Abstract
Recent studies suggest that individual hippocampal subregions perform distinct cognitive operations and are differentially targeted by aging and disease. Although originally developed to assess global hippocampal function, whether performance on standard memory tests used in neuropsychological batteries is associated with individual hippocampal subregions remains unknown. Here we addressed this issue by imaging 210 neuropsychologically-characterized subjects using a high-resolution variant of functional magnetic resonance imaging (fMRI) that generates maps reflective of basal hippocampal metabolism. Regression analysis revealed memory tests that differentially associate with 2 hippocampal subregions, the entorhinal cortex (EC) and the dentate gyrus (DG). Whereas performance on the delayed retention component of the Selective Reminding Test was associated with the EC, performance on the recognition component of the Benton Visual Retention Test was associated with the DG. Furthermore, elevation in blood glucose, previously shown to target the DG, was found to correlate selectively with the recognition component of the Benton Visual Retention Test. These findings provide further evidence that the hippocampal subregions perform distinct roles, and, interpreted in the context of previous neuropsychological and imaging studies, confirm that aging and Alzheimer’s disease target different hippocampal subregions.
INTRODUCTION
Nearly all studies investigating the effect aging and disease has on human cognition employ a battery of neuropsychological tests that have been characterized and standardized through decades of experience. Typically these batteries include memory tests putatively sensitive to the function of the hippocampal formation. Notably, these tests were originally developed to assess global hippocampal function, to contrast with performance on tests sensitive to the function in other general brain areas. Recent studies suggest, however, that each of the individual subregions that make up the hippocampal formation performs a distinct cognitive and computational operation (Nakazawa, Quirk et al. 2002; Kent, Hess et al. 2007; Leutgeb, Leutgeb et al. 2007; McHugh, Jones et al. 2007; Bakker, Kirwan et al. 2008; Brun, Leutgeb et al. 2008), and that individual subregions are differentially vulnerable to mechanisms of hippocampal dysfunction (West, Coleman et al. 1994; Small, Perera et al. 1999; Small, Chawla et al. 2004; Moreno, Wu et al. 2007; Wu, Brickman et al. 2008; Schobel, Lewandowski et al. 2009).
Whether standard memory tests used in neuropsychological batteries reflect the function of different hippocampal subregions remains unknown. Addressing this issue is important as it can provide additional evidence that hippocampal function can be parsed according to its individual subregions and can further clarify the distinct roles played by each subregion. Moreover, linking standardized memory tests to individual subregions will enhance the interpretive abilities of neuropsychological studies that have begun characterizing the cognitive profile associated with aging and hippocampal-dependent disorders such as the early stages of Alzheimer’s disease.
With these goals in mind, we generated functional maps of the hippocampal formation in 210 neuropsychologically-characterized subjects using a variant of functional magnetic resonance imaging (fMRI) that measures basal cerebral blood volume (CBV). As with other functional imaging variables, CBV is hemodynamically coupled to oxygen metabolism (Belliveau, Kennedy et al. 1991; Mandeville, Marota et al. 1998; Mandeville, Jenkins et al. 2001; Shen, Ren et al. 2008), and is tightly correlated with other basal measures of brain function, such as cerebral blood flow and deoxyhemoglobin as measured with MRI (Mandeville, Marota et al. 1998; Mandeville, Jenkins et al. 2001; Shen, Ren et al. 2008), or glucose uptake as measured with positron emission tomography (Gonzalez, Fischman et al. 1995). Among all functional imaging approaches, measuring steady-state basal CBV with MRI provides the highest spatial resolution (Lin, Celik et al. 1999), a feature that enhances the ability to visualize individual hippocampal subregions. Indeed, previous studies have used CBV mapping to pinpoint dysfunction in select regions of the hippocampal formation in aging (Small, Chawla et al. 2004), Alzheimer’s disease (Moreno, Wu et al. 2007), schizophrenia (Schobel, Lewandowski et al. 2009), and diabetes (Wu, Brickman et al. 2008).
The subjects all received a standard neuropsychological battery that contained a commonly-used verbal and non-verbal hippocampal-dependent test, the Selective Reminding Test (SRT) (Buschke and Fuld 1974) and the Benton Visual Retention Test (BVRT)(Benton 1994). In the recognition component of the BVRT subjects are briefly shown a visual pattern then asked to identify the pattern they saw among 3 other novel patterns. In the SRT, during a encoding phase subjects first learn a list of words presented aurally, and then after a 15 minutes delay are asked to freely recall the words. In a previous study using a smaller number of subjects that consisted of 11 Alzheimer’s disease patients and 11 controls, we examined ‘delayed recall’, the absolute number of words recalled after the delay (Moreno, Wu et al. 2007). Delayed recall, however, reflects both the number of words encoded during the learning phase and the number of words retained during the delay, the latter which is thought to be most sensitive to hippocampal function (Squire, Stark et al. 2004). In this study we focused on ‘delayed retention’, derived by normalizing the number of words recalled after delay by the number of words encoded after the learning phase. Delayed retention is particularly interesting because it has been shown to be affected in the earliest stages of Alzheimer’s disease, decades before the onset of disease (Elias, Beiser et al. 2000), and relatively unaffected by aging (Albert 1997).
After identifying a dissociation linking hippocampal subregions to different memory performance metrics, we then set out to confirm and extend our findings by exploiting previous imaging studies in humans, non-human primates, and mice showing that elevations in blood glucose levels targets the dentate gyrus (Wu, Brickman et al. 2008). Accordingly, we measured blood glucose levels in subset of subjects and tested for its association with the hippocampal-dependent memory tests.
METHODS
Subjects
Participants were part of a community-based study of elderly subjects (65 years and old) who together with brain imaging (Brickman, Schupf et al. 2008) received a detailed neuropsychological, neurological, and medical evaluation (Stern, Andrews et al. 1992). Within this cohort hippocampal CBV maps were generated in 210 subjects (mean age = 79, 63% female) who had: A) no evidence of Alzheimer’s disease or other neurological disorders, as determined by a consensus conference made up of neurologists and neuropsychologists; B) no evidence of preclinical Alzheimer’s disease, based on a specific cognitive profile of mild cognitive impairment that we have previously found to be indicative of the pre-dementia stage of the disease in this community-based population (Manly, Tang et al. 2008). Serum glucose measured in microIU/ml from serum collected within one month of the brain MRI and frozen at −70 °C. Glucose levels were measured on a Hitachi automated spectrophotometer (model 704, Hitachi Ltd, Tokyo, Japan) using commercial kits obtained from Wako Chemicals (Richmond, VA).
Memory tests
The Benton Visual Retention Test in an object recognition memory paradigm. Participants view a visual pattern and then are asked to select the target from an array of 4 patterns, 3 of which are distracters. There are ten trials on the task and the relevant outcome variable is the number of correct trials. In each trial, the subject is shown a visual object stimulus for 10 seconds, it is removed, and then a 2 × 2 array of 3 foils and 1 target is displayed. The subject’s task is to select the target stimulus. During a 10-trial BVRT matching block, subjects are shown the target stimulus and the 4-choice array simultaneously. Subjects are asked simply to indicate which of the 4 stimuli is identical to the target. Stimuli used during the matching trials are distinct from those used in the recognition trials. The Selective Reminding Test (Buschke and Fuld 1974) was administered to participants to assess verbal learning and memory. Participants are presented with 6 trials to learn 12 semantically unrelated words aurally. After each attempt to recall the list, subjects are reminded only of the words that were not recalled and then asked to recall the entire list (‘total recall’). Subjects are asked to recall as many words as possible from the list after a 15-minute delay. ‘Delayed recall’ is the total number of words recalled after delay, and ‘delayed retention’ is the total number of words recalled after delay normalized by number of items recalled on the last learning trial.
Imaging
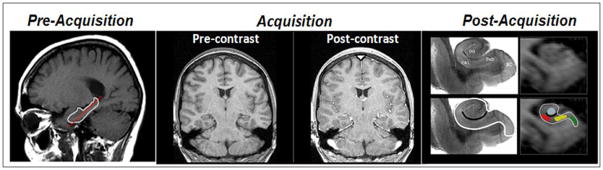
The technical and analytic details of how CBV maps of the human hippocampal formation were generated has been previously described (Moreno, Wu et al. 2007) (Fig. 1). Briefly, a 1.5 Tesla scanner (Philips Intera) was used to acquire oblique coronal 3D T1-weighted images (TR=20ms; TE=6ms; flip angle=25 degrees; in plane resolution=0.86mm × 0.86mm; slice thickness=3mm), perpendicular to the hippocampal long axis (Fig. 1B), before and 4-minutes after IV administration of gadolinium-pentate (Omniscan, 0.1mmol/kg). Then, the post-contrast images were subtracted from pre-contrast images, and the difference in the superior sagittal sinus, which serves as an estimate of the image intensity change of 100% blood, was recorded (Lin, Celik et al. 1999; Moreno, Wu et al. 2007). Finally, the subtracted image was divided by the difference in the top 4 pixels measured from the sagittal sinus and multiplied by 100 yielding percent CBV. In all cases, a single ideal slice was identified, anterior to the lateral geniculate nucleus and posterior to the uncus, that contains all hippocampal subregions and provides sufficient anatomical information to parse the subregions (Moreno, Wu et al. 2007) (Fig. 1B). Because spatial co-registration across subjects is problematic when evaluating small regions in clinical populations, strict anatomical criteria (Moreno, Wu et al. 2007) were used to identify the following regions-of-interest within the hippocampal formation: The entorhinal cortex, dentate gyrus, CA1 subfield, and the subiculum. The CA3 subregion cannot be reliably visualized because of age-related atrophy. On an individual basis, mean CBV values were measured for each hippocampal ROI and used for group data analysis.
Figure 1. Mapping cerebral blood volume (CBV) in the hippocampal formation.
Left panel: During pre-acquisition, slices were acquired perpendicular to the long axis of the hippocampus (indicated in red).
Middle panel: Images were acquired ‘pre’ and 4 minutes ‘post’ intravenous injection of gadolinium
Right panel: During post-acquisition, anatomical landmarks were used to identify hippocampal regions-of-interest. Upper and lower left panels show histological slice of the hippocampal formation. Upper right panel shows MRI slice of the hippocampal formation, Lower right panel shows regions-of-interest. Green=entorhinal cortex; blue=dentate gyrus; red=CA1 subfield; yellow=subiculum
Data analysis
To test for an association between cognitive performance and hippocampal subregions, a step-wise multiple regression model was constructed in which cognitive performance was included as the dependent variable, CBV measured from the 4 hippocampal subregions (EC, DG, CA1, SUB) were included as the independent variables and demographic data (age, gender, ethnicity, and education) were included as covariates.
RESULTS
By including the CBV values of hippocampal subregions into a single regression model, the dentate gyrus (DG) was found to be the only hippocampal subregion related to performance on the Benton recognition (beta=0.13, p=0.02) (Table 1 and Fig. 2). Among the other hippocampal subregions, CBV measured in the entorhinal cortex (EC), was least associated with performance (beta=0.02, p=0.68) (Table 1 and Fig. 2). In contrast, the EC was the only hippocampal subregion linked to delayed retention (beta=0.15, p=0.03), while the DG was least associated with performance (beta=−0.03, p=0.61) (Table 1 and Fig. 2).
Table 1.
Correlations between CBV measured in the hippocampal subregions and memory performance
| EC | DG | CA1 | SUB | ||
|---|---|---|---|---|---|
| Benton Recognition | Correlation | 0.02 | 0.13 | −0.06 | 0.10 |
| p value | 0.68 | *0.02 | 0.23 | 0.08 | |
| Delayed Retention | Correlation | 0.15 | −0.03 | 0.05 | 0.10 |
| p value | *0.03 | 0.61 | 0.45 | 0.15 |
Figure 2. Hippocampal subregions differentially associate with memory tests.
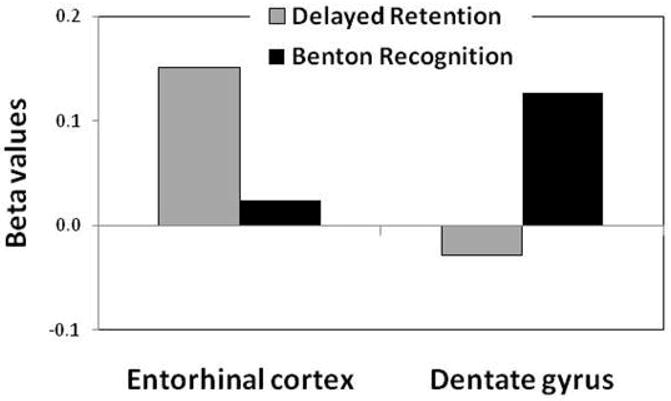
Cerebral blood volume (CBV) in the entorhinal cortex was differentially associated with delayed free retention, while CBV in the dentate gyrus was differentially associated with the Benton recognition. Y-axis=beta values taken from regression analysis.
To expand the focus, in a secondary analysis we used a partial correlational model in which included the CBV of 4 hippocampal subregions (EC, DG, CA1, Subiculum), 4 components of the selective reminding test (total recall, last-trial recall, delayed recall, and delayed retention), and the 2 components of the BVRT (recognition and matching), controlling for sex, age, education, and ethnicity. Beyond confirming the specificity of the effects, (supplementary table), this secondary analysis confirmed our previous observation that delayed recall is also associated with EC CBV (correlation coefficient=0.18; p=0.01). Importantly, immediate recall on the last trial was not significantly correlated with the EC (correlation coefficient=0.07; p=0.30) or other hippocampal subregions, suggesting that the association between EC and delay retention/recall is dependent on the delay period (i.e., forgetting) per se and not by the amount of information that was encoded. Additionally, there was not a significant association between performance on the BVRT matching trials and DG CBV (correlation coefficient =0.10; p=0.15) or any other hippocampal subregion, suggesting the association observed with BVRT recognition is not related to potential confounds in visual processing.
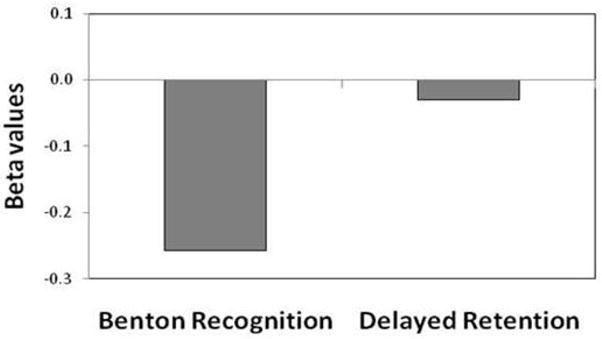
To extend the primary observations we measured blood glucose levels in 152 of subjects. Regression analysis showed that performance in the BVRT recognition, but not performance on delayed retention, was inversely and significantly linked to blood glucose (beta=−0.26, p=0.02) (Fig. 3). In secondary analyses we expanded the correlational analysis, and found no association between blood glucose and any other cognitive measure, including delayed recall (correlation coefficient = −0.62; p=0.46), last trial recall (correlation coefficient = −0.12; p=0.16), or BVRT matching (correlation coefficient = −0.13; p=0.12)
Figure 3. Blood glucose levels are differentially associated with Benton recognition.
DISCUSSION
Although neuropsychological tests were originally designed without the anatomical complexity of the hippocampal formation in mind, the results of this large-scale fMRI study suggest an anatomical dissociation between delayed retention on a word learning test and immediate recognition of novel visual patterns. Specifically, while delayed free recall/retention was associated with the EC, recognition of visual patterns was associated with the DG. The observation that elevation in blood glucose, previously found to target the DG not the EC, is inversely and selectively correlated with recognition of visual patterns further confirms these findings.
Although it important to note that the two memory tests differ in many ways, we can interpret our findings in the context of previous studies using non-standard memory tests that have begun uncovering distinct cognitive operations in individual hippocampal subregions. Influenced by computational and rodent studies (Leutgeb, Leutgeb et al. 2007; McHugh, Jones et al. 2007), human fMRI studies have established that the DG (Bakker, Kirwan et al. 2008), not the EC, plays in important role in pattern separation. Pattern separation is the computational process by which the hippocampal formation orthogonalizes the neural representation of similar stimuli. Importantly, previous studies have suggested that the cognitive operation that underlies pattern separation begins in the DG, not the EC (Colgin, Moser et al. 2008). The recognition component of the BVRT relies heavily on pattern separation, which can account for why performance on this task was found to associate with a functional measure of the DG. We note, however, that the BVRT was not explicitly designed to test pattern separation, and so this interpretation should be tested in future studies. In particular, a variation of this task that includes a greater number of items that vary by degree of similarity will be able to more formally test for pattern separation effects.
The observation that the EC CBV is selectively linked to delayed retention/recall but not immediate recall suggests that the EC might play a role during the delay period. Interestingly, a convergence of findings has implicated the EC in maintenance of memory representations across a delay. These observations were first found by unit recordings in monkeys and rats(Suzuki, Miller et al. 1997; Young, Otto et al. 1997), but have recently extended to include numerous human studies. By fMRI, studies have found that sustained EC activation during encoding predicts performance on delayed cue-recall (Fernandez, Brewer et al. 1999) or delayed recognition(Schon, Atri et al. 2005), and that sustained EC activation during the ‘delay’ in a delay-match-to-sample task (Schon, Hasselmo et al. 2004). Additionally, a recent unit recording study in the medial temporal lobe show that sustained activity in the EC in a delayed free recall task(Gelbard-Sagiv, Mukamel et al. 2008). The interpretation that the EC plays an active mnemonic role during the delay phase is mechanistically supported by a unique electrophysiological feature of the EC. Among all subregions, only the EC possesses ‘persistent firing’ neurons(Egorov, Hamam et al. 2002; Fransen, Alonso et al. 2004; Tahvildari, Fransen et al. 2007; Yoshida, Fransen et al. 2008), cells that are notable for their sustained firing, often many minutes post-stimulation and whose persistent firing is relatively resistant to distracters. Together, these two properties are ideally suited to play a role during delay periods, either by acting as a ‘memory buffer’ or by entraining plasticity in downstream subregions.
The importance of our findings is twofold. First, our study is the first to investigate the hippocampal formation using both a delay recall/retention task and a task dependent on pattern separation in the same subjects. The observed dissociation supports the emerging view about the differential roles played by the EC and the DG. At a practical level, our results suggest that standard memory tests used in most neuropsychological batteries can assess the functional integrity of individual hippocampal subregions, even in the absence of imaging or electrophysiological data. These results expand the interpretive power of neuropsychological testing in many studies that study the effect of aging and disease has on the hippocampal formation.
This ability is particularly useful in light of evidence showing that any process that causes hippocampal dysfunction typically does so by differentially targeting one hippocampal subregion over another (Small 2001). Alzheimer’s disease, a progressive process that anatomical spreads over time, is thought to begin in the EC before it spreads to involve other hippocampal subregions (Braak and Braak 1996). Importantly, a growing number of prospective neuropsychological studies have shown that delayed retention is a cognitive task that is affected in the earliest stages of Alzheimer’s disease-- remarkably over 2 decades before the onset of dementia (Elias, Beiser et al. 2000). At the same time, in contrast to Alzheimer’s disease, delayed retention is a hippocampal-dependent task relatively unaffected by aging (Albert 1996). Thus, by showing that delayed retention differentially localizes to the EC, our results support previous imaging studies showing that the EC is targeted by Alzheimer’s disease but spared in normal aging (Small, Chawla et al. 2004; Moreno, Wu et al. 2007).
Over time Alzheimer’s disease will involve other hippocampal subregions and therefore it is not surprising that the BVRT and pattern separation tasks are ultimately affected in the disease (Yassa, Stark et al.; Kawas, Corrada et al. 2003). Nevertheless, studies have shown that aging itself will affect the recognition component of the BVRT (Resnick, Trotman et al. 1995), other visual recognition tasks (Schacter, Cooper et al. 1992; Grady, McIntosh et al. 1995) and pattern separation (Toner, Pirogovsky et al. 2009) supporting the conclusion that aging without Alzheimer’s disease differentially targets the DG (West, Coleman et al. 1994; Gazzaley, Siegel et al. 1996; Small, Chawla et al. 2004).
Supplementary Material
Acknowledgments
NIH grants AG07232 and AG008702, and the McDonnell Foundation
References
- Albert MS. Cognitive and neurobiologic markers of early Alzheimer disease. Proc Natl Acad Sci U S A. 1996;93(24):13547–13551. doi: 10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS. The ageing brain: normal and abnormal memory. Philos Trans R Soc Lond B Biol Sci. 1997;352(1362):1703–1709. doi: 10.1098/rstb.1997.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, et al. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau JW, Kennedy DN, Jr, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Benton AL. Neuropsychological assessment. Annu Rev Psychol. 1994;45:1–23. doi: 10.1146/annurev.ps.45.020194.000245. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Brickman A, Schupf N, et al. Brain morphology in elderly African Americans, Caribbean Hispanics, and Caucasians from Northern Manhattan. Arch Neurol. 2008 doi: 10.1001/archneur.65.8.1053. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun VH, Leutgeb S, et al. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57(2):290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, et al. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31(9):469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, et al. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420(6912):173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Elias MF, Beiser A, et al. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57(6):808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Brewer JB, et al. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: a functional magnetic resonance imaging study with high acquisition rate. Hippocampus. 1999;9(1):35–44. doi: 10.1002/(SICI)1098-1063(1999)9:1<35::AID-HIPO4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fransen E, Alonso AA, et al. Ionic mechanisms in the generation of subthreshold oscillations and action potential clustering in entorhinal layer II stellate neurons. Hippocampus. 2004;14(3):368–384. doi: 10.1002/hipo.10198. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Siegel SJ, et al. Circuit-specific alterations of N-methyl-D-aspartate receptor subunit 1 in the dentate gyrus of aged monkeys. Proc Natl Acad Sci U S A. 1996;93(7):3121–3125. doi: 10.1073/pnas.93.7.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard-Sagiv H, Mukamel R, et al. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322(5898):96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RG, Fischman AJ, et al. Functional MR in the evaluation of dementia: correlation of abnormal dynamic cerebral blood volume measurements with changes in cerebral metabolism on positron emission tomography with fludeoxyglucose F 18. AJNR Am J Neuroradiol. 1995;16(9):1763–1770. [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, et al. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269(5221):218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Kawas CH, Corrada MM, et al. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology. 2003;60(7):1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- Kent K, Hess K, et al. CA3 NMDA receptors are required for experience-dependent shifts in hippocampal activity. Hippocampus. 2007;17(10):1003–1011. doi: 10.1002/hipo.20332. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, et al. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Lin W, Celik A, et al. Regional cerebral blood volume: a comparison of the dynamic imaging and the steady state methods. J Magn Reson Imaging. 1999;9(1):44–52. doi: 10.1002/(sici)1522-2586(199901)9:1<44::aid-jmri6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Jenkins BG, et al. Regional sensitivity and coupling of BOLD and CBV changes during stimulation of rat brain. Magn Reson Med. 2001;45(3):443–447. doi: 10.1002/1522-2594(200103)45:3<443::aid-mrm1058>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, et al. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med. 1998;39(4):615–624. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Tang MX, et al. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317(5834):94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Moreno H, Wu WE, et al. Imaging the abeta-related neurotoxicity of Alzheimer disease. Arch Neurol. 2007;64(10):1467–1477. doi: 10.1001/archneur.64.10.1467. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297(5579):211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Trotman KM, et al. Age-associated changes in specific errors on the Benton Visual Retention Test. J Gerontol B Psychol Sci Soc Sci. 1995;50(3):P171–178. doi: 10.1093/geronb/50b.3.p171. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Cooper LA, et al. Implicit and explicit memory for novel visual objects in older and younger adults. Psychol Aging. 1992;7(2):299–308. doi: 10.1037//0882-7974.7.2.299. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Atri A, et al. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci. 2005;25(40):9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, et al. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24(49):11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Ren H, et al. CBF, BOLD, CBV, and CMRO(2) fMRI signal temporal dynamics at 500-msec resolution. J Magn Reson Imaging. 2008;27(3):599–606. doi: 10.1002/jmri.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA. Age-related memory decline; current concepts and future directions. Archives of Neurology. 2001;58:360–364. doi: 10.1001/archneur.58.3.360. [DOI] [PubMed] [Google Scholar]
- Small SA, Chawla MK, et al. From The Cover: Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101(18):7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Perera GM, et al. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann Neurol. 1999;45(4):466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, et al. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stern Y, Andrews H, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49(5):453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Miller EK, et al. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78(2):1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- Tahvildari B, Fransen E, et al. Switching between “On” and “Off” states of persistent activity in lateral entorhinal layer III neurons. Hippocampus. 2007;17(4):257–263. doi: 10.1002/hipo.20270. [DOI] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, et al. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16(5):338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, et al. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344(8925):769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Wu E, Brickman A, et al. The brain in the age of old: The hippocampal formation is differentially affected by diseases of late-life. Annals of Neurology. 2008 doi: 10.1002/ana.21557. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Brickman AM, et al. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Ann Neurol. 2008;64(6):698–706. doi: 10.1002/ana.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, et al. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Fransen E, et al. mGluR-dependent persistent firing in entorhinal cortex layer III neurons. Eur J Neurosci. 2008;28(6):1116–1126. doi: 10.1111/j.1460-9568.2008.06409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Otto T, et al. Memory representation within the parahippocampal region. J Neurosci. 1997;17(13):5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.