Abstract
Akt signaling plays a central role in many biological functions, such as cell proliferation and apoptosis. Since Akt resides primarily in the cytosol, it is not known how these signaling molecules are recruited to the plasma membrane and subsequently activated by growth factor stimuli. Here, we found that the protein kinase Akt undergoes lysine 63 chain ubiquitination, which is important for Akt membrane localization and phosphorylation. TRAF6 was found to be a direct E3 ligase for Akt and was essential for Akt ubiquitination, membrane recruitment, and phosphorylation upon growth-factor stimulation. The human cancer-associated Akt mutant (E17K) displayed an increase in Akt ubiquitination, in turn contributing to the enhancement of Akt membrane localization and phosphorylation. Thus, Akt ubiquitination is an important step for oncogenic Akt activation.
Protein ubiquitination is an important posttranslational modification that regulates various biological functions (1, 2). Although ubiquitination often results in protein degradation, a certain type of ubiquitination is important for signaling activation and protein trafficking (1, 2). Ubiquitination through lysine 48 (K48) of the ubiquitin chain generally targets proteins for degradation, whereas ubiquitination through K63 plays a critical role in signaling activation and protein trafficking (1, 2). Akt (also known as Protein Kinase B) is an important component of cell signaling pathways regulating cell survival and metabolism. Although membrane recruitment of Akt by growth-factor stimuli is a critical step for Akt phosphorylation, it is not clear how Akt is recruited to the plasma membrane. As ubiquitination can regulate protein trafficking (1), we tested whether Akt is ubiquitinated in cells. Akt was ubiquitinated in the absence of proteasome inhibitor MG132 (fig. S1A). Ubiquitination occurred through K63 but not through K48 (Fig. 1A). Akt ubiquitination could be modified by K11-linked ubiquitin but not through K6 and K48 linkages (fig. S1B).
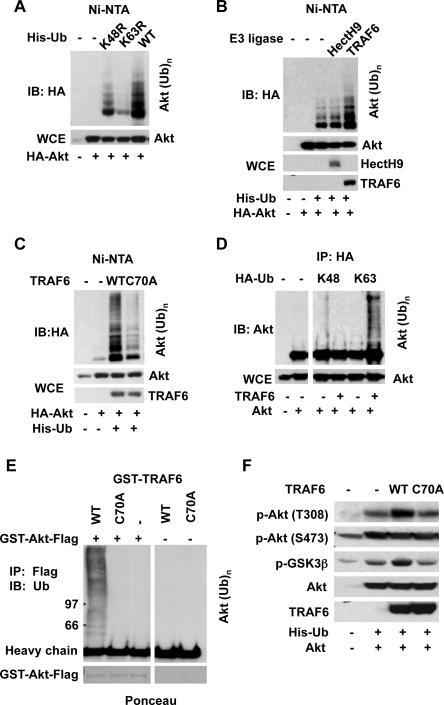
Fig. 1.
TRAF6 is an E3 ubiquitin ligase for Akt. (A) Immunoblot (IB) of lysed 293T cells transfected with Akt along with His-Ubiquitin (His-Ub), His-Ub K48R, or His-Ub K63R constructs. Ni-NTA, nickel bead pull-down; WCE, whole-cell extracts. (B) Immunoblot of lysed 293T cells transfected with HA-Akt, His-Ub, along with various E3 ligases. (C) Immunoblot of lysed 293T cells transfected with HA-Akt and His-Ub, along with TRAF6 or TRAF6 C70A. (D) Immunoblot of lysed 293T cells transfected with Akt and TRAF6, along with HA-Ub K48 (K48-only ubiquitin) or HA-Ub K63 (K63-only ubiquitin). (E) GST-Akt-Flag proteins were incubated with ATP, E1, E2 along with GST, GSTTRAF6, or GST-TRAF6 C70A proteins for in vitro ubiquitination of Akt. (F) WCE from 293T cells transfected with indicated plasmids was collected for immunoblot analysis.
We screened a panel of E3 ubiquitin ligases for Akt ubiquitination. Although Mdm2 E3 ligase interacts with Akt (3–5), it failed to promote Akt ubiquitination (fig. S2A), as did c-IAP-1, c-IAP2, Cbl-b, Itch, Smurf2, and Fbw7 (fig. S2A). Overexpression of c-IAP1 and c-IAP2 reduced Akt ubiquitination (fig. S2, A and B). Because Akt underwent K63 ubiquitination we focused on TRAF6 and HectH9, two E3 ubiquitin ligases that catalyze K63 ubiquitination and function in Toll-like receptor (TLR) or interleukin-1 (IL-1) signaling and oncogenic activation of Myc, respectively (6–8). TRAF6 promoted Akt ubiquitination, whereas HectH9 did not (Fig. 1B).
Activity of Akt was not required for TRAF6-mediated ubiquitination (fig. S2C). However, TRAF6 E3 ligase activity was required. The TRAF6 C70A mutant, which loses E3 ligase activity (8), had compromised activity (Fig. 1C), which was not due to a defect in Akt interaction (fig. S3A). Although TRAF6 induced Akt ubiquitination, it did not decrease the abundance of Akt (Fig. 1, B and C), suggesting it does not mediate K48 ubiquitination. Indeed, TRAF6 promoted K63 ubiquitination of Akt, but not K48 ubiquitination (Fig. 1D). TRAF6, but not TRAF6 C70A, induced Akt ubiquitination in vitro (Fig. 1E and fig. S2D). These results suggest that TRAF6 is an E3 ubiquitin ligase for Akt.
Coimmunoprecipitation experiments revealed that Akt interacted with overexpressed TRAF6 or TRAF6 C70A (fig. S3A). We detected interaction between endogenous Akt and TRAF6 in cells stimulated with insulin-like growth factor-1 (IGF-1) or IL-1β (fig. S3B), both of which activate Akt signaling. Glutathione-S-transferase (GST)-tagged TRAF6 interacted with Akt directly in vitro (Fig. S3C). Ubiquitination of the Akt1 and Akt2 isoforms, but not that of Akt3, was induced by TRAF6 (fig. S4A). Coimmunoprecipitation assay revealed that TRAF6 interacted with all Akt isoforms (fig. S4B).
Overexpression of TRAF6, but not that of TRAF6 C70A, enhanced phosphorylation of Akt at T308 but not at S473 and this enhancement was accompanied by increased Akt activity toward glycogen synthase kinase 3β (GSK3β) (Fig. 1F) (9, 10). A control E3 ligase HectH9 failed to regulate phosphorylation of Akt (fig. S5). Overexpression of c-IAP-1 and c-IAP-2, which slightly inhibited ubiquitination of Akt, reduced phosphorylation of Akt on T308 (fig. S2B). Thus, TRAF6 may enhance Akt phosphorylation by promoting Akt ubiquitination.
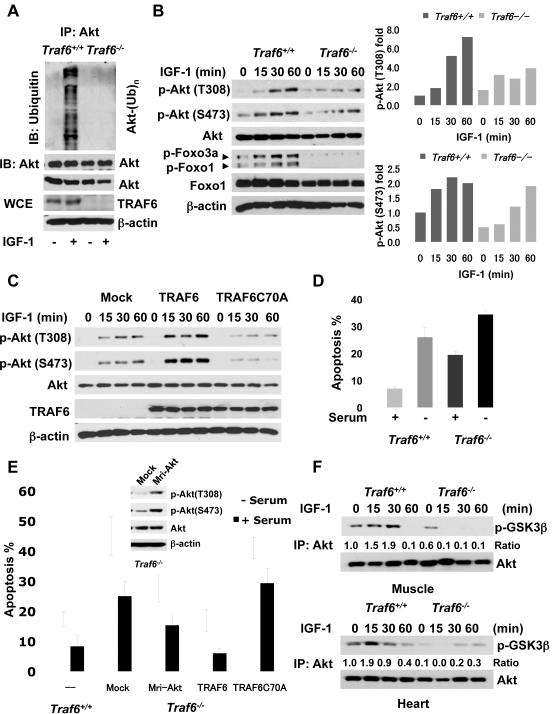
We compared Akt ubiquitination and phosphorylation in Traf6+/+ and Traf6−/− primary mouse embryonic fibroblasts (MEFs) treated with activators of Akt. Upon IGF-1 treatment, endogenous Akt ubiquitination was reduced in Traf6−/− MEFs compared to that in Traf6+/+ MEFs (Fig. 2A). Similarly, Akt ubiquitination was induced by 10% fetal bovine serum (FBS) or IL-1β in Traf6+/+ MEFs but not in Traf6−/− MEFs (fig. S6A). Consequently, Akt phosphorylation at T308 and S473 after IGF-1 treatment was reduced in Traf6−/−MEFs compared with that in Traf6+/+ MEFs (Fig. 2B). This defect in Akt phosphorylation in Traf6−/− MEFs was correlated with an impairment in phosphorylation of Foxo1 and Foxo3a, two Akt substrates (9, 10). Ubiquitination of TRAF6 was also induced by IGF-1 stimulation (fig. S7A). TRAF6 interacted with IGF-1 receptor (IGF-1R) in serum-free conditions, but not after IGF-1 treatment (fig. S7B). Thus, activated IGF-1 receptor may directly engage TRAF6 activation.
Fig. 2.
TRAF6 is required for ubiquitination and phosphorylation of Akt. (A) Traf6+/+ and Traf6−/− MEFs were serum-starved and were treated with or without IGF-1 for 30 min; WCE were collected for immunoprecipitation with Akt, followed by immunoblot analysis. (B) MEFs were serum-starved, treated with IGF-1 for various time points, and harvested for immunoblot analysis. The quantification results were shown on the right panel. (C) Primary Traf6−/− MEFs infected with Mock, TRAF6, or TRAF6 C70A mutant were treated with IGF-1 at various time points and harvested for immunoblot analysis. (D) MEFs were cultured in 10% FBS or serum-starved for 2 days, and apoptosis was determined by Annexin V staining, followed by flow cytometry analysis. Results are presented as mean values ±standard deviation (S.D.) (E) Primary MEFs were infected with Mock, constitutively active Akt (Mri-Akt), TRAF6, or TRAF6 C70A and apoptosis and immuno blot analysis were determined. Results are presented as mean values ± S.D. (F) Heart and skeletal muscle isolated from WT and Traf6−/− mice (n=4) injected with IGF-1 at various time points was subjected to an in vitro Akt kinase assay and immunoblot analysis.
Phosphorylation of Akt T308 and S473 was also inhibited in Traf6−/− MEFs treated with 10% FBS (fig. S6B). In contrast, phosphorylation of ERK1 and ERK2 was comparable in Wt and Traf6−/− MEFs (fig. S6B), suggesting that TRAF6 selectively affects Akt activation. IL-1 and lipopolysaccharide (LPS) activate the IL-1 receptor (IL-1R) and Toll-like receptor 4 (TLR4), respectively. As TRAF6 is a critical regulator for both IL-1R and TLR4 signaling (7, 8), we tested whether IL-1β-induced and LPS-induced phosphorylation of Akt acts through TRAF6. LPS and IL-1β induced phosphoylation of Akt at T308 in Traf6+/+ MEFs in the presence of 10% FBS; this effect was reduced in Traf6−/−MEFs (fig. S6, C and D). Neither LPS nor IL-1β was sufficient to trigger phosphorylation of Akt at either T308 or S473 in serum-starved conditions (0.1% FBS) in multiple primary cell lines (fig. S8), suggesting that IL-1β and LPS may require cooperation with growth-factor receptor signaling to induce Akt activation. Restoration of TRAF6 expression in Traf6−/− MEFs rescued the defect of Akt phosphorylation in cells stimulated with IGF-1 or IL-1β, whereas restoration ofTRAF6 C70A did not (Fig. 2C and fig. S6E). Thus TRAF6 is critical for Akt phosphorylation and activation through induction of Akt ubiquitination.
Because Akt influences cell survival and apoptosis (9, 10), we tested whether Traf6 deficiency sensitized cells to apoptosis after serum withdrawal. Apoptosis in Traf6−/− MEFs was higher than in Traf6+/+ MEFs in the presence or absence of serum (Fig. 2D and fig. S9). The active, cleaved form of caspase 3, a mediator of apoptosis, was more abundant in Traf6−/− MEFs than in Traf6+/+ MEFs (fig. S10A). Restoration of TRAF6, but not of TRAF6 C70A, rescued Traf6−/− MEFs from apoptosis (Fig. 2E). A constitutively active form of Akt partially rescued cells from apoptosis (Fig. 2E). Thus other signaling pathways may be also involved. As apoptosis inducers such as DNA damage agents induce phosphorylation and activation of Akt (11, 12), we tested whether TRAF6 was also involved. Doxorubicin (Dox)- and cisplatin (Cis)-induced phosphorylation of Akt T308 in WT MEFs was impaired in Traf6−/− MEFs (fig. S10B). The impairment of Akt phosphorylation was correlated with increased caspase 3 activation in Traf6−/− MEFs (fig. S10C).
TRAF6 is expressed in most mouse tissues (13, 14). We compared Akt activity in skeletal muscle and heart tissues from WT and Traf6−/− mice. Steady-state Akt activity in heart muscle, but not skeletal muscle, was lower in Traf6−/− mice than in WT mice (fig. S11). Akt activation in animals injected with IGF-1 was impaired in both forms of muscles in Traf6−/− mice (Fig. 2F). These results suggest that TRAF6 plays a critical role in Akt activation in vivo.
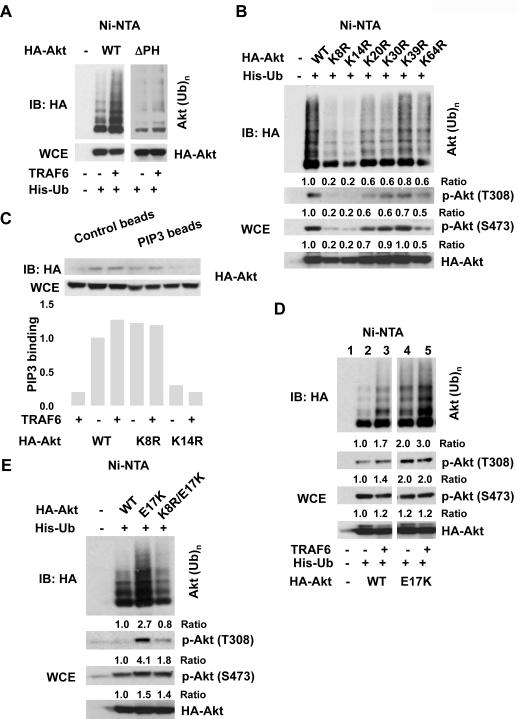
As the pleckstrin homology (PH) domain of Akt is critical for phosphatidylinositol (3,4,5)-trisphosphate (PIP3) lipid binding and protein-protein interaction (15, 16), we analyzed whether the PH domain of Akt influenced TRAF6-mediated ubiquitination of Akt. TRAF6 failed to promote ubiquitination of the Akt mutant devoid of the PH domain (Fig. 3A). Of the six lysine residues within the PH domain of Akt, mutation on either K8 or K14 to arginine (R) most substantially reduced Akt ubiquitination and Akt phosphorylation at T308 and S473 (Fig. 3B). The K8 and K14 residues are well conserved from Drosophila to humans (fig. S12), suggesting that the ubiquitination of Akt may be evolutionarily conserved.
Fig. 3.
Identification of Akt ubiquitination sites. (A) Immunoblot of lysed 293T cells transfected with His-Ub along with HA-Akt or HA-Akt ΔPH. (B) 293T cells transfected with His-Ub along with HA-Akt or various HA-Akt mutants were lysed for ubiquitination and phosphorylation of Akt. (C) WCE from 293T cells transfected with HA-Akt or various Akt mutants were incubated with control or PIP3 beads for overnight, washed, and subjected to immunoblot analysis. The PIP3 binding was calculated as the ratio between levels of Akt bound with PIP3 beads and total levels of Akt. (D, E) Immunoblot of lysed 293T cells transfected with indicated plasmids.
Akt K8R bound effectively to isolated PIP3, but Akt K14R did not (Fig. 3C). The K14 residue lies within the PIP3 lipid-binding pocket (17–19). Overexpression of TRAF6 did not enhance the binding of Akt to PIP3 (Fig. 3C). Thus, Akt ubiquitination by TRAF6 appears not to influence PIP3 lipid binding and the defect in phosphorylation of Akt K8R is not due to its impairment in PIP3 binding.
A mutation in the PH domain (E17K) of Akt has been identified in human cancer patients, including those with breast cancer (18). This cancer-associated Akt mutant exhibited constitutive Akt phosphorylation at T308 but not at S473 and had greater oncogenic potential. Basal ubiquitination of the E17K mutant was much higher than that of WT Akt (Fig. 3D). Overexpression of TRAF6 still increased ubiquitination of this mutant, but to a lesser extent than WT Akt (Fig. 3D). The E17K mutant displayed higher Akt phosphorylation at T308 but not at S473 (18), and this was not increased by TRAF6 overexpression (Fig. 3D). Ubiquitination of a K8R/E17K Akt mutant in vivo was reduced and correlated with a reduction in phosphorylation of Akt T308 (Fig 3E). Thus, increased Akt ubiquitination apparently contributes to the hyperactivation of Akt observed in the Akt E17K mutant.
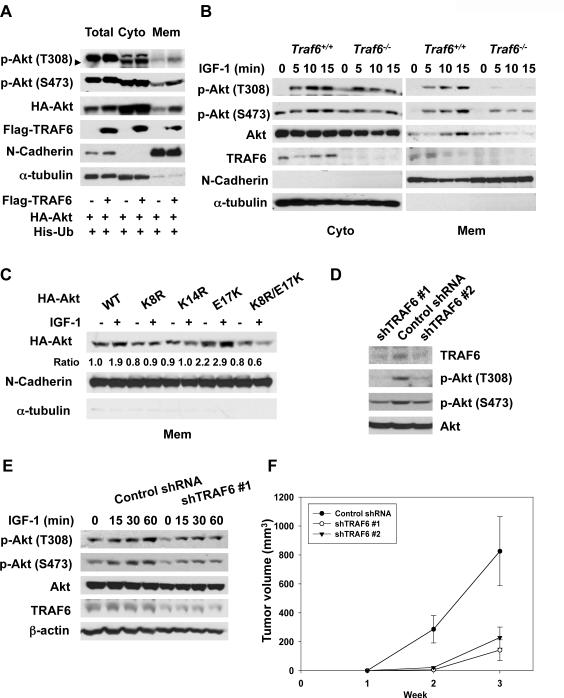
As K63 ubiquitination regulates protein trafficking, we tested whether TRAF6 influenced membrane recruitment of Akt. Overexpression of TRAF6 increased Akt membrane localization, which correlated with an increase in phosphorylation and ubiquitination of Akt (Fig. 3A). IGF-1-induced Akt membrane localization and T308 phosphorylation in WT MEFs was abolished in Traf6−/− MEFs (Fig. 4B). Thus, TRAF6 is required for Akt membrane recruitment and phosphorylation upon IGF-1 stimulation.
Fig. 4.
Ubiquitination of Akt is required for membrane recruitment of Akt. (A) 293T was transfected with mock or TRAF6, and the membrane and cytosolic fractions were subjected to immunoblot analysis. (B) MEFs were serum-starved, treated with IGF-1, and the membrane and cytosolic fractions were isolated for immunoblot analysis (C) COS-1 cells were transfected with indicated plasmids, serum-starved, treated with IGF-1 for 15 min, and the membrane fractions were isolated for immunoblot analysis. (D) PC-3 cells silenced with control or TRAF6 shRNAs were harvested for immunoblot analysis. (E) PC-3 cells silenced with control or TRAF6 shRNA were serum-starved, treated with IGF-1 for various times, and harvested for immunoblot analysis. (F) PC-3 cells silenced with control or TRAF6 shRNAs were injected into nude mice (n=6 for each group) and monitored for tumorigenesis. Results are presented as mean values ± S.D. *p<0.05, using Student's t-test.
We also compared the membrane recruitment of WT Akt and Akt mutants (K8R and K14R), which are defective in Akt ubiquitination. Membrane recruitment of Akt K8R and K14R upon IGF-1 treatment was reduced (Fig. 4C and fig. S13). The Akt E17K mutant localized to the membrane, even without IGF-1 stimulation, although IGF-1 stimulation further increased membrane recruitment (Figs. 4C and fig. S13). The Akt K8R/E17K mutant showed impaired association with the membrane (Figs. 4C and fig. S13). This suggests that Akt ubiquitination contributes to membrane recruitment and phosphorylation of Akt.
As deregulated Akt can contribute to cancer development (20, 21), we depleted TRAF6 in PC-3 prostate cancer cells using short hairpin RNAs (shRNAs). Depletion of TRAF6 reduced Akt phosphorylation at T308 and S473 (Fig. 4D). In cells treated with IGF-1, Akt phosphorylation in TRAF6 knockdown cells was impaired (Fig. 4E). In xenograft tumor models the two stable TRAF6 knockdown cells had lower tumorigenic potential than control cells (Fig. 4F and fig. S14). Thus, TRAF6 appears to influence tumorigenesis in this model.
TRAF6 has an important role in TLR signaling and the innate immune response. Our results expand its known function to include the survival and oncogenic signaling pathways (Fig. S15). We suggest that TRAF6 may be a previously uncharacterized oncogene that may serve as an important therapeutic target for human cancers.
Supplementary Material
Acknowledgments
We thank Drs. M.C. Hung, D. Bohmann, M. Eiliers, A.M. Weissman, S. Lipkowitz, J.L. Wrana, X. Yang, W.C. Yeh, and T. Mak for reagents. We are grateful to Drs. M.C. Hung, D. Sarbassov, and M.H. Lee for insightful comments and suggestions. Special thanks to Dr. H. Li and S. Zhang for their technical support and sharing reagents. We also thank Dr. Z. Han for his assistance in confocal microscopy, Mohlere, V.M., and T. Locke for their critical reading and editing of our manuscript. This work was supported by the M. D. Anderson Trust Scholar Fund to H.K.L.
References and notes
- 1.Mukhopadhyay D, Riezman H. Science. 2007 Jan 12;315:201. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 2.Pickart CM. Annu Rev Biochem. 2001;70:503. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 3.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Embo J. 2002 Aug 1;21:4037. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayo LD, Donner DB. Proc Natl Acad Sci U S A. 2001 Sep 25;98:11598. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou BP, et al. Nat Cell Biol. 2001 Nov;3:973. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 6.Adhikary S, et al. Cell. 2005 Nov 4;123:409. doi: 10.1016/j.cell.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZJ. Nat Cell Biol. 2005 Aug;7:758. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamothe B, et al. J Biol Chem. 2007 Feb 9;282:4102. doi: 10.1074/jbc.M609503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta SR, Brunet A, Greenberg ME. Genes Dev. 1999 Nov 15;13:2905. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 10.Luo J, Manning BD, Cantley LC. Cancer Cell. Oct 2003;4:257. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 11.Bozulic L, Surucu B, Hynx D, Hemmings BA. Mol Cell. 2008 Apr 25;30:203. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Yu HG, et al. Int J Cancer. 2008 Jan 15;122:433. doi: 10.1002/ijc.23049. [DOI] [PubMed] [Google Scholar]
- 13.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. Nature. 1996 Oct 3;383:443. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura M, Naito S. Biol Pharm Bull. 2005 May;28:886. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- 15.Brazil DP, Park J, Hemmings BA. Cell. 2002 Nov 1;111:293. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 16.Varnai P, et al. J Cell Sci. 2005 Oct 15;118:4879. doi: 10.1242/jcs.02606. [DOI] [PubMed] [Google Scholar]
- 17.Bellacosa A, et al. Oncogene. 1998 Jul 23;17:313. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 18.Carpten JD, et al. Nature. 2007 Jul 26;448:439. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 19.Rong SB, et al. J Med Chem. 2001 Mar 15;44:898. doi: 10.1021/jm000493i. [DOI] [PubMed] [Google Scholar]
- 20.Cantley LC. Science. 2002 May 31;296:1655. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 21.Vivanco I, Sawyers CL. Nat Rev Cancer. 2002 Jul;2:489. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.