced right middle frontal volumes compared to both Normal Controls (t = 4.33, df = 46, p = .0001) and Substance Use Controls (t = 2.48, df = 40, p = .018). Nevertheless, APDs also showed significantly reduced left middle frontal volumes compared to both Normal Controls (t = 2.19, df = 46, p = .033) and Substance Use controls (t = 2.29, df = 40, p = .027).
A group x hemisphere interaction was also found for rectal gyral volumes, F = 3.24, (2,69), p = .045, eta2 = .086, indicating that groups differences were significantly stronger in the right than the left hemisphere. APDs had significantly reduced right rectal gyral volumes compared to both Normal Controls (t = 2.80, df = 46, p = .008) and Substance Use controls (t = 2.54, df = 40, p = .015), with no group differences for left hemisphere volumes (p > .38).
Antisocial behavior in neuroanatomically-defined groups
In addition to APD individuals having reduced orbitofrontal and middle frontal volumes, the converse was also observed. That is, individuals with relatively low orbitofrontal and middle frontal grey volumes were significantly more antisocial than those with relatively high volumes. The base rate for APD in this sample was 22.6%. This cut-point was therefore used to define groups with either high (top 22.6%) or low (bottom 22.6%) volumes for each of the four frontal regions. While those with either low total orbitofrontal or low total middle frontal grey volumes had significantly higher scores on both diagnostic and self-report antisocial measures, no such differences were found for superior or inferior frontal gyral volumes (see Table 1).
Table 1.
Mean scores for low (bottom 22.6%) and high (top 22.6%) prefrontal volume groups on diagnostic and self-report assessments of antisocial personality and behavior. Standard deviations are in parentheses
| Antisocial Personality Disorder | ||||||
|---|---|---|---|---|---|---|
| Low Volume (N = 17) |
High Volume (N = 17) |
t | df | p | d | |
| Orbito-frontal | 16.5 (10.2) |
9.7 (6.0) |
2.35 | 32 | .025 | 1.05 |
| Middle | 14.7 (11.7) |
8.8 (6.4) |
2.03 | 32 | .05 | 0.65 |
| Rectal | 13.8 (8.7) |
9.7 (6.4) |
1.59 | 32 | .15 | 0.37 |
| Superior | 11.1 (11.7) |
10.5 (6.8) |
0.19 | 32 | .853 | 0.06 |
| Inferior | 12.8 (8.5) |
9.5 (9.2) |
1.13 | 32 | .279 | 0.37 |
| Self-report Crime | ||||||
| Low Volume (N = 17) |
High Volume (N = 17) |
t | df | p | d | |
| Orbito-frontal | 25.88 (17.01) |
17.64 (10.3) |
1.71 | 32 | .097 | 1.62 |
| Middle | 28.1 (16.7) |
13.3 (9.6) |
3.24 | 32 | .003 | 1.81 |
| Rectal | 21.1 (15.8) |
18.3 (7.9) |
0.63 | 32 | .53 | 0.37 |
| Superior | 22.3 (16.4) |
18.7 (14.5) |
0.73 | 32 | .473 | 0.23 |
| Inferior | 20.5 (13.2) |
20.6 (16.1) |
−.03 | 32 | .977 | −0.01 |
Frontal gray - antisocial relationships in women and men
In females, reduced orbito-frontal volume was associated with increased antisocial behavior (r = −.67, p = .02) and personality (r = −58, p = .048, two-tailed). Reduced middle frontal volume was associated with increased antisocial behavior (r = −62, p = .032) but not with increased antisocial personality (r = −41, p = .19). Similar significant relationships were observed in males in the same direction, but at a lower level of magnitude (see Table 2). No such relationships were observed for other frontal regions.
Table 2.
Correlations between prefrontal gray matter volumes and self-report and diagnostic interviewer measures of antisocial behavior.
| Males (N = 72) | Females (N = 12) | |||
|---|---|---|---|---|
| Antisocial Behavior | Antisocial Behavior | |||
| Diagnostic | Self-report | Diagnostic | Self-report | |
| Orbito-frontal | −.37** | −.27* | −.58* | −.66* |
| Middle frontal | −.25* | −.34** | −.41 | −.62* |
| Rectal frontal | −.20+ | −.10 | −.12 | −.11 |
| Superior frontal | −.09 | −.18 | −.22 | −.11 |
| Inferior frontal | −.09 | .07 | −.14 | −.06 |
p < .01, two-tailed test
p < .05, two-tailed test
Gender difference in antisocial behavior and brain volume
Males as a whole were more antisocial than females on diagnostic and self-report measures of antisocial behavior, F(5,78) = 3.11, p = .013, eta2 = .166 (see Table 3a). A multivariate ANOVA also indicated that all males compared to all females in the sample had reduced whole-brain corrected frontal volumes, F(5,84) = 3.48, p = .007, eta2 = .007. More specifically, males compared to females had reduced whole-brain corrected orbitofrontal gray volumes (F(1,82) = 10.85, p = .001, eta2 = .117), reduced middle frontal gray volume (F (1,82) = 4.44, p = .039, eta2 = .051), and a trend for reduced rectal gyral volumes (F (1,82) = 3.37, p = .07, eta2 = .039) (see Table 3b). Controlling for orbitofrontal gray alone reduced the gender differences in antisocial behavior by 65.9% (F (2,80) = 1.20, p = .30, eta2 = .030). Controlling for middle frontal gray rendered the gender effect marginally significant, reducing it by 34.1% (F (2,79) = 2.45, p = .093, eta2 = .058). Controlling for rectal gyral gray also rendered the gender effect marginally significant, reducing it by 21.6% (F (2,79) = 2.92, p = .06, eta2 = .069). Controlling for all three brain regions largely abolished the gender difference in antisocial behavior, reducing it by 77.3% (F (2,77) = 0.94, p = .46, eta2 = .020).
Table 3.
Sex differences in (a) diagnostic (antisocial personality disorder) and self-report (crime perpetration) measures of antisocial behavior, and (b) prefrontal gray regional volumes expressed as a function of whole brain volume. SDs are in parentheses.
| (a) Antisocial personality / behavior | ||||||
|---|---|---|---|---|---|---|
| Males | Females | t | df | p | d | |
| Self-report | 20.28 (13.56) |
10.75 (7.37) |
2.37 | 81 | .02 | 0.76 |
| Diagnostic | 11.89 (8.28) |
4.92 (6.43) |
2.79 | 81 | .007 | 0.86 |
| (b) Frontal gray volumes | ||||||
| Males (N = 72) |
Females (N = 12) |
t | df | p | d | |
| Orbito-frontal | 0.0198 (.0023) |
0.0223 (.0030) |
3.29 | 82 | .001 | 1.02 |
| Middle | .0206 (.0037) |
.0232 (.0049) |
2.10 | 82 | .039 | 0.68 |
| Superior | .0433 (.0083) |
.0417 (.0084) |
−0.64 | .82 | .526 | − 0.19 |
| Inferior | .0134 (.0025) |
.0139 (.0028) |
0.58 | 82 | .564 | 0.20 |
| Rectal | .0048 (.0009) |
.0053 (.0008) |
1.84 | 82 | .07 | 0.63 |
Controlling for other psychiatric comorbidity
Although differences between APDs and Substance Dependents indicated that the frontal gray deficits are not an artifact of comorbidity in APDs for alcohol and substance dependence, it is possible that these deficits could be attributed to comorbid affective and schizophrenia-spectrum disorders also present in the APDs and which have been shown to have prefrontal structural deficits 37. This possibility was tested by comparing APDs to the Psychiatric Control group.
The APD group again showed significant reductions in orbito-frontal, middle and rectal frontal gray, but not white, volumes. The previous group x region x hemisphere interaction remained significant (F (4,31) = 4.12, p = .006, eta2 = .347). A breakdown of this interaction revealed significant main group effects for orbito-frontal gray (F (1,34) = 6.99, p = .012, eta2 = .171), middle frontal gray (F (1,34) = 10.45, p = .003, eta2 = .235), and rectal gyral gray (F (1,34) = 4.58, p = .04, eta2 = .119), but not for superior (p = .68) or inferior (p = .28) frontal sites. While the APD group had lower volumes bilaterally for middle and orbital regions, the reduction in rectal gyral volumes was lateralized to the right hemisphere (t(34) = 2.93, p = .006), with no group difference in left volumes (p = .34). Groups did not differ on whole brain volume (F (1,34) = 0.81, p = .38) or prefrontal white matter (F(1,34) = 1.09, p = .31).
Controlling for head injury and trauma exposure
Ventral prefrontal cortex is particularly sensitive to head injury, and trauma exposure could also be an environmental contribution to volume loss. The potential contribution of these factors to antisocial-frontal gray relationships was examined by entering history of head injury and trauma exposure as covariates in the above analyses. The APD group again showed significant reductions in orbito-frontal, middle frontal, and rectal frontal gray, but not white, volumes. The previous group x region remained significant, F(8,130) = 4.28, p = .0001, eta2 = .21.) Significant main group effects were again obtained for orbito-frontal gray (F (1,34) = 6.99, p = .012, eta2 = .171), middle frontal gray (F (1,34) = 10.45, p = .003, eta2 = .235), and rectal gyral gray (F (1,34) = 4.58, p = .04, eta2 = .119), but not for superior (p = .68) or inferior (p = .28) frontal sites.
The group x region x hemisphere interaction also remained significant (F (8,130) = 3.10, p = .003, eta2 = .16). While bilateral volume reductions were observed for both left orbito-frontal gray (F(2,67) = 3.58, p = .03, eta2 = .10) and for right orbitofrontal gray (F(2,67) = 3.86, p = .03, eta2 = .10), group effects were stronger for middle frontal gray on the right hemisphere (F (2,67) = 10.2, p = .0001, eta2 = .23) than on the left (F (2,67) = 2.87, p = .06, eta2 = .08), with similar laterality effects for rectal gyral gray (F (2,67) = 4.48, p = .02, eta2 = .12 for right and F(2,67) = 0.38, p = .69, eta2 = .01, for left).
Prediction of group membership
In a logistic regression in which APDs were compared to Controls, the three variables of total orbitofrontal, right middle, and right rectal frontal gray volumes predicted 55.1% of the variance in group membership (χ2 = 24.88, df = 2, p < .0001), and predicted group membership with an accuracy of 83.3%. Similarly, in predicting APD vs Psychiatric Control group membership, these measures accounted for 48.0% of the variance (χ2 = 16.08, p < .0001), and again correctly classified 83.3% of group members. Furthermore, in predicting APD vs Substance Dependent Control group membership, these measures accounted for 31.6% of the variance (χ2 = 11.25, p < .004), and correctly classified 69.0% of group members.
Independence from psychosocial risk factors
Prefrontal deficits were independent of psychosocial deficits in the APD group. This was demonstrated by entering ten demographic and psychosocial risk factors for antisocial personality (parental social class, early parental divorce, parental verbal arguments, parental criminality, parental physical fights, family size, physical abuse, sexual abuse, raised in an institution, raised by foster parents) into a logistic regression in a single block using forward entry, after which APD versus Control group differences remained significant for the three frontal gray (middle, orbital, rectal) measures (χ2 = 37.38, df=3, p < .0001). In a similar analysis comparing the APDs with the Substance Dependent group, effects remained significant for frontal gray measures after controlling for the 10 psychosocial measures (χ2 = 13.7, df = 3, p = .003). These analyses indicate that frontal deficits in APDs cannot be attributed to psychosocial deficits.
The prefrontal measures added substantially to the prediction of APD vs. Control group membership over and above psychosocial measures. The 10 psychosocial variables in the above logistic regression accounted for 54.1% of the variance. After the additional entry of the three prefrontal gray measures into the regression equation, the amount of group variance explained increased significantly (χ2 = 37.39, p < .00001) to 100%. Prediction of group membership also increased from 73.9% correctly classified to 100% after including frontal measures. Similarly, in a comparison of APD versus Substance Dependent groups, the psychosocial variables explained 74.7% of the variance, which increased significantly to 58.8% of the variance after entry of the three frontal variables. Accuracy of group prediction increased from 78.0% to 92.7%
Discussion
This study delineates a structural deficit to gray matter localized to middle frontal, orbito-frontal and rectal gyral regions of the frontal cortex in those with APD compared to normal controls. Middle frontal and rectal gyral structural impairments were stronger in the right than left hemisphere. The same findings were observed when comparisons were made to both Substance Abuse and other Psychiatric Disorder control groups. Reversing the study design, those with low volumes of orbital and middle frontal gray volumes showed significantly higher levels of antisocial behavior, indicating robustness of antisocial-neuroanatomical relationships. These deficits were also independent of major psychosocial risk factors for APD, and significantly added to the prediction of group membership over and above psychosocial predictors. Males showed a reduced volume of orbitofrontal and middle frontal gray volumes, and controlling for this anatomical gender difference rendered the gender difference in antisocial behavior non-significant. Findings give rise to the hypothesis that part of the gender difference in antisocial behavior is attributable to gender differences in the prefrontal cortex.
How may structural impairments to ventral and middle frontal gray matter translate into increased risk for APD? Regarding the functional neuroanatomy of the ventral prefrontal cortex (lateral and medial regions) this region is centrally involved in decision-making 38, controlling and correcting punishment-related behavior 39, fear conditioning 39, passive avoidance learning 40, response perseveration 41, the representation of the reward value of reinforcers 39 40, emotion-regulation 42 40, behavioral inhibition 43 44, compassion and caring for others 45, reduced expression of negative affect during parent-child interactions 44, affective theory of mind 46, sensitivity to others' emotional states 47, and lack of insight 48. In parallel with these brain-function relationships, antisocial children and adults have been found to show impaired decision-making 38, poor fear conditioning 49, poor passive avoidance learning 50, poor emotion-regulation 11, behavioral disinhibition 51, callousness 52, response perseveration 53, more negative affect expression to parents, lack of empathy 54, and lack of self-insight 55.
With respect to the middle frontal gyrus (BA 10, 46, 9) this region constitutes a component of the neural circuitry subserving fear conditioning 56, failure to alter punished behavior 57, contingency awareness during aversive classical conditioning 58, response inhibition 59, moral decision-making 60, choosing delayed rewards as opposed to immediate rewards 61, empathy to pain stimuli 62, and introspective evaluation of one's thoughts and feelings 63. Correspondingly, offenders have been found to show poor fear conditioning and lack of fear 64 49, disinhibited behavior 51, have impaired moral judgment and break moral boundaries 65, are less able to delay gratification 66,67 lack empathy 54, and lack self-insight 55. Taking both dorsal and ventral structures together, there is reason to believe that structural impairments to the middle frontal and ventral prefrontal cortex may give rise to a constellation of social, cognitive, and emotional risk factors which increase the likelihood of antisocial behavior and personality. The fact that both ventral and middle frontal brain regions contribute to some of the same functional risk factors for antisocial behavior (poor fear conditioning, lack of insight, disinhibition) suggests both the salience of these well-replicated neurocognitive risk factors and also that outcome for antisocial behavior may be especially likely when both of these regions are structurally compromised.
When expressed as a function of whole brain volumes, unselected males had a volume of orbito-frontal gray matter that was 12.6% lower than that of unselected females. This difference is similar to the 16.7% reduction in whole-brain corrected OFC volumes observed in males 5. Reduced OFC volumes in men have also been reported by 7, while 8 have reported significantly lower gray matter OFC concentrations in males compared to females. A large study of 465 normal adults using VBM similarly observed significantly smaller right OFC volumes in males compared to females 6. Furthermore, males have been reported to show lower activation of the OFC compared to females when performing a variety of tasks, including verbal fluency 68, working memory 69, processing unambiguous threat stimuli 70, and working memory during a negative emotion context 71. Not all studies have observed this male inferiority 72. For example, one study did not observe reduced OFC volumes in males, although a 10.3% volume reduction in males in the neighboring ventromedial (straight gyrus) region was found, similar to the non-significant 10.4% reduction observed in the rectal gyrus in males in the current study 73. Furthermore, some studies have observed sex differences in adults but not adolescents 70, arguing for a transition in differential brain development between the sexes from adolescence to adulthood 74. Males have also been reported to show stronger age-related volume reductions in orbitofrontal volumes compared to women 75. Future imaging studies on children and adolescents could test whether sexual dimorphism is similarly observed and accounts for sex differences in adolescent antisocial behavior.
Findings indicate that a significant proportion (77.3%) of the gender difference in antisocial behavior can be accounted for by gender differences in ventral prefrontal gray matter. Strikingly, gender differences were found in frontal sectors that are associated with antisocial behavior, but not in those sectors that were not associated with antisocial behavior. While findings of this study satisfy the three evidential criteria for causation 15, we caution that the gender difference in antisocial behavior likely has multiple neurobiological contributions, with ventral gray volume being only one of them. Furthermore, these findings do not rule out some role for socio-cultural explanations founded on differential socialization of prosocial and antisocial behavior 2. Findings do however provide additional support for the contribution of prefrontal impairments, particularly ventral and middle frontal gray, in the etiology of antisocial personality disorder.
The question of what causes the structural prefrontal gray loss in those with APD cannot be resolved in this study, and remains a critically important issue to address in future studies. Initial hypotheses can however be developed that can be tested in future studies which assess putative causal agents in association with brain-behavior measures. For example, exposure to mother's smoking during pregnancy is a well-replicated risk factor for later criminal and violent offending 75, and exposure to smoking has also been found to thin gray matter in the middle frontal gyrus 45. Lead exposure is both associated with both conduct disorder / juvenile delinquency 76 and also reduced volume of the lateral ventral prefrontal cortex, particularly in males 77. The ventral prefrontal cortex is particularly susceptible to head injury, and impulsive antisocial individuals are more susceptible to accidents and injuries; while we failed to support head injury as a mediating factor, early infant abuse cannot be ruled out as a potential contributing causal agent. Alternatively, because behavioral genetic studies have shown that 90-95% of the variance in prefrontal gray is under genetic control 78, and given the findings from over 100 twin and adoption studies showing that 40-50% of the variance in antisocial behavior is also under genetic control, a genetic etiological contribution to the ventral gray - antisocial relationship cannot be ruled out.
Hemisphere influences were observed for middle and rectal gyral volumes, with group differences being stronger for the right hemisphere. Interpretation of this hemisphere interaction must proceed cautiously because robust, replicable asymmetries have not generally been observed in the literature for these frontal regions. Nevertheless, right (but not left) middle frontal activation has been found for empathic processing of pain79, as well as self-awareness80. Similarly, right but not left ventromedial activation has been associated with successfully inhibiting responses to unpredictable events 81. Consequently, reduced volumes of right middle and rectal regions may help explain the indifference to the pain of others, lack of self-awareness of their actions on others, and the impulsive-aggressive behavior found to characterize individuals with APD.
It should be emphasized that the sample size of females is small and like all initial findings, they need to be replicated and extended. At the same time, the small female sample size biases towards type II error rather than Type I error and does not explain why the antisocial-prefrontal gray correlational findings in males are replicated in an independent sample of females. Furthermore, it is re-emphasized that findings should not be interpreted as ruling out cultural and socialization influences on the gender difference in antisocial behavior, but instead as suggesting that neurobiological influences may play an additional significant role. Despite these limitations, findings give rise to a hypothesis, testable in future studies, that structural differences in the ventral and middle frontal gray act as both a risk factor for antisocial personality disorder and as a partial explanation for sex differences in APD and crime.
Acknowledgments
This study was supported by NIH grants to the first author (K02 MH01114 and RO3 MH50940), the second author (1F31MH079592), the third author (K01MH073990). The third and fourth authors were also supported by grants from the National Center for Research Resources (P41 RR13642), the NIH Roadmap Initiative (P20 RR020750), the National Library of Medicine (R01 LM05639), and the NIH Roadmap for Medical Research, Grant U54 RR021813 entitled Center for Computational Biology (CCB). The authors wish to thank Samantha Henry, Elizabeth Culley, Donna Kha, Reimar Macaranas, Henry Wu, Lydia Lee, Sum-yan Ng, Lori Lacasse, and Todd Lencz, for assistance in data collection and analysis.
References
- 1.Moffitt TE, Caspi A, Rutter M, Silva PA. Sex differences in antisocial behaviour: Conduct disorder, delinquency, and violence in the Dunedin longitudinal study. Cambridge University Press; Cambridge: 2001. [Google Scholar]
- 2.Eme RF. Sex differences in childhood psychopathology – A review. Psychological Bulletin. 1979;86(3):574–595. [PubMed] [Google Scholar]
- 3.Rutter M, Giller H, Hagell A. Antisocial Behavior by Young People. Cambridge University Press; Cambridge: 1998. [Google Scholar]
- 4.Moffitt TE, Caspi A, Rutter M, Silva PA. Sex differences in antisocial behavior. Cambridge University Press; Cambridge: 2001. [Google Scholar]
- 5.Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11(6):490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 6.Good CD, Johnsrude I, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 7.Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cerebral Cortex. 2002;12(9):998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Falgueras A, Junque C, Gimenez M, Caldu X, Segovia S, Guillamon A. Sex differences in the human olfactory system. Brain Res. 2006;1116:103–111. doi: 10.1016/j.brainres.2006.07.115. [DOI] [PubMed] [Google Scholar]
- 9.Damasio A. Descartes' error: Emotion, reason, and the human brain. GP Putnam's Sons; New York: 1994. [Google Scholar]
- 10.Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. 2000;57(2):119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- 11.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289(5479):591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 12.Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165(4):429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiihonen J, Rossi R, Laakso MP, Hodgins S, Testa C, Perez J, et al. Brain anatomy of persistent violent offenders: More rather than less. Psychiatry Research-Neuroimaging. 2008;163(3):201–212. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Ignacio FA, Tovar-Moll F, et al. Psychopathy as a disorder of the moral brain: Fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40(3):1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 15.Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. Journal of Child Psychology and Psychiatry. 2003;44(8):1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- 16.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for Axis I DSM-IV disorders (SCID, Version 2.0) New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin L. Structured clinical interview for DSM-IV Axis II personality disorders (SCID-II, Version 2.0) Version 2.0 ed. New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- 18.Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79(2):163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 19.Elliott DS, Ageton S, Huizinga D, Knowles B, Canter R. National Youth Survey. Behavior Research Institute; Boulder, CO: 1983. The prevalence and incidence of delinquent behavior: 1976-1980. (Report No. 26). Ref Type: Report. [Google Scholar]
- 20.Raine A, Mellingen K, Liu JH, Venables P, Mednick SA. Effects of environmental enrichment at ages 3-5 years on schizotypal personality and antisocial behavior at ages 17 and 23 years. Am J Psychiatry. 2003;160(9):1627–1635. doi: 10.1176/appi.ajp.160.9.1627. [DOI] [PubMed] [Google Scholar]
- 21.Raine A, Lencz T, Taylor K, Hellige JB, Bihrle S, LaCasse L, et al. Corpus callosum abnormalities in psychopathic antisocial individuals. Arch Gen Psychiatry. 2003;60(11):1134–1142. doi: 10.1001/archpsyc.60.11.1134. [DOI] [PubMed] [Google Scholar]
- 22.Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, et al. Hippocampal structural asymmetry in unsuccessful psychopaths. Biol Psychiatry. 2004;55(2):185–191. doi: 10.1016/s0006-3223(03)00727-3. [DOI] [PubMed] [Google Scholar]
- 23.Hollingshead AB. Four factor index of social status. Connecticut; New Haven: 1975. [Google Scholar]
- 24.Rex DE, Ma JQ, Toga AW. The LONI Pipeline Processing Environment. Neuroimage. 2003;19:1033–1048. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- 25.Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6:129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- 26.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. Ieee Transactions on Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 27.Mazziotta JC. Brain mapping: its use in patients with neurological disorders. Revue Neurologique. 2001;157(8-9):863–871. [PubMed] [Google Scholar]
- 28.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A Probabilistic atlas of the human brain – Theory and rationale for its development. Neuroimage. 1995;2(2):89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- 29.Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998a;22:139–52. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 30.Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: Ii. Intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography. 1998b;22:153–65. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 31.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13(5):856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald D, Avis D, Evans A. Proceedings of the International Society for Optical Engineering (SPIE) Conference on Visualization in Biomedical Computing. SPIE; Rochester: 1994. Multiple surface identification and matching in magnetic resonance images. In: Robb RA, editor; pp. 160–169. [Google Scholar]
- 33.Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: An MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161(1):99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- 34.DeArmond J, Fusco MM, Dewey MM. Structure of the Human Brain. 3rd ed. Oxford University Press; New York: 1989. [Google Scholar]
- 35.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. Academic Press; San Diego: 1997. [Google Scholar]
- 36.Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI, and Blood Supply. 2nd ed. Springer; New York: 1999. [Google Scholar]
- 37.Raine A. Schizotypal personality: Neurodevelopmental and psychosocial trajectories. Annual Review of Clinical Psychology. 2006;2:291–326. doi: 10.1146/annurev.clinpsy.2.022305.095318. [DOI] [PubMed] [Google Scholar]
- 38.Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–1294. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- 39.Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 40.Blair RJR. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363(1503):2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York Academy of Sciences. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 42.Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103(16):6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Whittle S, Yap MBH, Yucel M, Fornito A, Simmons JG, Barrett A, et al. Prefrontal and amygdala volumes are related to adolescents' affective behaviors during parent-adolescent interactions. Proc Natl Acad Sci U S A. 2008;105(9):3652–3657. doi: 10.1073/pnas.0709815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toro R, Leonard G, Lerner JV, Lerner RM, Perron M, Pike GB, et al. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacology. 2008;33(5):1019–1027. doi: 10.1038/sj.npp.1301484. [DOI] [PubMed] [Google Scholar]
- 46.Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cognitive and Behavioral Neurology. 2005;18(1):55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- 47.Schirmer A, Escoffier N, Zysset S, Koester D, Striano T, Friederici AD. When vocal processing gets emotional: On the role of social orientation in relevance detection by the human amygdala. Neuroimage. 2008;40(3):1402–1410. doi: 10.1016/j.neuroimage.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Developmental Neuropsychology. 2000;18(3):355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- 49.Fairchild G, Van Goozen SH, Stollery SJ, Goodyer IM. Fear conditioning and affective modulation of the startle reflex in male adolescents with early-onset or adolescence-onset conduct disorder and healthy control subjects. Biol Psychiatry. 2008;63(3):279–285. doi: 10.1016/j.biopsych.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 50.Newman JP, Schmitt WA. Passive avoidance in psychopathic offenders: A replication and extension. Journal of Abnormal Psychology. 1998;107(3):527–532. doi: 10.1037//0021-843x.107.3.527. [DOI] [PubMed] [Google Scholar]
- 51.Patrick CJ. Psychophysiological correlates of aggression and violence: an integrative review. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363(1503):2543–2555. doi: 10.1098/rstb.2008.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frick PJ, Cornell AH, Bodin SD, Dane HE, Barry CT, Loney BR. Callous-unemotional traits and developmental pathways to severe conduct problems. Developmental Psychology. 2003;39(2):246–260. doi: 10.1037//0012-1649.39.2.246. [DOI] [PubMed] [Google Scholar]
- 53.Seguin JR, Arseneault L, Boulerice B, Harden PW, Tremblay RE. Response perseveration in adolescent boys with stable and unstable histories of physical aggression: the role of underlying processes. Journal of Child Psychology and Psychiatry. 2002;43(4):481–494. doi: 10.1111/1469-7610.00039. [DOI] [PubMed] [Google Scholar]
- 54.Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37(1):335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 55.Happe F, Frith U. Theory of mind and social impairment in children with conduct disorder. British Journal of Developmental Psychology. 1996;14:385–398. [Google Scholar]
- 56.Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. Journal of Neuroscience. 2004;24(1):218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raine A. The psychopathology of crime: Criminal behavior as a clinical disorder. Academic Press; San Diego: 1993. [Google Scholar]
- 58.Carter RM, O'Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage. 2006;29(3):1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 59.Mcnab F, Leroux G, Strand F, Thorell L, Bergman S, Klingberg T. Common and unique components of inhibition and working memory: An fMRI, within-subjects investigation. Neuropsychologia. 2008;46(11):2668–2682. doi: 10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 60.Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Social, Cognitive, and Affective Neuroscience. 2006;1:203–213. doi: 10.1093/scan/nsl033. Ref Type: Generic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 62.Gu XS, Han SH. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. 2007;36(1):256–267. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 63.Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 64.Patrick CJ. Emotion and psychopathy: Startling new insights. Psychophysiology. 1994;31(4):319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 65.Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 66.Dolan M, Fullam R. Behavioural and psychometric measures of impulsivity in a personality disordered population. Journal of Forensic Psychiatry & Psychology. 2004;15(3):426–450. [Google Scholar]
- 67.Miller JD, Lynam DR. Psychopathy and the five-factor model of personality: A replication and extension. Journal of Personality Assessment. 2003;81(2):168–178. doi: 10.1207/S15327752JPA8102_08. [DOI] [PubMed] [Google Scholar]
- 68.Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, et al. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. Journal of Neurology Neurosurgery and Psychiatry. 1998;64(4):492–498. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. Journal of Neuroscience. 2005;25(40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, et al. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry. 2004;55(11):1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 71.Koch K, Pauly K, Kellermann T, Seiferth NY, Reske M, Backes V, et al. Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia. 2007;45(12):2744–2754. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 72.Blanton RE, Levitt JG, Peterson JR, Fadale D, Sporty ML, Lee M, et al. Gender differences in the left inferior frontal gyrus in normal children. Neuroimage. 2004;22(2):626–636. doi: 10.1016/j.neuroimage.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 73.Wood JL, Heitmiller D, Andreasen NC, Nopoulos P. Morphology of the ventral frontal cortex: Relationship to femininity and social cognition. Cerebral Cortex. 2008;18(3):534–540. doi: 10.1093/cercor/bhm079. [DOI] [PubMed] [Google Scholar]
- 74.Cowell PE, Sluming VA, Wilkinson ID, Cezayirli E, Romanowski CAJ, Webb JA, et al. Effects of sex and age on regional prefrontal brain volume in two human cohorts. European Journal of Neuroscience. 2007;25(1):307–318. doi: 10.1111/j.1460-9568.2006.05281.x. [DOI] [PubMed] [Google Scholar]
- 75.Brennan PA, Grekin ER, Mednick SA. Maternal smoking during pregnancy and adult male criminal outcomes. Arch Gen Psychiatry. 1999;56(3):215–219. doi: 10.1001/archpsyc.56.3.215. [DOI] [PubMed] [Google Scholar]
- 76.Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. JAMA: Journal of the American Medical Association. 1996;275(5):363–369. [PubMed] [Google Scholar]
- 77.Cecil KM, Brubaker CJ, Adler CM, Dietrich KN, Altaye M, Egelhoff JC, et al. Decreased brain volume in adults with childhood lead exposure. Plos Medicine. 2008;5(5):741–750. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 79.Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. NeuroImage. 2007;36:256–267. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 80.Platek SM, Keenan JP, Gallup GG, Mohamed FB. Where am I? The neurological correlates of self and other. Cognitive Brain Research. 2004;19:114–122. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 81.Fassbender C, Murphy K, Fox JJ, Wylie GR, Javitt DC, Robertson IH, Garavan H. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Cognitive Brain Research. 2004;20:132–143. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]