Abstract
Study Objective
Atrial fibrillation (AF) affects over 2 million people in the United States and accounts for nearly 1% of emergency department (ED) visits. Physicians have little information to guide risk stratification of patients with symptomatic AF and admit more than 65%. Our aim was to assess whether data available in the ED management of symptomatic AF can estimate a patient's risk of experiencing a 30-day adverse event.
Methods
We systematically reviewed the electronic medical records of all ED patients presenting with symptomatic AF between August 2005 and July 2008. Predefined adverse outcomes included 30-day ED return visit, unscheduled hospitalization, cardiovascular complication or death. We performed multivariable logistic regression to identify predictors of 30-day adverse events. The model was validated using 300 bootstrap replications.
Results
During the 3-year study period, 914 patients accounted for 1228 ED visits. Eighty patients were excluded for non-AF related complaints and 2 patients had no follow-up recorded. Of 832 eligible patients, 216 (25.9%) experienced at least one of the 30-day adverse events. Increasing age (odds ratio [OR] and [95% CI]: 1.20 per decade [1.06, 1.36]), complaint of dyspnea (OR: 1.57 [1.12, 2.20]), smokers (OR: 2.35 [1.47, 3.76]), inadequate ventricular rate control (OR: 1.58 [1.13, 2.21]), and patients taking beta-blockers (OR: 1.44 [1.02, 2.04]) were independently associated with higher risk for adverse events. C-index was 0.67.
Conclusion
In ED patients with symptomatic AF, increased age, inadequate ED ventricular rate control, dyspnea, smoking, and beta-blocker treatment were associated with an increased risk of a 30-day adverse event.
INTRODUCTION
Background
Atrial fibrillation (AF) affects over 2 million people in the United States and the combination of increasing AF prevalence, high admission rate and emergency department (ED) crowding will severely burden the healthcare System.1, 2 The AF prevalence is projected to double by 2020 and increase to 5.6 million by 2050.2 AF increases with age; 5.9% of those over 65 years of age and 9% of those over 80 years are diagnosed with the arrhythmia.3 The proper management of patients with AF is critical due to the well-documented association with heart failure and stroke.2, 4–8, 9, 10
The number of ED visits for complaints related to AF increased by 88% between 1993 and 2003 and now account for nearly 1% percent of all ED visits in the United States.11, 12 More than 65% of these AF visits result in hospital admission and over $6.65 billion in expenditures.11, 13 Over the past 20 years, hospital admissions for AF have increased by 66%.14–16
Importance
Previous studies have suggested that incorporation of ED practice guidelines for AF management, the presence of observation units and expedited cardioversion have been successful in reducing the AF admission rates without compromising patient safety.9, 17–19 A strategy to better define the ED management of patients presenting with AF, especially one that categorizes patients in low and high risk, is required.11 A recent study that reviewed 12-years of ED visits for AF from the National Hospital Ambulatory Medical Care Survey (NHAMCS) database found that patients hospitalized with symptomatic AF were similar to those discharged home from the ED with respect to age, sex and whether ED rate control, cardioversion or anticoagulation were attempted.11 The development of a highly accurate prediction rule will assist ED physicians in the risk stratification of patients with symptomatic AF.
Goals of This Investigation
We developed our prediction rule through a systematic review of the electronic medical records of all patients treated for symptomatic AF at an urban, academic ED. This study's goal is to identify predictors of 30-day adverse events in ED patients evaluated for symptomatic AF. We hypothesize that data available in the ED management of symptomatic AF can estimate a patient's risk of experiencing a 30-day adverse event. The development of a highly accurate prediction rule may significantly advance the management of AF in the ED.
MATERIALS AND METHODS
Study Design and Setting
We performed a retrospective, observational cohort study using a query of our electronic medical record (EMR) archives and identified all patients ≥ 18 years of age with a primary or supporting ICD-9 ED discharge diagnosis of AF treated in the adult ED between August 1, 2005 and July 31, 2008. Our facility is an urban, academic, tertiary care referral center with an adult ED that treats 50,000 patients annually. The ED attending physician evaluates every patient in our ED and the attending physician's dictated ED history and physical document is subsequently transcribed into the EMR. The results of laboratory, radiographic, electrocardiogram and other diagnostic studies are also available in the EMR. Our medical center's institutional review board approved this study.
Two investigators (TWB and ARM) systematically reviewed the EMR for corresponding data adhering to strict chart review methodology guidelines.30 The study's principal investigator, an ED faculty physician, and a fourth year medical student researcher were the 2 data abstractors. Both abstractors trained on a set of 10 records. Cases were selected based on the strict inclusion and exclusion criteria discussed below. We selected potentially important predictor variables a priori based on clinical expertise and a review of the related literature.11, 16, 17, 19–29 We recorded information on patient medical history, home medications, physical exam findings, and diagnostic test results. Our computer query system automatically populates some data (triage complaint, triage vital signs, ED and hospital diagnostic (ICD-9) and procedural (CPT) codes) into our database. We used a standardized electronic data abstraction form and entered directly into a statistical database (SPSS, Version 17.0, Chicago, IL). We held twice monthly meetings to review data collection and resolve any disputes. When there was a question regarding a record, the principal investigator reviewed the entire EMR and often clarified the dispute. In rare instances, a question regarding data collection was discussed with the other study investigators for final determination. In an attempt to minimize missing data, we reviewed ED attending and resident history and physical examination documents, ED nursing notes, consultant notes, hospital records, outpatient clinic notes, diagnostic study reports and electronic clinical communications. Reviewers were not blinded to the study's objective or the outcomes of interest. The reviewers, however, always entered the information on candidate predictors prior to recording whether the patient experienced a 30-day adverse event.
Both investigators independently reviewed a random sample of 46 (5%) records to measure interrater reliability for this structured medical record review. We calculated the interrater agreement with Cohen's kappa statistic.
Selection of Participants
All adult patients treated in our ED for AF or atrial flutter were eligible for inclusion in our cohort regardless of ED disposition (eg, discharge, hospitalization). We included patients with atrial flutter as it may degenerate into AF and atrial flutter commonly occurs together in patients with AF. The 2006 and 2008 ACC/AHA guidelines group the two arrhythmias together with regard to management and performance measures recommendations.15, 16 Previous landmark trials regarding treatment of AF have included patients with atrial flutter.31–33 Inclusion criteria required documented evidence of AF or atrial flutter on an ED electrocardiogram or rhythm strip. The patient also must have signs (tachycardia, dyspnea) or symptoms (palpitations, chest pain, shortness of breath, weakness, lightheadedness, pre-syncope, or syncope) consistent with primary symptomatic AF documented in the EMR. We also included patients whose initial presenting complaint was not directly related to their AF diagnosis (e.g. evaluation for febrile illness, gastrointestinal complaint, injury) but had a secondary complaint consistent with symptomatic AF that required ED evaluation. We extensively reviewed the patient's entire EMR related to that ED evaluation and determined whether the patient underwent an evaluation for their AF in addition to their primary complaint. Patients with the following were included in the study: new AF diagnosis, AF associated with inadequate rate control (based on prior studies and our clinical experience, we defined adequate ventricular rate control as a resting heart rate less than 100 beats per minute [bpm]),31–33 AF associated with heart failure symptoms, AF in the setting of a cerebrovascular accident (CVA) or transient ischemic attack (TIA), AF associated with other thromboembolic complications. We excluded patients when our review of their EMR determined that AF was unrelated to the ED complaints and did not require evaluation in the ED. When a patient had multiple ED visits during the study period, we included only their first visit to the ED in the analysis.
Candidate Predictors
Candidate predictor variables were selected based on an extensive review of the medical literature and clinical expertise.11, 16, 17, 19–29 Candidate predictors need to be biologically plausible for the predictive rule to maintain face validity and be realistically available to most emergency physicians.34 Invasive studies or laboratory results that do not return within 2 hours will result in an ED rule rarely used. To that end, we recorded information on 50 variables that included patient history of present illness, past medical history, home medications, physical exam findings, ED treatments and diagnostic test results.
Given a set of candidate predictors, many published rules erroneously use a stepwise selection of predictors that is based on analyzing whether the association of each predictor with the outcome is statistically significant using bivariate analysis and p-values. Stepwise methods may lead to instability of predictor selection, biased estimates of coefficients, exaggeration of p-values and worse predictive quality than using the full model without selection.34, 35 We selected 12 predictor variables for inclusion in our prediction rule from the larger set based on clinical relevance and the results of baseline descriptive statistics in our cohort of ED patients with symptomatic AF. Specifically, we reviewed the baseline characteristics of the patients who did and did not experience a 30-day adverse event and selected the 12 predictors for inclusion in the model from these 50 candidate predictors based on apparent differences in predictor representation between the two groups, clinical relevance, and sensibility. Collinearity of predictors can lead to inclusion of extraneous predictors and inflated standard errors for the regression coefficients.36 Therefore, in order to limit collinearity and ensure a parsimonious model, Spearman's correlations were calculated between the clinically sensible associations within our 12 predictor variables. Specifically, Spearman's correlations were calculated between the following clinically sensible associations: 1) history of hypertension status and beta-blocker and diuretic use 2) history of heart failure and beta-blocker home use, diuretic home use, peripheral edema on physical exam and dyspnea in the ED.
Adequate rate control in the ED was one of the predictors selected for consideration in the rule. For the purposes of analysis, we defined a priori that adequate rate control in the ED would be a ventricular rate less than 100 bpm. The documented ventricular heart rate at the time of ED disposition determined whether patients were classified as having adequate ventricular rate control (pulse < 100 bpm) or not (pulse >/=100 bpm). We obtained this data from reviewing the electronic and scanned nursing records for documentation of the patient's heart rate at the time of ED disposition. These included heart rate at time of transfer to floor or ICU and recorded heart rate prior to discharge from ED. We did not continuously track ventricular rate but recorded the single measurement at time of ED disposition. Patients were subsequently classified as adequate or inadequate rate control based on whether that data point was less than 100 bpm.
Outcome Measures
The primary outcome measure was the occurrence of 1 or more adverse events within 30-days of the patient's ED visit. Predetermined adverse outcome measures were: 30-day ED return visit for an AF-related complaint, unscheduled hospital admission for an AF-related complaint, 30-day cardiovascular complication, and patient death secondary to an AF-related problem. We defined an AF-related complaint as one of the following: ED visit or hospitalization for signs (tachycardia, dyspnea) or symptoms (palpitations, chest pain, shortness of breath, weakness, lightheadedness, pre-syncope, or syncope) consistent with primary symptomatic AF, an AF-related medication adverse effect (e.g. bradycardia due to excess beta-blockade, supratherapeutic anticoagulation or warfarin-associated bleeding), or an ED evaluation for a cardiovascular complication (e.g. arrhythmia, acute heart failure exacerbation, acute coronary syndrome). We defined cardiovascular complications as the occurrence of one of the following: atrial fibrillation with rapid ventricular response, acute heart failure exacerbation, acute coronary syndrome, acute atrial and/or ventricular arrhythmia requiring evaluation, thromboembolic CVA, cardiogenic shock, or cardiac arrest. Cardiovascular complications that occurred during an admitted patient's index hospitalization were not counted as positive outcomes. When a patient died within the 30-day period, we reviewed the death summary and certificate (when available) to evaluate AF's role in causing the patient's death.
Data Collection and Processing
We reviewed patients’ EMR to record whether an adverse event occurred within 30 days of their ED visit. The observation period for patients discharged from the ED included the 30 days subsequent to the date of initial ED visit. The observation period for admitted patients spanned the 30-days from the initial ED visit minus the days spent in the hospital. The only exception was death related to AF during the first 30 days was considered an event even if the patient was in the hospital. The majority of our center's patients with AF follow as outpatients in our cardiology or internal medicine clinics resulting in excellent follow-up information on this patient cohort. When patient's returned to the ED within 30 days of their initial visit, we reviewed the ED record and admission documentation (if applicable) to verify that the visit was AF-related. In instances where the visit or admission was for a non-AF related reason, this visit was not considered an adverse event. We specifically reviewed all cardiology and primary care clinic notes within 6 months of the patient's ED visit for mention of any adverse event, ED visit, or hospitalization that might have occurred at an outside hospital. Data were entered directly into a statistical database (SPSS, Version 17.0, Chicago, IL).
Data Analysis
To avoid overfitting and ensure a reliable prediction rule, we adhered to the accepted formula that there must be 15 events per predictor degree of freedom (i.e. per regression coefficient estimated).34,35,37 Based on a query of our ED visit database, we anticipated approximately 300 individual patient visits for AF annually; therefore, we chose to review three years of ED medical records to guarantee adequate sample size for formulating the model.
Descriptive statistics on baseline variables are presented as median (interquartile range [IQR]) or % (N) as appropriate. We analyzed the association of the a priori selected variables with 30-day adverse events using multivariable logistic regression from which we derived the original model's beta coefficients. Clinically meaningful interactions were included in the model. Their significance was tested as a group to avoid inflating Type I error. All interaction terms were removed as a group and the model refit if results were non-significant. Specifically, interactions between home use of beta-blockers and diuretics and between edema on physical exam and a history of heart failure were tested. The primary outcome was based on 30-day adverse event status. We assumed that missing values occurred at random and used multiple imputation to derive predictions for missing values of selected Variables.38–40 All analyses were done using the statistical programming language R, version 2.8.1.40–42 Predictive discrimination was assessed using the C-statistic and a histogram of predicted probabilities.
Prediction models need to be validated and calibrated. Internal validation estimates the likely performance of the rule on a new sample of patients from the same patient stream. Calibration measures a rule’s accuracy of the predicted probability of the outcome and the observed outcome frequency. This may be demonstrated with a smooth nonparametric calibration curve or scatter plot of predicted versus observed outcome, which illustrates the bias in predicted values. We internally validated and calibrated the model using 300 bootstrap resamples. Bootstrapping, a more efficient technique for model validation and calibration than data-splitting techniques, preserves the sample size leading to more precision and power.43 Each bootstrap resample involved randomly sampling a new set of patients from the original set with replacement. Thus, in a given resample, some patients might be represented multiple times, and others not at all. Each coefficient was averaged over the 300 bootstrap resamples to build the bootstrap model. The difference between the original and bootstrap model predictive probabilities provides a sense for how the original maximum likelihood model results which are presented in Table 3 would perform on future patient samples in our facility.
Table 3.
(A) Prediction model for 30-day adverse events in ED patients with symptomatic AF Primary Model (B) Secondary model testing composite outcome that excluded 25 return visits to the ED that did not result in hospitalization or patient death (C) Secondary model without and with Hospital Disposition included as additional covariate.
| Predictor | (A) Primary Model Odds Ratio |
95% CI | (B) Secondary Model Odds Ratio |
95% CI | (C) Secondary Model Odds Ratio |
95% CI |
|---|---|---|---|---|---|---|
| Smoker | 2.35 | (1.47, 3.76) | 2.23 | (1.34, 3.70) | 2.35 | (1.47, 3.76) |
| Age (1 year increment) | 1.02 | (1.01,1.03) | 1.02 | (1.01,1.04) | 1.02 | (1.01, 1.03) |
| Inadequate heart rate control in ED |
1.58 | (1.13, 2.21) | 1.88 | (1.32, 2.67) | 1.55 | (1.10, 2.20) |
| Complaint of dyspnea in ED | 1.57 | (1.12, 2.20) | 1.63 | (1.14, 2.33) | 1.55 | (1.10, 2.19) |
| Home use of beta-blockers | 1.44 | (1.02, 2.04) | 1.37 | (0.95, 1.96) | 1.44 | (1.02, 2.03) |
| Heart failure history | 1.35 | (0.92, 1.98) | 1.53 | (1.02, 2.28) | 1.34 | (0.97,1.97) |
| Edema on physical exam | 1.28 | (0.89, 1.85) | 1.46 | (1.00, 2.14) | 1.27 | (0.88, 1.84) |
| Hypertension history | 1.21 | (0.82, 1.79) | 1.48 | (0.97,2.27) | 1.21 | (0.82, 1.79) |
| Female | 1.11 | (0.79, 1.56) | 1.02 | (0.71, 1.46) | 1.11 | (0.79, 1.56) |
| Palpitations in the ED | 0.90 | (0.63,1.30) | 0.94 | (0.64,1.38) | 0.91 | (0.63, 1.31) |
| COPD history | 1.08 | (0.69,1.69) | 1.03 | (0.64,1.66) | 1.07 | (0.69, 1.68) |
| Home use of diuretic | 1.00 | (0.69,1.44) | 0.91 | (0.62, 1.33) | 1.00 | (0.69, 1.43) |
| Admitted to Hospital | 1.10 | (0.70, 1.72) |
We performed two additional secondary analyses with our prediction model. We included patient disposition (e.g. hospitalization, discharge) as an additional variable in the model to test for the potential confounding of hospitalization on the association between the predictors and adverse events. We compared the beta-coefficients and model's discrimination and calibration with and without inclusion of the disposition predictor variable to measure the impact of the hospitalization. We also performed a sensitivity analysis testing our original model on a more refined composite outcome that only included death, hospitalization and cardiovascular complication within 30 days. This outcome focuses on the most severe adverse events and excludes patients with a return visit to the ED who do not require admission. Finally, agreement between EMR reviewers was assessed on 30-day adverse events and model predictors using Cohen's kappa statistic.
RESULTS
During the 3-year study period, 914 patients accounted for 1228 ED visits. Eighty patients were excluded for non-AF related complaints and 2 who had no follow-up recorded resulting in a study population of 832 patients. The most common non-AF related complaints included: trauma evaluations (n=26), dehydration/ general malaise (n=13), infectious complaints (n=10), and abdominal/flank pain (n=10). The baseline characteristics for the subjects are presented in Table 1. Of the 832 patients, 717 (86%) had isolated AF, 95 (11%) had atrial flutter and 20 (2.4%) had both AF and atrial flutter.
Table 1.
Subjects' characteristics
| Variable | N | N missing (total) |
No 30-day Adverse Event (N=616) |
Experienced a 30-day Adverse Event (N=216) |
|---|---|---|---|---|
| Age (in years) | 832 | 0 (0%) | 67 (55, 78) | 72 (61, 81) |
| Age at time of initial diagnosis of AF (in years) |
676 | 156 (19%) | 62 (49, 73) | 68 (56, 79) |
| Sex: Female | 832 | 0 (0%) | 244 (40%) | 98 (45%) |
| Classification of AF | 810 | 22 (2.6%) | ||
| New Diagnosis | 222 (37%) | 74 (35%) | ||
| Paroxysmal/Persistent | 265 (44%) | 81 (38%) | ||
| Permanent | 111 (19%) | 57 (27%) | ||
| Maximum pulse rate in ED (bpm) |
804 | 28 (3.4%) | 123 (97, 144) | 130 (98, 148) |
| Adequate heart rate control in ED: Yes |
825 | 7 (0.8%) | 386 (63%) | 114 (53%) |
| Body mass index (m2/kg) | 681 | 151 (18%) | 27 (24, 31) | 25 (22, 30) |
| ≥ 2 AV nodal blockers: Yes | 832 | 0 (0%) | 90 (15%) | 36 (17%) |
| Home beta-blocker use: Yes |
832 | 0 (0%) | 254 (41%) | 114 (53%) |
| Home diltiazem/ verapamil use: Yes |
832 | 0 (0%) | 93 (15%) | 37 (17%) |
| Home digitalis use: Yes | 832 | 0 (0%) | 101 (16%) | 30 (14%) |
| Home diuretic use: Yes | 832 | 0 (0%) | 279 (45%) | 114 (53%) |
| Home amiodarone use: Yes | 832 | 0 (0%) | 25 (4%) | 14 (6%) |
| Home sotalol use: Yes | 832 | 0 (0%) | 42 (7%) | 7 (3%) |
| Home warfarin use: Yes | 832 | 0 (0%) | 205 (33%) | 78 (36%) |
| Home statin use: Yes | 832 | 0 (0%) | 200 (32%) | 75 (35%) |
| Home ACEI/ARB use: Yes | 832 | 0 (0%) | 236(38%) | 90 (42%) |
| Current smoker: Yes | 830 | 2 (0.2%) | 73 (12%) | 42 (20%) |
| Current alcohol drinker: Yes |
830 | 2 (0.2%) | 64 (10%) | 23 (11%) |
| Reported history of cocaine use: Yes |
830 | 2 (0.2%) | 14 (2%) | 4 (2%) |
| History of myocardial infarction |
828 | 4 (0.5%) | 102 (17%) | 33 (15%) |
| History of coronary artery disease |
830 | 2 (0.2%) | 197 (32%) | 75 (35%) |
| History of COPD | 829 | 3 (0.4%) | 82 (13%) | 44 (21%) |
| History of hypertension | 832 | 0 (0%) | 401 (65%) | 160 (74%) |
| History of valvular heart disease |
831 | 1 (0.1%) | 106 (17%) | 56 (26%) |
| History of heart failure | 832 | 0 (0%) | 140 (23%) | 76 (35%) |
| History of renal insufficiency |
831 | 1 (0.1%) | 66 (11%) | 40 (19%) |
| History of insulin- dependent diabetes: Yes |
830 | 0 (0%) | 42 (7%) | 18 (8%) |
| History of non-insulin- dependent diabetes: Yes |
831 | 1 (0.1%) | 98 (16%) | 41 (19%) |
| Pacemaker: Yes | 830 | 2 (0.2%) | 56 (9%) | 27 (13%) |
| Family history of AF: Yes | 826 | 6 (0.7%) | 37 (6%) | 11 (5%) |
| Family history of coronary artery disease: Yes |
826 | 6 (0.7%) | 255 (42%) | 98 (46%) |
| Family history of valvular heart disease: Yes |
826 | 6 (0.7%) | 11 (2%) | 1 (0%) |
| Complaint of palpitations in ED: Yes |
830 | 2 (0.2%) | 261 (42%) | 75 (35%) |
| Complaint of shortness of breath in ED: Yes |
830 | 2 (0.2%) | 261 (42%) | 123 (57%) |
| Complaint of neurologic deficit in ED: Yes |
829 | 3 (0.4%) | 51 (8%) | 33 (15%) |
| Presence of edema on physical exam in ED: Yes |
832 | 0 (0%) | 154 (25%) | 75 (35%) |
| Presence of cardiac murmur on physical exam in ED: Yes |
832 | 0 (0%) | 91 (15%) | 40 (19%) |
| Presence of pulmonary rales on physical exam in ED: Yes |
832 | 0 (0%) | 122 (20%) | 80 (37%) |
N equal total number of non-missing responses for each variable. Categorical variables presented as number followed by percentage in parentheses. Continuous variables are represented as the median with interquartile range in parentheses.
Abbreviations in table: ACEI - angiotensin converting enzyme inhibitor; ARB - angiotensin receptor blocker; COPD - chronic obstructive pulmonary disease
Two hundred sixteen patients (25.9%) had at least one of the following 30-day AF-related adverse events: ED return visit (124, 14.9%), unscheduled hospital admission (130, 15.6%), cardiovascular complication (128, 15.3%) or death (54, 6.5%). Of the 130 unscheduled hospitalizations, 98 (75.3%) were admitted through the ED. The most common cardiac complications and reasons for hospitalization were recurrent AF with rapid ventricular response and acute heart failure exacerbations. Heart failure and intracranial hemorrhage were the most common causes of death. All 7 of the patients who died of an intracranial hemorrhage were taking warfarin. A detailed listing of the outcome measurements is presented in Table 2. Adverse events occurred in 181 of the 638 (28.4%) admitted patients and 35 of the 192 (18.2%) patients discharged from the ED. Two patients died in the ED. The median hospital lengths of stay for admitted patients who did and did not experience an adverse event were 4 days (IQR: 2 to 7.5 days) and 3 days (IQR: 2 to 5.75 days), respectively. The median time to adverse event among discharged patients was 10 days (IQR: 6 to 19 days).
Table 2.
Description of Specific 30-day Adverse Event Outcomes
| Adverse Event Category | N | Frequency |
|---|---|---|
| Reason for return visit to ED | 124 | |
| Shortness of Breath | 29 (23%) | |
| Chest Pain | 19 (15%) | |
| Palpitations | 16 (13%) | |
| Weakness | 15 (12%) | |
| Tachycardia | 7 (6%) | |
| Altered Mental Status | 6 (5%) | |
| Syncope | 6 (5%) | |
| Extremity edema | 5 (4%) | |
| Cerebrovascular accident (CVA) | 4 (3%) | |
| Arrhythmia | 4 (3%) | |
| Abdominal Pain | 3 (2%) | |
| Abnormal Bleeding | 3 (2%) | |
| Hypotension | 2 (2%) | |
| Nausea | 2 (2%) | |
| Other | 3 (2%) | |
| Hospital admission diagnosis | 130 | |
| AF with rapid ventricular response | 42 (32%) | |
| Heart failure | 28 (22%) | |
| Chest Pain/Acute coronary syndrome | 11 (7%) | |
| Symptomatic AF/atrial flutter | 8 (6%) | |
| Shortness of breath/Hypoxia | 7 (5%) | |
| CVA/Transient ischemic attack | 6 (5%) | |
| Malaise | 6 (5%) | |
| Hypotension/Syncope | 5 (4%) | |
| Tachycardia | 8 (6%) | |
| Bradycardia | 4 (3%) | |
| Palpitations | 2 (2%) | |
| Acute limb ischemia | 1 (1%) | |
| Other | 2 (2%) | |
| Cardiovascular complication | 128 | |
| AF with rapid ventricular response | 43 (34%) | |
| Heart Failure | 32 (25%) | |
| Embolic complications | 10 (8%) | |
| AF with rapid ventricular response and heart failure |
9 (7%) | |
| Acute coronary syndrome | 7 (5%) | |
| Atrial flutter with rapid ventricular response |
6 (5%) | |
| Syncope | 5 (4%) | |
| Pacemaker dysfunction | 4 (3%) | |
| Bradycardia | 4 (3%) | |
| Adverse medication reaction | 3 (3%) | |
| Cardiac arrest | 3 (3%) | |
| Other | 2 (2%) | |
| Cause of death | 54 | |
| Heart Failure | 9 (17%) | |
| Intracranial hemorrhage | 7 (13%) | |
| Respiratory failure | 7 (13%) | |
| Complications of metastatic cancer | 7 (13%) | |
| Cardiac arrest | 7 (13%) | |
| Sepsis | 6 (11%) | |
| Ischemic Stroke | 4 (7%)) | |
| Thoracic aortic disease | 2 (4%) | |
| Pneumonia | 2 (4%) | |
| Complications of renal failure | 2 (4%) | |
| Myelodysplasia | 1 (2%) |
AF or atrial flutter was the primary reason for the ED visit in 651 (78%) of our cohort. AF or atrial flutter was a complicating secondary diagnosis in the remainder. The most common triage complaints were chest pain (16.9%), shortness of breath (12.9%), and palpitations/arrhythmia (21.5%). More than half of the cohort, 494 patients (59.4%), achieved successful ventricular rate control at the time of ED disposition. A continuous AV nodal blocker infusion was administered in the ED to 144 (17.3%) patients. Among the 301 patients admitted who failed to achieve adequate rate control in the ED, 44 (14.6%) had a return visit to the ED within 30 days. Of these, 16 returned to the ED for AF with rapid ventricular response and all but one were readmitted.
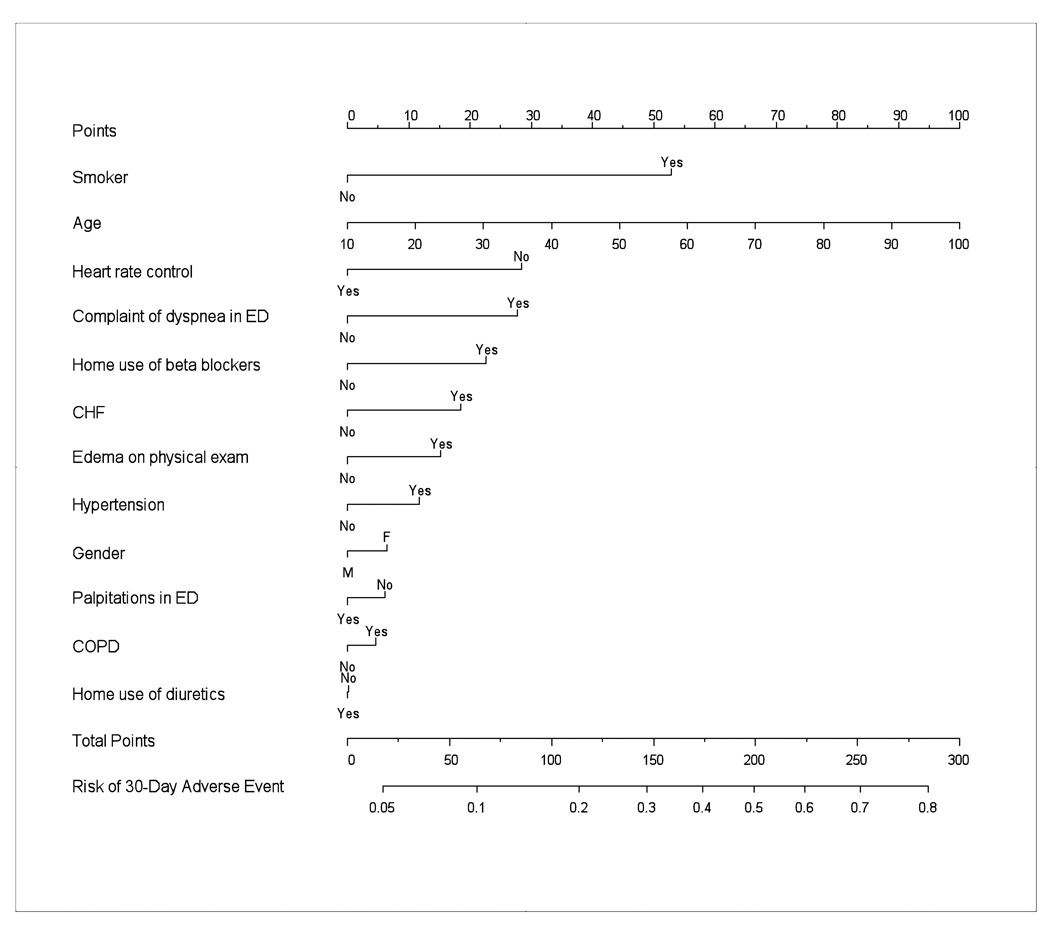
We selected 12 predictor variables for inclusion in the rule based on clinical relevance and a review of baseline descriptive statistics. No variables were removed from the a priori list due to overlapping information. Clinically meaningful interaction terms among these 12 predictor variables were tested as a group and failed to show significant contributions to the model. Therefore, they were not included in the final prediction rule. The odds ratios and 95% confidence intervals (95% CI) for the selected predictors’ impact on risk of 30-day adverse event in ED patients with symptomatic AF are presented in Table 3. Five of the 12 predictors met statistical significance at an α-level of 0.05. Increased age, inadequate ED ventricular rate control, ED complaint of dyspnea, smoking, and beta-blocker treatment were associated with an increased risk of a 30-day adverse event. Gender, diuretic use, heart failure, lower extremity edema, COPD, hypertension and a complaint of palpitations were not found to be statistically significant. Figure 1 provides a nomogram of our rule's predicted probabilities for 30-day adverse events. Table 4 gives predicted probabilities for 30-day adverse events that can be computed from the nomogram for 5 hypothetical patient examples with various risk factors.
Figure 1.
30-day adverse event prediction rule nomogram. Points are assigned for each of the 12 predictors. The total points correspond to an absolute predicted risk for 30-day adverse events. (This nomogram should not be used in clinical practice until an independent validation is completed).
Table 4.
Hypothetical patient examples with the rule's calculated predicted probability of 30-day adverse event
| Case | Age | Sex | Dyspnea in ED |
Smoker | Use of Beta Blocker |
Use of Diuretic |
Heart Failure history |
Peripheral edema on |
Adequate rate control in ED |
History of COPD |
History of Hypertension |
Palpitations in ED |
Calculated Probability of 30-day adverse event |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | F | NO | NO | NO | NO | NO | NO | YES | NO | NO | YES | 0.08 |
| 2 | 52 | M | YES | YES | NO | NO | NO | NO | YES | YES | NO | NO | 0.27 |
| 3 | 72 | F | YES | NO | YES | YES | YES | YES | YES | NO | YES | YES | 0.39 |
| 4 | 77 | M | NO | YES | NO | YES | YES | NO | NO | YES | YES | NO | 0.49 |
| 5 | 86 | M | YES | YES | YES | YES | YES | YES | NO | YES | YES | NO | 0.77 |
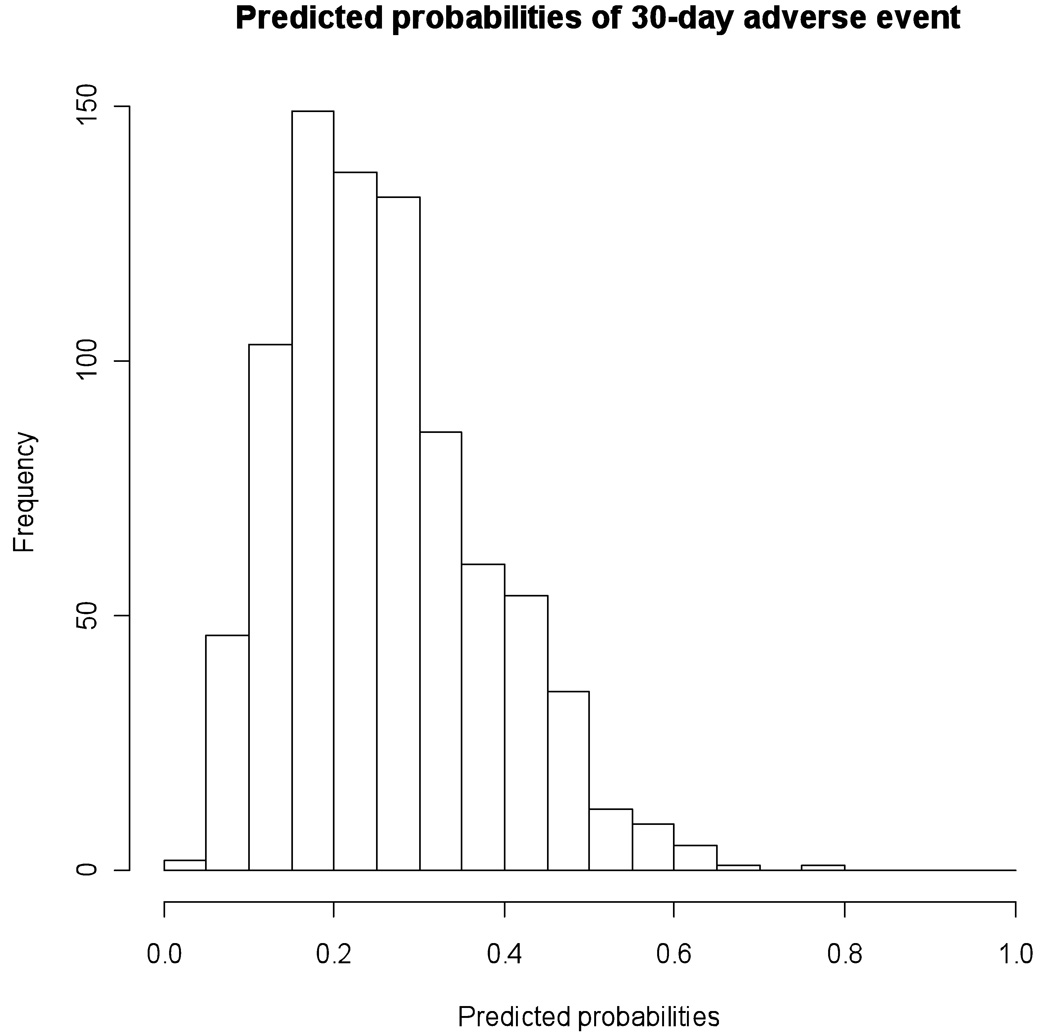
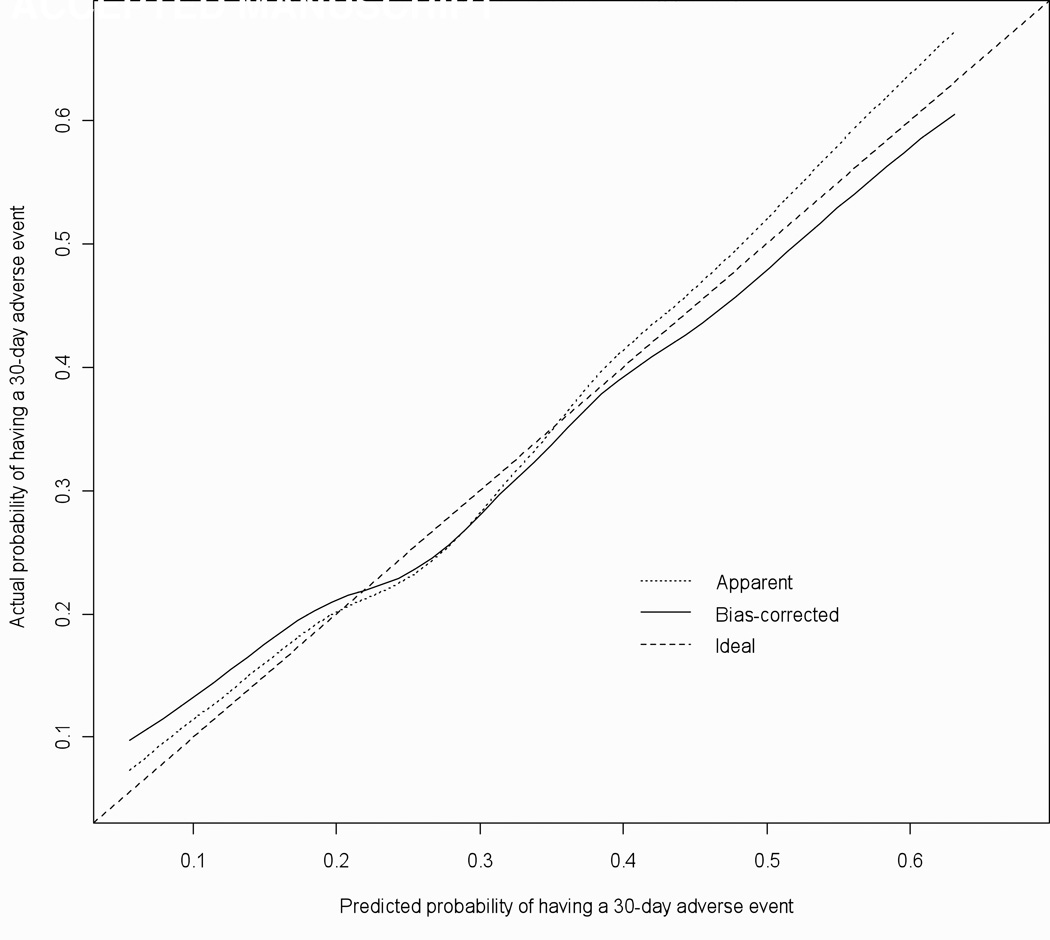
The AF rule's predictive discrimination was modest with a C-statistic of 0.67 (95% CI =0.63, 0.71). Figure 2 illustrates the histogram of predicted probabilities from the model. Figure 3 depicts the prediction rule’s calibration curve.41, 43 The calibration accuracy for the original maximum likelihood model ('Apparent') and the bootstrap model ('Bias-corrected') would be perfect if both lines fell along the 'Ideal' line of unity for actual and predicted probabilities of having a 30-day adverse event. In Figure 3, we see that the 'bias-corrected' estimate is slightly non-linear but only slightly worse than the 'apparent' calibration. The 0.9 quantile of absolute error in predicted probabilities between the 'bias corrected' and 'apparent' model is 0.03 suggesting only a small degree of bias from overfitting in the original model.
Figure 2.
Histogram of predicted probabilities of 30-day adverse events This figure illustrates the histogram of predicted probabilities from the model and shows that 3.4% of subjects had predicted probabilities > 0.50 and 5.8% had predicted probabilities < 0.10.
Figure 3.
Calibration Plot for AF Clinical Prediction Model This plot illustrates the calibration accuracy of the original model ("Apparent") and the boot-strap model ("Bias-corrected") for 30-day adverse events with lowess smoothing used to model the relationship between actual and predicted probabilities.. As can be seen, the model's calibration function estimate is slightly non-linear with the corrected calibration showing good agreement with the apparent calibration.
As a sensitivity analysis, we measured the prediction rule's performance on a more refined serious adverse event outcome that excluded the 25 return ED visits not requiring hospitalization. The model's adjusted odds ratios are presented in column B) of Table 3 and the rule's predictive discrimination C-statistic was 0.70. This revised model had very similar odds ratios and 95% CI for the predictors with only patient history of heart failure replacing home use of beta-blocker medication as the fifth significant predictor.
We further examined whether hospitalization impacted an individual's odds of experiencing a 30-day adverse event. This secondary analysis showed no difference in model results or its predictive discrimination [column C) of Table 3]. A description of the inpatient diagnostic and therapeutic procedures is listed in Table 5. Interrater agreement between EMR reviewers ranged from moderate to perfect agreement (0.69 – 1.00). The interrater agreement for the composite outcomes was perfect for all (kappa= 1.0) except cardiovascular complication with a kappa of 0.73.
Table 5.
Summary of Inpatient Procedures during Initial Hospitalization (Total number of patients hospitalized = 638)
| Inpatient Procedure | N (total) |
No 30-day Adverse Event (N=457) |
Experienced a 30-day Adverse Event (N=181) |
|---|---|---|---|
| Thoracentesis | 9 | 3 | 6 |
| Insertion of Coronary artery stent |
14 | 12 | 2 |
| Cardiac catheterization |
47 | 31 | 16 |
| Electrophysiologic study |
9 | 9 | 0 |
| Ablation | 11 | 9 | 2 |
| Pacemaker Insertion | 26 | 20 | 6 |
| Pacemaker Revision | 5 | 4 | 1 |
| Hemodialysis | 14 | 12 | 2 |
| Transthoracic Echocardiogram |
60 | 44 | 16 |
| Transfusion of blood products |
51 | 31 | 20 |
| Atrial cardioversion | 18 | 15 | 3 |
| Other cardioversion | 31 | 25 | 6 |
| Intubation | 14 | 7 | 7 |
| Required continuous intravenous AV nodal blocking infusion in ED |
144 | 106 | 38 |
LIMITATIONS
To our knowledge, this study is the first to develop a clinical prediction model for 30-day adverse events among ED patients evaluated for AF. The results of this study cannot be used to draw any conclusions about the safety of discharging patients with symptomatic AF from the ED. The study was a retrospective cohort analysis and therefore is subject to the inherent limitations of such studies. We did not prospectively collect data on predictors or the outcomes and there is the potential that missing data might bias our results.
We limited our candidate predictors to data that is available to ED physicians early in the patient evaluation. The prediction model did not include laboratory studies, such as troponin and brain natriuretic peptide, that were measured in only a minority of patients as there is likely selection bias in the physician ordering of these studies. Patients might have experienced additional events within the 30 days that were treated at other hospitals and not recorded in our database. We did examine follow-up clinic notes, electronic and telephone clinical communication reports and searched for mention of any events since the original ED visit. Internists or cardiologists at Vanderbilt follow the majority of our patients closely. There were only 2 patients in the study that were out-of-state visitors and had no further records following their ED visit. The potential for undocumented adverse events might result in an underestimate of the actual incidence of 30-day adverse events. In addition, this study was conducted at a single tertiary referral center ED that might introduce selection and referral bias and limit applicability to patients treated in other settings.
Our decision to include all ED patients treated for symptomatic AF might be criticized as clearly patients with an acute CVA will not be candidates for ED discharge. The majority (78%) of patients in our cohort visited the ED for primary AF-related complaints. Our definition of adverse events that included an AF-related return visit to the ED or unscheduled hospitalization might be criticized as overly conservative. We chose these conservative outcome definitions so that our model would identify the lowest risk patients. Given the significant practice variation in the management of AF, high admission rate for AF, and that this is an initial study in the development of a novel ED-based AF prediction rule, we decided to measure all important predictors and potential serious outcomes in all eligible patients from our study cohort. We intend our clinical prediction model to assist, not replace, physician decision making. We would expect that physician gestalt take precedence over the prediction model when patients are unstable and result in appropriate hospitalization. The results of this paper cannot be used to determine appropriateness of discharge or to derive guidelines about appropriate utilization. All prediction rules, including this AF rule, must be prospectively validated in an independent diverse patient population prior to use in patient care. This rule developed in a primarily inpatient cohort, if validated, will require further study to determine whether outpatient treatment is safe in the patients identified as low risk. Our hope is that this prediction rule will be validated and would assist ED physicians with the disposition decision-making in stable patients.
Heart rate fluctuation is the norm for AF and there is the potential for misclassification bias with regard to adequate rate control. We recorded only the heart rate at time of ED disposition and did not continuously record heart rates throughout the ED stay. There is potential that patients might have been misclassified as inadequate rate control based on a single falsely elevated measurement. This might result in adequate rate control being a less reliable predictor in the model.
Patient disposition might have potentially impacted the primary outcome. The decision to hospitalize patients with AF is often subjective and multi-factorial based on the patient's acute and chronic conditions. The incidence of adverse events was 10% greater among admitted patients than those discharged from the ED. This might reflect that hospitalized patients represent a sicker cohort at higher risk for adverse events despite treatments initiated in the hospital. Furthermore, the inpatient hospital workup is not standardized and patients underwent various diagnostic and therapeutic interventions while hospitalized. An inpatient intervention, such as a pacemaker placement, might reduce the risk of a 30-day adverse event whereas another intervention (i.e. initiating a new antiarrhythmic medication) might increase the risk of an event. We examined the impact of hospitalization on our prediction rule's performance and found no difference in the model's performance. We also recorded the time to adverse event among the patients discharged from the ED to investigate whether hospitalization might have prevented the outcome or resulted in patient reclassification (i.e. a cardiovascular complication that occurs during the initial hospitalization [not counted as positive outcome] rather than as outpatient). The median time to adverse event was 1 week longer than the median hospital length of stay demonstrating that these outcomes did not take place while the admitted patients were still hospitalized.
DISCUSSION
We found 5 significant predictors of 30-day adverse events - age, smoking, complaint of dyspnea, inadequate heart rate control in the ED, and home beta-blocker use. We limited predictors to those variables that would be readily available to treating physicians during their initial evaluation. The ultimate goal of our research is to accurately identify patients who are low risk for adverse outcomes and can be safely discharged from the ED. This study is the initial step in the development of a prediction rule to achieve that goal. Our prediction rule should not be used to determine whether a patient is appropriate for ED discharge until it is prospectively validated.
Presently, in the United States, more than 2 out of every 3 patients presenting to an ED with symptomatic AF are hospitalized.14–16 Significant practice variation occurs between US regions with 76% admission rates in the Northeast versus 48% in the West.11 Despite this regional variation, however, the admission rate is more than double the 29% admission rates reported in a large European study.44 The ACC/AHA/ESC 2006 guidelines for the management of patients with AF state that management involves 3 objectives: rate control, prevention of thromboembolism, and correction of the rhythm disturbance.16 According to the guidelines, a patient with a first-documented episode of AF, who achieves adequate rate control, does not need to be hospitalized.16 In our study, 84% of patients with a new AF diagnosis were hospitalized despite nearly half (48%) of these patients achieving successful ventricular rate control in the ED.
Emergency physicians need to feel confident identifying stable, low risk patients with AF. A highly accurate, easy to use, prediction rule based on validated risk assessments is needed to accomplish this practice change. The incorporation of previous decision rules into emergency medicine practice has resulted in decreased admissions for low-risk patients with acute chest pain and community-acquired pneumonia.45, 46 AF prediction rules have primarily focused on maintenance of sinus rhythm, reducing the risk of stroke and overall Mortality.21–24, 28, 47–56 One such example is the validated CHADS2 score for predicting the stroke risk in AF patients.21 Patients with age ≥ 75 years of age with hypertension, diabetes, or prior stroke/TIA are at moderate to high risk of subsequent stroke.21 Similarly, a prospective analysis of the Framingham Heart Study found that advancing age, female sex, increasing systolic blood pressure, prior stroke or TIA, and diabetes were also associated with an increased risk of stroke in individuals with AF.28 While these outpatient studies provide excellent candidate predictors, they do not address the acutely symptomatic ED patient.
Determining severity of AF exacerbations in the ED is difficult and imprecise. Many patients have significant cardiac and non-cardiac co-morbidities serving as precipitants or contributors to patient instability.11, 44 For example, AF is known to occur with acute myocardial infarction; patients are frequently admitted to the hospital to exclude acute coronary syndrome as the cause of their AF.19, 29, 57 Previous ED-based studies found that patients with AF and without evidence of significant ST-segment changes (ST-segment elevation or >2mm ST- depression) are at very low risk for acute myocardial infarction and that AF did not change the relative risk of acute coronary syndrome in patients at an urban ED with chest pain syndromes.29, 57
Physicians currently have no validated clinical prediction rules to assist with the decision to hospitalize an ED patient with symptomatic AF. The first branch point in this decision process often is whether a patient can be successfully rate controlled in the ED. Inadequate ventricular rate control in the ED increases the risk for a 30-day adverse event in our prediction model. In this study, physicians hospitalized 20% fewer patients who achieved successful ventricular rate controlled in the ED although the admission rate for these patients remained high at 65%.
This prediction rule identified 5 variables that are associated with a patient having an increased risk of experiencing an adverse event within 30 days of their ED visit. Previous studies have linked increasing age, smoking, and a complaint of dyspnea with AF-associated adverse events including stroke and death.21, 22, 28 Patients who were unable to be adequately rate controlled in the ED had increased risk of adverse events. This may be the result of associated illness (infections, dehydration) that triggered or exacerbated their AF, inadequate rate control with current AV nodal blocking drugs, or suboptimal acute treatment of the AF in the ED. Patients on beta-blockers were at increased risk for adverse events. This surprising result might reflect inadequate rate control with their current AV nodal blocking drug regimen, associated heart failure or hypertension, or some other unmeasured predictor. We intend to further study these associations in a prospective study.
In summary, our study identified 5 important predictors for experiencing a 30-day adverse event among patients presenting to the ED with symptomatic AF. This study suggests that patients with increased age, smoking history, complaint of dyspnea, inadequate ventricular rate control in the ED, and home beta-blocker therapy are more likely to experience an AF-related adverse event within 30 days.
Acknowledgments
No industry financial support or compensation was received for conducting this study. The study was entirely funded by the Vanderbilt University Medical Center Physician Scientist Development Program (supported in part by Vanderbilt CTSA grant 1 UL1 RR024975 from NCRR/NIH) and the Department of Emergency Medicine Research Division. Dr. Darbar and Dr. Roden are supported in part by NIH grants U01 HL65962 and R01 HL092217.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reprints not available from the authors.
Paper presented at 2009 Academic College of Emergency Physicians Scientific Assembly, Boston, Massachusetts, October 5, 2009.
References
- 1.Benjamin EJ, Chen PS, Bild DE, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Feinberg WM, Blackshear JL, Laupacis A, et al. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 5.Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 6.Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 7.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Dawber TR, Thomas HE, Jr, et al. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28:973–977. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 9.Koenig BO, Ross MA, Jackson RE. An emergency department observation unit protocol for acute-onset atrial fibrillation is feasible. Ann Emery Med. 2002;39:374–381. doi: 10.1067/mem.2002.122785. [DOI] [PubMed] [Google Scholar]
- 10.Lip GY, Watson T, Shantsila E. Anticoagulation for stroke prevention in atrial fibrillation: is gender important? Eur Heart J. 2006;27:1893–1894. doi: 10.1093/eurheartj/ehl140. [DOI] [PubMed] [Google Scholar]
- 11.McDonald AJ, Pelletier AJ, Ellinor PT, et al. Increasing US emergency department visit rates and subsequent hospital admissions for atrial fibrillation from 1993 to 2004. Ann Emerg Med. 2008;51:58–65. doi: 10.1016/j.annemergmed.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Scott PA, Pancioli AM, Davis LA, et al. Prevalence of atrial fibrillation and antithrombotic prophylaxis in emergency department patients. Stroke. 2002;33:2664–2669. doi: 10.1161/01.str.0000035260.70403.88. [DOI] [PubMed] [Google Scholar]
- 13.Coyne KS, Paramore C, Grandy S, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 14.Friberg J, Buch P, Scharling H, et al. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003;14:666–672. doi: 10.1097/01.ede.0000091649.26364.c0. [DOI] [PubMed] [Google Scholar]
- 15.Estes NA, 3rd, Halperin JL, Calkins H, et al. ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation): developed in collaboration with the Heart Rhythm Society. Circulation. 2008 Feb 26;117(8):1101–1120. doi: 10.1161/CIRCULATIONAHA.107.187192. [DOI] [PubMed]
- 16.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cordial. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed]
- 17.Decker WW, Smars PA, Vaidyanathan L, et al. A prospective, randomized trial of an emergency department observation unit for acute onset atrial fibrillation. Ann Emerg Med. 2008;52:322–328. doi: 10.1016/j.annemergmed.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Stiell IG, Clement CM, Symington C, et al. Emergency department use of intravenous procainamide for patients with acute atrial fibrillation or flutter. Acad Emerg Med. 2007;14:1158–1164. doi: 10.1197/j.aem.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Zimetbaum P, Reynolds MR, Ho KK, et al. Impact of a practice guideline for patients with atrial fibrillation on medical resource utilization and costs. Am J Cardiol. 2003;92:677–681. doi: 10.1016/s0002-9149(03)00821-x. [DOI] [PubMed] [Google Scholar]
- 20.Dankner R, Shahar A, Novikov I, et al. Treatment of Stable Atrial Fibrillation in the Emergency Department: A Population-Based Comparison of Electrical Direct-Current versus Pharmacological Cardioversion or Conservative Management. Cardiology. 2008;112:270–278. doi: 10.1159/000151703. [DOI] [PubMed] [Google Scholar]
- 21.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 22.Khumri TM, Idupulapati M, Rader VJ, et al. Clinical and echocardiographic markers of mortality risk in patients with atrial fibrillation. Am J Cardiol. 2007;99:1733–1736. doi: 10.1016/j.amjcard.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 23.Loricchio ML, Cianfrocca C, Pasceri V, et al. Relation of C-reactive protein to long-term risk of recurrence of atrial fibrillation after electrical cardioversion. Am J Cardiol. 2007;99:1421–1424. doi: 10.1016/j.amjcard.2006.12.074. [DOI] [PubMed] [Google Scholar]
- 24.Mahe I, Drouet L, Simoneau G, et al. D-dimer can predict survival in patients with chronic atrial fibrillation. Blood Coagul Fibrinolysis. 2004;15:413–417. doi: 10.1097/01.mbc.0000114440.81125.bd. [DOI] [PubMed] [Google Scholar]
- 25.Miyasaka Y, Barnes ME, Gersh BJ, et al. Changing trends of hospital utilization in patients after their first episode of atrial fibrillation. Am J Cardiol. 2008;102:568–572. doi: 10.1016/j.amjcard.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrea DN, Ekmektzoglou KA, Vlachos IS, et al. A formula for the stratified selection of patients with paroxysmal atrial fibrillation in the emergency setting: A retrospective pilot study. J Emerg Med. 2008 doi: 10.1016/j.jemermed.2008.02.062. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Vaglio JR, Kucera S, Stubblefield G, et al. Genetic and clinical predictors of response to rate control therapy in patients with atrial fibrillation. Circulation. 2008;118:S_828. [Google Scholar]
- 28.Wang TL, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 29.Zimetbaum PJ, Josephson ME, McDonald MJ, et al. Incidence and predictors of myocardial infarction among patients with atrial fibrillation. J Am Coll Cardiol. 2000;36:1223–1227. doi: 10.1016/s0735-1097(00)00828-7. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert EH, Lowenstein SR, Koziol-McLain J, et al. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med. 1996;27:305–308. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690–1696. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 32.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 33.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 34.Steyerberg EW. Clinical Prediction Models - A practical approach to development, validation, and updating. New York: Springer; 2009. [Google Scholar]
- 35.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Glantz SA, Slinker BK. Primer of Applied Regression and Analysis of Variance. New York: McGraw-Hill; 1990. [Google Scholar]
- 37.Moons KG, Donders AR, Steyerberg EW, et al. Penalized maximum likelihood estimation to directly adjust diagnostic and prognostic prediction models for overoptimism: a clinical example. J Clin Epidemiol. 2004;57:1262–1270. doi: 10.1016/j.jclinepi.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 38.Donders AR, van der Heijden GJ, Stijnen T, et al. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 39.van der Heijden GJ, Donders AR, Stijnen T, et al. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol. 2006;59:1102–1109. doi: 10.1016/j.jclinepi.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Harrell FE. Hmisc: Harrell Miscellaneous. R package version 3.6-0. 2009 and with contributions from many other users http://CRAN.R-proiect.org/package=Hmisc.
- 41.Harrell FE. Design: Design Package. R package version 2.2-0. 2009 http://CRAN.R-project.orR/package=Design.
- 42.R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2009. R: A language and environment for statistical computing. ISBN 3-900051-07-0, URL http://www.R-prroject.org. [Google Scholar]
- 43.Harrell FE Jr, editor. Sections 5.2.5 and 10.9 In: Regression Modeling Strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 44.del Arco C, Martin A, Laguna P, et al. Analysis of current management of atrial fibrillation in the acute setting: GEFAUR-1 study. Ann Emerg Med. 2005;46:424–430. doi: 10.1016/j.annemergmed.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Gibler WB, Runyon JP, Levy RC, et al. A rapid diagnostic and treatment center for patients with chest pain in the emergency department. Ann Emerg Med. 1995;25:1–8. doi: 10.1016/s0196-0644(95)70347-0. [DOI] [PubMed] [Google Scholar]
- 46.Marrie TL, Lau CY, Wheeler SL, et al. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA. 2000;283:749–755. doi: 10.1001/jama.283.6.749. [DOI] [PubMed] [Google Scholar]
- 47.Dogan A, Avsar A, Ozturk M. P-wave dispersion for predicting maintenance of sinus rhythm after cardioversion of atrial fibrillation. Am J Cardiol. 2004;93:368–371. doi: 10.1016/j.amjcard.2003.09.064. [DOI] [PubMed] [Google Scholar]
- 48.Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 49.Lip GY, Patel JV, Hughes E, et al. High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke. 2007;38:1229–1237. doi: 10.1161/01.STR.0000260090.90508.3e. [DOI] [PubMed] [Google Scholar]
- 50.Liu T, Li G, Li L, et al. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol. 2007;49:1642–1648. doi: 10.1016/j.jacc.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 51.Novo G, Mansueto P, La Franca ML, et al. Risk factors, atrial fibrillation and thromboembolic events. Int Angiol. 2008;27:433–438. [PubMed] [Google Scholar]
- 52.Shimizu H, Murakami Y, Inoue S, et al. High plasma brain natriuretic polypeptide level as a marker of risk for thromboembolism in patients with nonvalvular atrial fibrillation. Stroke. 2002;33:1005–1010. doi: 10.1161/hs0402.105657. [DOI] [PubMed] [Google Scholar]
- 53.Vene N, Mavri A, Kosmelj K, et al. High D-dimer levels predict cardiovascular events in patients with chronic atrial fibrillation during oral anticoagulant therapy. Thromb Haemost. 2003;90:1163–1172. doi: 10.1160/TH03-06-0363. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe D, Shizuka K, Koyama S, et al. Plasma brain natriuretic peptide levels indicating thromboembolism in very elderly patients with nonvalvular atrial fibrillation. Circ J. 2007;71:1446–1451. doi: 10.1253/circj.71.1446. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe E, Arakawa T, Uchiyama T, et al. High-sensitivity C-reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion. Int J Cardiol. 2006;108:346–353. doi: 10.1016/j.ijcard.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 56.Zohar P, Kovacic M, Brezocnik M, et al. Prediction of maintenance of sinus rhythm after electrical cardioversion of atrial fibrillation by non-deterministic modelling. Europace. 2005;7:500–507. doi: 10.1016/j.eupc.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Brown AM, Sease KL, Robey JL, et al. The risk for acute coronary syndrome associated with atrial fibrillation among ED patients with chest pain syndromes. Am J Emerg Med. 2007;25:523–528. doi: 10.1016/j.ajem.2006.09.015. [DOI] [PubMed] [Google Scholar]