Abstract
Neural circuit assembly during development involves a series of highly regulated steps. While genetically pre-determined programs play key roles in the early steps including neurogenesis, migration, and initial growth and guidance of axons; increasing evidence indicates that as the axons reach their targets, the late steps of neuronal differentiation and connectivity formation may be influenced or even specified by target-derived signals. Here we attempt to provide a brief synthesized review on the roles of retrograde neurotrophin and growth factor signaling in regulating the final stages of neural circuit specificity such as axonal projection, dendritic patterning, neurotransmitter phenotype acquisition, and synapse formation.
Introduction
The assembly of complex yet specific neural circuits is controlled by both genetic programs and experience-dependent changes, i.e. nature plus nurture. Genetically encoded programs can be further divided into pre-specified and target-dependent mechanisms. The pre-specified programs control the transcriptional profiles of neural progenitor cells and early postmitotic neurons, which reflect the intrinsic cell fate differences and are independent of target contact. These programs determine the early steps of neural circuit assembly including directed neuronal migrations, the initial axon pathfinding toward the target region, and the initiation of dendritic growth [1–3]. At the same time, the pre-specified programs also include the expressions of specific receptors for factors released by the neurons’ axonal targets (intermediary or final targets). Interestingly, in many types of neurons, target-derived signals can induce subsequent molecular and cellular changes, which are essential for the late steps of assembly and maturation of the relevant neural circuits [4]. Since the primary functions of axons are to deliver/forward information from the soma to their synaptic targets, this direction is generally called anterograde, and therefore the signals received by axons are considered as “retrograde”.
We will focus on neurotrophins and those growth factors that have the potentials to elicit long-range retrograde signaling from axon terminal to neuronal soma. Other cell surface proteins that act as “recognition molecules” to guide the local interactions between growth cones and their targets are not subjects of this review. Moreover, we will not discuss the roles of neurotrophins as neuronal survival factors or as synaptic transmission modulators. Readers are referred to previously published excellent reviews for details on intracellular mechanisms of retrograde neurotrophin signaling [5,6], local neurotrophin signaling in axon outgrowth [7,8], and on neurotrophin-mediated rapid modulation of synaptic transmission and plasticity [9–11]. We will review the studies related to retrograde specification of neuronal connections and circuit properties, highlight recent findings, and discuss the advantages afforded by the target-dependent mechanisms.
Retrograde regulation of axon projections: target-derived signals received by peripheral axons control the projections of central axons in the somatosensory system
Sensory neurons in the mammalian somatosensory system have a pseudo-unipolar axon that stems from the cell body and bifurcates to form two main branches: one innervating a peripheral target and the other projecting into the spinal cord or the brainstem. The central axons of these sensory neurons have two important tasks: terminate onto distinct laminae/layers in the developing spinal cord according to their sensory modalities (functional classes), and project topographically based on their peripheral targets’ locations thereby forming a somatotopic map of the body surface. Considerably, increasing evidence suggests that both laminar-specific termination and topographic mapping depend on gene expressions induced by periphery-derived retrograde signals.
The signals regulating layer-specific innervations into the spinal cord from three different classes of sensory neurons are summarized in Figure 1A. Non-peptidergic nociceptive sensory neurons require skin-derived NGF/TrkA signaling to induce/maintain the expression of Runx1 transcription factor, which then regulates the lamina-specific targeting in the spinal cord. In the absence of Runx1, the afferents from these neurons move from innervating the more ventral lamina II to the most dorsal lamina I in the dorsal spinal cord [12,13]. The rapid adapting (RA) low threshold mechanosensory neurons were recently shown to derive from the early Ret-receptor expressing neurons [14,15]. The Ret tyrosine kinase is a signaling receptor for the glial-derived neurotrophic factor (GDNF) family of ligands that are dynamically expressed in the peripheral tissues. Ret signaling is partially required for inducing and maintaining the expression of transcription factor MafA [14]. Importantly, Ret signaling and MafA expression are necessary for the central axons of these rapid adapting mechanosensory neurons to innervate lamina III-V of the dorsal horn, and to ascend ipsilaterally within the dorsal column to innervate the gracile and cuneate nuclei [14,15]. In the case of proprioceptive sensory neurons, muscle derived retrograde NT-3/TrkC signaling is required for inducing the expression of transcription factor Er81, which is essential for enabling the proprioceptive central axons to project ventrally and to establish connections with motor neurons [16,17].
Figure 1. Schematic summaries of retrograde regulation of axon projections.
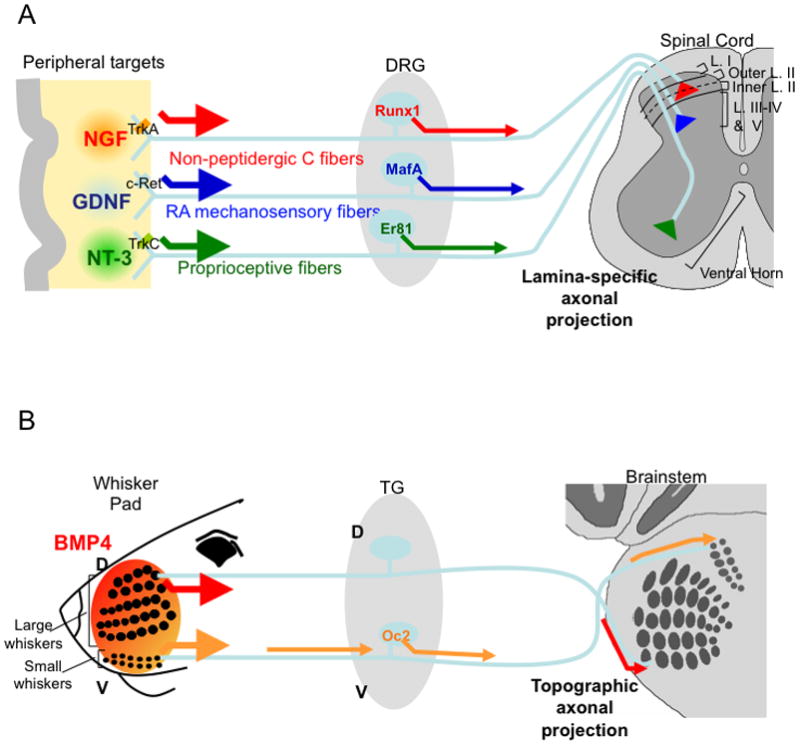
A. Retrograde neurotrophin signaling regulates laminar-specific termination of distinct classes of somatosensory neurons.
B. Topographic projection of trigeminal sensory neurons into the brainstem is dependent on target-derived retrograde BMP4 signaling (arrows).
DRG, dorsal root ganglion; L., lamina; TG, trigeminal ganglia.
The role of retrograde signals in regulating the formation of somatotopic sensory maps came from studies of the mouse trigeminal sensory neurons that innervate and map the face somatosensory organs onto the brain (Figure 1B). It was shown that after initial axon outgrowth into the developing face, BMP4, a member of the TGFβ superfamily growth factors, which is differentially expressed by diverse areas of the face, retrogradely signals to trigeminal sensory neurons. This target-derived BMP4 signaling induces spatially patterned expressions of several transcription factors along the dorsoventral axis of the trigeminal ganglion. Deleting one of the BMP4-regulated gene, Onecut2, led to errors in the mapping of small whiskers located on the ventral regions of the face [18]. Whisker-derived TGFβ signaling was further shown to be essential for the precise topographic segregations of trigeminal central axons representing neighboring whiskers into separate neural structures in the brainstem (da Silva et al, manuscript submitted). In both laminar-specific projection and topographic mapping, the downstream axon guidance molecules that are controlled by target-derived retrograde neurotrophin or TGFβ signaling remain to be identified.
Retrograde regulation of dendritic patterns: axonal target-derived signals control the growth and patterning of dendrites
Different classes of neurons have vastly diverse dendrite patterns. Dendritic morphogenesis is tied to neurons’ physiological functions and is thus an essential and integral part of neural circuit assembly. For an in-depth discussion on intrinsic programs regulating dendritic branching, including transcription factors, cell surface molecules and intracellular membrane trafficking mechanisms, please see the recent review by Jan and Jan [19]. Here we focus on several examples of axonal target-derived signals that control the growth and patterning of dendrites in a retrograde manner.
A classic example of axonal target regulating dendritic arbor growth came from studies of the sympathetic ganglion neurons (also called postganglionic sympathetic neurons). The size of the peripheral target field is positively correlated with the size and complexity of the dendritic arbors of these neurons [20]. In this case, target-derived NGF signaling is not only essential for the survival for sympathetic neurons, but also necessary for promoting the growth of dendritic branches in vivo [21–23]. In addition, BMPs have also been implicated in regulating the growth of these dendrites [24,25]. Another interesting example came from studies in Xenopus which examined the dendritic arborization of retinal ganglion cells (RGCs) (Figure 2A). It was shown that BDNF expressed at the target of RGCs, the optic tectum, not only promoted terminal axon arborization, but also led to the increase of RGC dendrite branching in the retina through retrograde signaling. Surprisingly, if BDNF was applied locally in the retina, it inhibited RGC dendrite arborization [26]. How target-derived and local BDNF might exert opposite effects on dendritic growth is still not completely understood.
Figure 2. Schematic representations for retrograde regulation of dendritc growth and patterns.
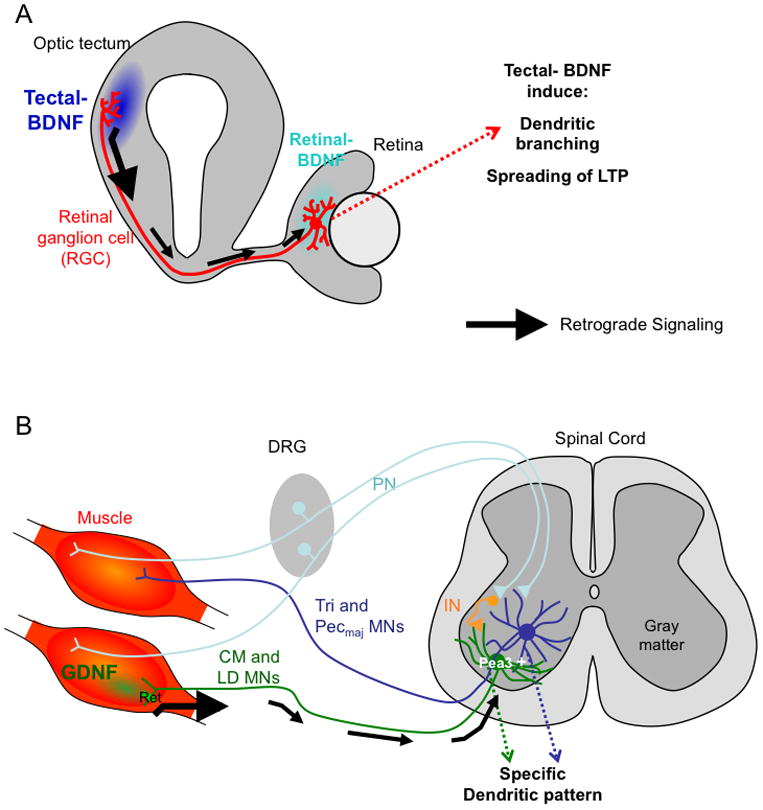
A. Retrograde signaling of BDNF derived from tectum to retinal ganglion cells (RGCs) promotes dendritic arborization of RGCs, and helps spread LTP from tectum to retina.
B. Specific muscle-derived retrograde GDNF signaling enables motor neurons innervating the cutaneous maximus (CM) muscle or the latissimus dorsi (LD) mucle to form laterally extended dendrites, which is different from the radial dendrites formed by motor neurons innervating the triceps brachii muscles (Tri) or the pectoralis major muscle (Pecmaj).
IN, interneuron.
In addition to dendritic growth, the patterns of dendritic arborization may also be controlled by axonal targets (Figure 2B). Motor neurons receiving mono-synaptic input from proprioceptive sensory neurons have radially extended dendritic arbors whereas motor neurons receiving poly-synaptic input project their dendrites laterally along the borders of the ventral horn avoiding the central gray matter [27]. Neurons with lateral dendrites express Ets domain transcription factor Pea3. Importantly, the expression of Pea3 is dependent on and induced by muscle derived GDNF signaling through the motor axons (which express the Ret receptor). Disruption of this GDNF-Ret-Pea3 signaling pathway resulted in radial-dendritic patterns for all motor neurons [27]. Motor neuron cell body positioning and sensory-motor connectivity is also altered in Pea3 mutants.
Target-dependent retrograde regulation of neurotransmitter phenotypes
Acquiring the mature neurotransmitter phenotype is an important step during the establishment of appropriate functional connections between neurons and their targets. There are a few well-studied developmental cases in which the neurotransmitter phenotypes are induced or determined by target-derived signals.
Neurotransmitter acquisition in the postganglionic sympathetic neurons has been an active research area since the 1980s. The majority of the sympathetic neurons that innervate blood vessels, smooth muscles and other internal organs utilize noradrenalin (norepinephrine) as the neurotransmitter (Figure 3A). Aorta-derived BMPs and the transcription factors Phox2b/2a, Hand2 and Gata2/3, whose expressions are induced by the retrograde BMP signaling, are required for establishing the noradrenergic phenotype (see [28] and the references therein). Another small population of sympathetic neurons that innervate sweat glands and periosteum uses acetylcholine as the transmitter. One of the main mechanisms underlying the acquisition of the cholinergic phenotype is the trans-differentiation of noradrenergic into cholinergic neurons, which is dependent on target-derived signals. Neurotrophic cytokines of the CNTF family (including CNTF, LIF, cardiotrophin, cardiotrophin-like-cytokine, neuropoietin and others) expressed in sweat glands or periosteum elicit gp130 dependent signaling in the immature noradrenergic sympathetic neurons, and induce them to switch to the cholinergic phenotype (again, see [28] and the references therein) (Figure 3B).
Figure 3. Schematic representations for retrograde regulation of neurotransmitter phenotypes in postganglionic sympathetic neurons and synapse maturation in spinal interneurons.
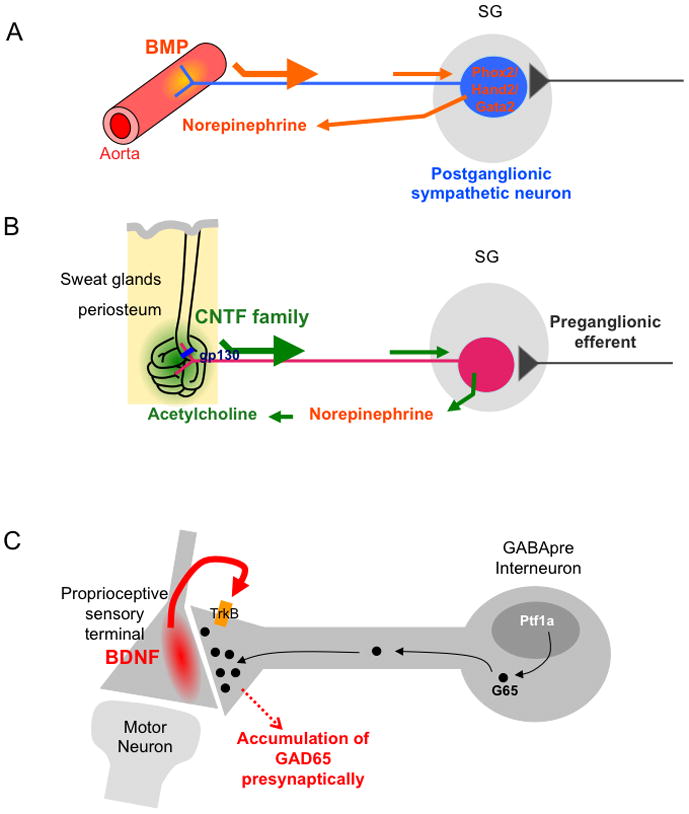
A. Aorta-derived BMP signaling is required for the acquisition of norepinephrine (noradrenalin) transmitter phenotype.
B. CNTF family ligands expressed by sweat glands and periosteum induce sympathetic neurons to trans-differentiate from noradrenergic to cholinergic phenotype through a gp130-dependent retrograde signaling pathway.
C. BDNF derived from proprioceptive sensory afferents induces GAD65 localization to the synapses formed between the GABApre interneurons and the sensory axons.
SG, sympathetic ganglion.
Expression of neuropeptide phenotypes can also be regulated by target-derived signals. In the somatosensory system, the peptidergic nociceptive neurons express Calcitonin gene-related peptide (CGRP). Release of CGRP from sensory axons delivers pain information in the spinal cord and leads to blood vessel dilation at periphery. During embryonic development, expression of CGRP in sensory neurons depends on skin-derived NGF signaling [29]. Notably, the aforementioned NGF-Runx1 signaling in non-peptidergic neurons actually results in suppressing CGRP expression [12]. Thus, the outcome of NGF signaling is context dependent. Interestingly in adult, skin injury or inflammation significantly increases the expression of Activin A, another member of the TGFβ family growth factors. Retrograde Activin A signaling in turn leads to ectopic expression of CGRP in many types of adult sensory neurons including non-peptidergic neurons, thereby contributing to greatly enhanced pain responses [30]. The retrograde induction of neuropeptide expression is also seen in invertebrates. Target-derived BMP signaling is required both for induction and for maintenance of the neuropeptide FMRFamide (FMRFa) expression in Drosophila Tv neurons [31–33]. Furthermore, retrograde neurotrophin signaling has also been implicated in regulating ion channel expressions and other electrophysiological properties of diverse types of neurons which are not reviewed here.
Retrograde regulation of synapse formation, growth and global synaptic plasticity
Formation of synapses between pre- and post-synaptic neurons or between neurons and their targets is a highly regulated step. Sanes and Yamagata summarized ten mechanisms that are employed by the nervous system to control the specificity of synaptogenesis [34]. In several systems, postsynaptic target-derived growth factor and neurotrophin signaling have been shown to regulate synapse formation, synaptic growth, and the global spreading of synaptic plasticity.
BMP7 (which is also called Gbb, again another member of the TGFβ family) in Drosophila neuromuscular junction (NMJ), and Wnt7a and FGF22 in cerebellar glomerular rosette are the best-characterized examples of non-neurotrophin growth factors that act as retrograde regulators at their respective synapses, and these studies have been extensively reviewed [35–37]. Only BMP7/Gbb was shown to elicit long-range retrograde signaling from axons to cell bodies, which requires the BMP-receptor activated transcription factor Mad [35]. This muscle-derived BMP signaling stimulates motor neurons to form more synaptic boutons to keep pace with the tremendous growth of the muscles at the Drosophila larval NMJ. Recently, it was discovered that the BMP-Mad retrograde signaling leads to Mad mediated transcription of Trio, which encodes a RAC GEF protein. Trio in turn stimulates the GTPase activity of Rac, and Rac-mediated cytoskeleton changes promote synaptic expansion. Furthermore, transgenic over-expression of Trio in larval mutants for BMP signaling partially rescues the NMJ defects [38]. This exciting new study not only identified Trio as the first target in the BMP-Mad pathway but also provided a cellular mechanism for synaptic growth. Moreover, retrograde BMP-Mad signaling is also required during development to confer competence of motoneurons to express homeostatic synaptic plasticity, independent of the growth control signaling [39]. The downstream targets in the synaptic homeostasis pathway are unknown at present.
Neurotrophins have also been shown to promote the formation and differentiation of synapses in general [11, 40]. An example for postsynaptic target-derived neurotrophin inducing synapse maturation was shown elegantly in a recent study [41]. In the spinal cord, a subpopulation of GABAergic interneurons form inhibitory synapses onto the afferent axons of sensory neurons (axo-axonic synapses). These presynaptic GABAergic neurons show striking synaptic specificity. In the absence of the sensory axons as post-synaptic targets, these neurons did not switch to form synapses with other targets, such as the motor neurons. Instead, they completely fail to undergo presynaptic differentiation and retract their axons. Although the actual specific synapse inducing molecules expressed by the sensory afferents are not yet known, the authors did discover that target-derived BDNF is required for the presynaptic localization of the GABA synthesizing enzyme GAD65, a defining differentiation feature of these axo-axonic synapses (Figure 4) [41]. Other than the trafficking of GAD65, other synaptic features of these GABApre interneurons are not affected by the loss of sensory-derived BNDF.
Target-derived BDNF was also shown to induce the long-range spreading of synaptic plasticity. Long-term potentiation (LTP) is a well-characterized form of synaptic plasticity. LTP is usually expressed locally as synapse-specific “imprint”. In the retinotectal projections of Xenopus tadpoles, Poo and colleagues showed that LTP induced in the retinotectal synapses at the optic tectum, can be rapidly spread to the retina, resulting in potentiation of the synapses formed between bipolar cells and the dendrites of RGCs. This long-range retrograde propagation of LTP through the RGCs required signaling initiated by BDNF expressed in the tectum [42,43] (Figure 2B). Notably, this global spreading of synaptic plasticity could be linked to the aforementioned retrograde BDNF signaling by regulation of dendritic arborization of RGCs [26]. The function of such spreading might be to coordinate the development and/or the refinement of the output synaptic strength with that of the input synapses.
Concluding remarks
Although we have discussed the roles of retrograde neurotrophin and growth factor signaling separately according to individual steps of neural circuit assembly, these processes of axon targeting, dendritic patterning, selection of neurotransmitters, and synapse formation and growth happen at overlapping times during development together with the natural occurring apoptosis of neurons. Unsurprisingly, the same retrograde factor can exert effects on multiple steps of neural circuit assembly, depending on their specific localization and cellular context. It is interesting to note that retrograde regulation of neuronal connectivity and functional differentiation is most prominently associated with circuits concerning peripheral sensory (somatosensory, retina), autonomic, and motor functions. This correlation supports the hypothesis that by employing target-dependent retrograde signaling mechanisms in addition to the pre-determined programs, the peripheral nervous system is able to respond to changes of the peripheral organs occurring during natural variation and selection, such that they can wire the central circuits accordingly. In other words, retrograde signaling allows the nervous system to be both hardwired and adaptive.
Acknowledgments
F.W is supported by the McKnight Scholar Award, the Wings for Life Foundation, and NIH grants from NIDCR. S. da S. was supported by a predoctoral fellowship from the Foundation for Science and Technology, Portugal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 2.Salie R, Niederkofler V, Arber S. Patterning molecules; multitasking in the nervous system. Neuron. 2005;45:189–192. doi: 10.1016/j.neuron.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- 4.Hippenmeyer S, Kramer I, Arber S. Control of neuronal phenotype: what targets tell the cell bodies. Trends Neurosci. 2004;27:482–488. doi: 10.1016/j.tins.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Cosker KE, Courchesne SL, Segal RA. Action in the axon: generation and transport of signaling endosomes. Curr Opin Neurobiol. 2008;18:270–275. doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol. 2002;12:268–274. doi: 10.1016/s0959-4388(02)00326-4. [DOI] [PubMed] [Google Scholar]
- 7.Polleux F, Snider W. Initiating and growing an axon. Cold Spring Harb Perspect Biol. 2010;2:a001925. doi: 10.1101/cshperspect.a001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou FQ, Snider WD. Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci. 2006;361:1575–1592. doi: 10.1098/rstb.2006.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovalchuk Y, Holthoff K, Konnerth A. Neurotrophin action on a rapid timescale. Curr Opin Neurobiol. 2004;14:558–563. doi: 10.1016/j.conb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 11.Vicario-Abejon C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nat Rev Neurosci. 2002;3:965–974. doi: 10.1038/nrn988. [DOI] [PubMed] [Google Scholar]
- 12.Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–377. doi: 10.1016/j.neuron.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Ladle DR, Pecho-Vrieseling E, Arber S. Assembly of motor circuits in the spinal cord: driven to function by genetic and experience-dependent mechanisms. Neuron. 2007;56:270–283. doi: 10.1016/j.neuron.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 14•.Bourane S, Garces A, Venteo S, Pattyn A, Hubert T, Fichard A, Puech S, Boukhaddaoui H, Baudet C, Takahashi S, et al. Low-threshold mechanoreceptor subtypes selectively express MafA and are specified by Ret signaling. Neuron. 2009;64:857–870. doi: 10.1016/j.neuron.2009.12.004. This study identified a transcription factor MafA that is expressed in low-threshold mechanosensory neurons. MafA expression depends on GDNF-Ret signaling, and MafA mutant sensory neurons have abnormal central projections. [DOI] [PubMed] [Google Scholar]
- 15•.Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron. 2009;64:841–856. doi: 10.1016/j.neuron.2009.11.003. This study identified “early Ret+” sensory neurons that are low-throshold rapid adapting mechanoreceptors. The central axonal projections from these mechanosensory neurons into layer III–V of spinal cord and to subdomains of the dorsal column nuclei require GDNF-Ret signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen HH, Hippenmeyer S, Arber S, Frank E. Development of the monosynaptic stretch reflex circuit. Curr Opin Neurobiol. 2003;13:96–102. doi: 10.1016/s0959-4388(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 17.Patel TD, Kramer I, Kucera J, Niederkofler V, Jessell TM, Arber S, Snider WD. Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron. 2003;38:403–416. doi: 10.1016/s0896-6273(03)00261-7. [DOI] [PubMed] [Google Scholar]
- 18.Hodge LK, Klassen MP, Han BX, Yiu G, Hurrell J, Howell A, Rousseau G, Lemaigre F, Tessier-Lavigne M, Wang F. Retrograde BMP signaling regulates trigeminal sensory neuron identities and the formation of precise face maps. Neuron. 2007;55:572–586. doi: 10.1016/j.neuron.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voyvodic JT. Peripheral target regulation of dendritic geometry in the rat superior cervical ganglion. J Neurosci. 1989;9:1997–2010. doi: 10.1523/JNEUROSCI.09-06-01997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller FD, Kaplan DR. Signaling mechanisms underlying dendrite formation. Curr Opin Neurobiol. 2003;13:391–398. doi: 10.1016/s0959-4388(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 22.Snider WD. Nerve growth factor enhances dendritic arborization of sympathetic ganglion cells in developing mammals. J Neurosci. 1988;8:2628–2634. doi: 10.1523/JNEUROSCI.08-07-02628.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 24.Beck HN, Drahushuk K, Jacoby DB, Higgins D, Lein PJ. Bone morphogenetic protein-5 (BMP-5) promotes dendritic growth in cultured sympathetic neurons. BMC Neurosci. 2001;2:12. doi: 10.1186/1471-2202-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo X, Lin Y, Horbinski C, Drahushuk KM, Kim IJ, Kaplan PL, Lein P, Wang T, Higgins D. Dendritic growth induced by BMP-7 requires Smad1 and proteasome activity. J Neurobiol. 2001;48:120–130. [PubMed] [Google Scholar]
- 26.Lom B, Cogen J, Sanchez AL, Vu T, Cohen-Cory S. Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J Neurosci. 2002;22:7639–7649. doi: 10.1523/JNEUROSCI.22-17-07639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452. doi: 10.1016/j.cell.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 28.Apostolova G, Dechant G. Development of neurotransmitter phenotypes in sympathetic neurons. Auton Neurosci. 2009;151:30–38. doi: 10.1016/j.autneu.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Patel TD, Jackman A, Rice FL, Kucera J, Snider WD. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 30.Xu P, Van Slambrouck C, Berti-Mattera L, Hall AK. Activin induces tactile allodynia and increases calcitonin gene-related peptide after peripheral inflammation. J Neurosci. 2005;25:9227–9235. doi: 10.1523/JNEUROSCI.3051-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allan DW, St Pierre SE, Miguel-Aliaga I, Thor S. Specification of neuropeptide cell identity by the integration of retrograde BMP signaling and a combinatorial transcription factor code. Cell. 2003;113:73–86. doi: 10.1016/s0092-8674(03)00204-6. [DOI] [PubMed] [Google Scholar]
- 32.Eade KT, Allan DW. Neuronal phenotype in the mature nervous system is maintained by persistent retrograde bone morphogenetic protein signaling. J Neurosci. 2009;29:3852–3864. doi: 10.1523/JNEUROSCI.0213-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marques G, Haerry TE, Crotty ML, Xue M, Zhang B, O’Connor MB. Retrograde Gbb signaling through the Bmp type 2 receptor wishful thinking regulates systemic FMRFa expression in Drosophila. Development. 2003;130:5457–5470. doi: 10.1242/dev.00772. [DOI] [PubMed] [Google Scholar]
- 34.Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- 35.Marques G. Morphogens and synaptogenesis in Drosophila. J Neurobiol. 2005;64:417–434. doi: 10.1002/neu.20165. [DOI] [PubMed] [Google Scholar]
- 36.Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- 37.Shen K, Scheiffele P. Genetics and cell biology of building specific synaptic connectivity. Annu Rev Neurosci. 2010;33:473–507. doi: 10.1146/annurev.neuro.051508.135302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Ball RW, Warren-Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, Haghighi AP. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron. 2010;66:536–549. doi: 10.1016/j.neuron.2010.04.011. This study identified Trio as the first downstream transcriptional target of the retrograde BMP signaling at drosophila neuromuscular junction. Trio is a Rac GEF that activates Rac GTPase activity, thereby directly links retrograde BMP signaling to cytoskeleton remodeling that promotes the synaptic growth. [DOI] [PubMed] [Google Scholar]
- 39.Goold CP, Davis GW. The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron. 2007;56:109–123. doi: 10.1016/j.neuron.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci. 2002;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Betley JN, Wright CV, Kawaguchi Y, Erdelyi F, Szabo G, Jessell TM, Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell. 2009;139:161–174. doi: 10.1016/j.cell.2009.08.027. The authors showed that the GABAergic interneurons mediating presynaptic inhibition in the spinal sensorimotor circuit have stringent synaptic specificity and only synapse with sensory afferent axons. They further discovered that sensory axon derived BDNF is required for the maturation of these GABApre synapses by inducing the synaptic localization of GABA-synthesizing enzyme GAD65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du JL, Poo MM. Rapid BDNF-induced retrograde synaptic modification in a developing retinotectal system. Nature. 2004;429:878–883. doi: 10.1038/nature02618. [DOI] [PubMed] [Google Scholar]
- 43.Du JL, Wei HP, Wang ZR, Wong ST, Poo MM. Long-range retrograde spread of LTP and LTD from optic tectum to retina. Proc Natl Acad Sci U S A. 2009;106:18890–18896. doi: 10.1073/pnas.0910659106. [DOI] [PMC free article] [PubMed] [Google Scholar]


