Abstract
Improving the time resolution in microdialysis coupled to high performance liquid chromatography (HPLC) requires that the volume of the separation system be decreased. A low-volume separation permits smaller microdialysate volumes to be injected without suffering a sensitivity loss from dilution. Thus, improved time resolution can be achieved with offline analysis simply by decreasing the separations system volume. For online (near real-time) analysis, there is a further requirement. The separation speed must be at least as fast as the sampling time. Here, the combined use of high column pressures and temperatures, sub-2-μm stationary phase particles, capillary columns, and sensitive, low dead-volume detection resulted in a retention time for the neurotransmitter serotonin of less than one min in a 500 nL dialysate sample volume. Two sensitive detectors, photoluminescence following electron transfer (PFET) and electrochemical, were used for the detection of sub-nanomolar concentrations of serotonin in brain microdialysate samples. The general principles developed are applicable to a wide range of separations with the additional advantages of increases in sample throughput and decreases in mobile phase usage.
Monitoring extracellular levels of serotonin (5-hydroxytryptamine; 5-HT) in the central nervous system with higher temporal resolution than typically practiced (~ 10–30 min) will be a key to understanding how the dynamics of serotonergic signaling modulate normal and disease-associated brain function1. The most commonly used method for in vivo measurement of extracellular serotonin concentration is microdialysis, a sampling method, coupled to high performance liquid chromatography (HPLC)2. Newton and Justice reported that one-minute time resolution was possible for microdialysis sampling of dopamine (DA)3. Indeed, recently, Wang et al. demonstrated 2-s microdialysis time resolution4. Thus, microdialysis is not currently the limiting factor in defining the time resolution of microdialysis/HPLC. High speed has recently been attained by HPLC. Approaches to improving speed include the use of high pressure to increase the linear velocity of the mobile phase, elevating column temperatures, and using shorter columns packed with smaller particles5. However, these applications relied on typical injection volumes of several μL. In microdialysis, the sample volume is defined by the product of dialysate flow rate and the sampling time. Because typical microdialysis flow rates are in the low- or sub-μL/min range, a sampling time (and thus time resolution) of 10–30 min is generally required to accumulate enough volume of sample (and thus enough mass of analyte) to make an injection regardless of detector (electrochemical6, mass spectrometric7, or chemiluminescence8). Newton and Justice3 used 0.5 or 1.0 μL samples and a 500 μm ID column to determine DA in microdialysate. Jung, et al.9, using a 100 μm inside diameter (ID) column, performed dopamine (DA) determinations in microdialysate with 0.5 μL samples. The use of capillary columns with much smaller peak volumes than standard or microbore (1 mm diameter) HPLC columns minimizes the dilution of small volume samples10. However, neither of the DA separations mentioned above approached the one-minute timeframe. The shortest reported retention time for serotonin is ~3.5 min by Ji et al.11 using a UHPLC system. However, the injection volume of 20 μL still rendered the overall experimental temporal resolution a modest 20 min. Thus, the rate limiting step in online microdialysis/HPLC is the separation.
The Poppe plot is a useful tool to understand how operational parameters affect speeds achievable with a particular number of theoretical plates12. Aided by a Poppe plot, we selected capillary columns packed with 1.7 μm diameter particles to conduct HPLC separations of serotonin at up to 80 °C using a splitless pump capable of providing a maximum pressure of 10,000 psi. The goal of this work was to achieve 1-min separation and quantification of brain extracellular serotonin levels in microdialysate samples obtained from unanesthetized mice using an offline chromatographic separation for initial expediency.
2. Experimental
Chemicals and materials
Acetonitrile (AN) - used for the preparation of mobile phases was HPLC grade (Fisher, Springfield, NJ). Sources of chemicals are as follows: Isopropanol, trifluoroacetic acid (TFA), sodium 1-octanesulfonate (SOS), serotonin hydrochloride, 5-hydroxyindole-3-acetic acid (5-HIAA), dopamine hydrochloride, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 3-methoxytyramine hydrochloride (3-MT), and 5-hydroxy-N-methyltryptamine: Sigma (St. Louis, MO); lead dioxide: EM Science (Gibbstown, NJ); disodium EDTA: Fisher Scientific (Fairlawn, NJ); sodium perchlorate: GFS Chemicals (Columbus, OH); sodium acetate: MCB (Cincinnati, Ohio). Deionized water was obtained from a Millipore Milli-Q Synthesis A10 system (Bedford, MA) or was purchased from Cayman Chemical Co. (Ann Arbor, MI). All chemicals from commercial sources were used as received without further purification. The PFET reagent Os(bpy)3(PF6)2 (bpy-2,2′-bipyridine) was prepared according to a previously reported procedure9, 13. Fused silica capillaries were purchased from Polymicro Technologies, LLC (Phoenix, AZ).
Chromatography
Stock solutions of neurotransmitters and metabolites with known concentrations near 1 mM were prepared in 0.1 M acetic acid and stored frozen. 5-Hydroxy-N-methyltryptamine (1 mM) was prepared in deionized water. Aqueous standards for chromatography were prepared by successive 10-fold dilutions of stock solutions with 0.1 M acetic acid, except for the final dilution where artificial cerebrospinal fluid (aCSF) was used. The aCSF solution contained (in mM) 147 NaCl, 3.5 KCl, 1.0 CaCl2, 1.2 MgCl2, 1.0 NaH2PO4 and 2.5 NaHCO3 at pH 7.414.
Liquid chromatography experiments were performed using a NanoLC Ultra pump (Eksigent Technologies, Dublin, CA) that delivers mobile phase at flow rates from 0.1–10 μL/min without a splitter and generates operating pressures as high as 10,000 psi. A Valco 10-port valve, which can withstand pressures up to 15,000 psi, was used for manual injections. The fused silica capillary sample loop was 500 nL. Capillary columns were packed by the slurry method9, 15 using a custom-built high-pressure packing system. Frits were made by sintering borosilicate particles (2 μm, Thermo Fisher Scientific, Indianapolis, IN), which were packed at the ends of the column. Fused-silica capillaries with 100 μm i.d. and 365 μm o.d. were used as column blanks. Stationary phases were 2.6 μm XTerra C18 MS and 1.7 μm BEH C18 bonded (Waters, Milford MA). The packing pressure was increased gradually during the packing process with a final pressure of ~12,000 psi. Column lengths were in the range of 4 – 5.5 cm for the 1.7 μm packing material, and 7.5 cm for the 2.6 μm packing material. Particular lengths are provided in figure legends. Flow rates were approximately 4 μL/minute for columns with 1.7 μm packing and 6 μL/minute for columns with 2.6 μm packing.
Two heaters were designed and built, one for heating the injection valve and the other for the column. The injection valve was wrapped with an etched foil-like Kapton heating unit (Minco Products, inc., Minneapolis, MN), and the temperature sensor was attached to the center of the top of the valve. The column heater was made of an aluminum cylinder wrapped with another foil-like heating unit having a small hole in the center to accommodate capillary columns. The temperature sensor for the column heater was embedded inside the wall of the aluminum cylinder. These two heaters were controlled independently by two temperature controllers (Minco Products, inc., Minneapolis, MN), which were designed to fit the power requirements of the heating units.
Mobile phases were prepared by mixing acetonitrile with aqueous buffers containing 100 mM sodium acetate, 0.15 mM disodium EDTA, and 10 mM SOS. The pH of the aqueous buffer was adjusted with glacial acetic acid or concentrated NaOH solution to the desired pH (as stated below). The mobile phase was passed through a Nylon filter with 0.20 μm pores prior to use (Fisher Scientific, Pittsburgh, PA).
PFET detection
The photoluminescence following electron transfer (PFET) detector has been previously reported9, 13 and was further modified for enhanced sensitivity. An iBEAM-488-S-3V3 single-mode diode laser (Toptica Photonics AG, Gräfelfing, Germany) with a maximum optical output of 20 mW was used to provide a 488 nm laser beam as the excitation source. Fluorescence intensity was measured by an IR-sensitive photomultiplier tube (R374, Hamamatsu, Bridgewater, NJ) and a 6485 picoammeter (Keithley, Cleveland, Ohio), which was followed by a low pass programmable analog filter (Model SIM965, Stanford Research Systems, Inc., Sunnyvale, CA 94089). Data were collected by a PeakSimple Chromatographic Data System (SRI Instruments, Torrance, CA). Custom mixers were similar to those reported previously but with capillaries of smaller i.d. to reduce band dispersion16. Fused silica capillaries of 25 μm i.d. were used as the inlet arms of the mixer and 39.5 μm i.d. capillaries were used as the mixing/reacting arms. The detection window was ~30 cm from the mixing point. A 1 mM stock solution of Os(bpy)3(PF6)2 was prepared in acetonitrile. This was diluted with an acidic electrolyte solution (0.2% TFA and 0.1 M NaClO4 in acetonitrile or water) to prepare the PFET reagent solution.
Electrochemical detection
The electrochemical detector was composed of a BASi radial-style thin-layer auxiliary electrode (West Lafayette, IN), a 13-μm thick Teflon spacer and a custom-built 1 mm diameter glassy carbon electrode block. This thin spacer provides a detector volume that minimizes band broadening in the flow cell. The glassy carbon electrode was secured in the center of a Kel-F block with Spurr low viscosity epoxy resin (Polysciences, Inc., Warrington, PA), which was allowed to cure at 70 °C for at least 8 h. Electrical contact was made by connecting the glassy carbon electrode to a metal pin using silver epoxy H20E (Epoxy Technology Inc., Billerica, MA), which was cured at 80 °C overnight. Before assembling the flow cell, the electrode block was wet polished with 0.05 μm γ-alumina (Buehler Ltd., Lake Bluff, IL, USA), rinsed, and sonicated with deionized water. The electrochemical detector was housed in a Faraday cage. A short length of 25 μm i.d. fused silica capillary acted as a connecter between the column and the detector. A BASi Epsilon potentiostat controlled the detection potential at +0.7 V vs. Ag/AgCl (3 M NaCl).
Microdialysis
Dialysates contain a number of interfering peaks not found in standards. Thus, it was necessary to assess our approach to the separation of serotonin using actual microdialysis samples. Nine-week-old male mice (~ 40 g) with constitutive loss of serotonin transporter expression (SERT−/−) and wildtype mice (SERT+/+) were used for these experiments1, 14. All procedures involving animals were approved by The Pennsylvania State University Institutional Animal Care and Use Committee and strictly adhered to National Institutes of Health Animal Care and Use Guidelines.
Guide cannulae for CMA/7 microdialysis probes (CMA Microdialysis, Solna, Sweden) were implanted in the right ventral hippocampus at A-2.8, L-3.5, V-2.2 under isoflurane anesthesia2 Three to five days later, mice were briefly anesthetized and a microdialysis probe (CMA 7, 2 mm × 240 μm cuprophane membrane; 6,000 MW cutoff) was slowly inserted into the cannula. Ascorbate (7 μM) was added to aCSF to prevent oxidation of serotonin. Artificial cerebrospinal fluid was vacuum-filtered using 0.45 μm Millipore filters and loaded into 1 mL syringes to be used with a CMA 102 programmable infusion pump. The pump and probe were connected via a zero dead-volume CMA 110 liquid switch.
Two mice were studied: one SERT+/+ and one SERT−/−. Before experiments began, probes were perfused with aCSF overnight at 1.1 μL/min in vivo. The following morning, baseline samples were collected from each mouse at either 1 μl/min for 3 h or 3 μL/min for 1 h (i.e., samples were 180 μL) into borosilicate glass HPLC microsampler vials (CMA Microdialysis, North Chelmsford, MA). Following this, probes were perfused with aCSF containing isoosmotic 120 mM K+ for 20 min (3 μL/min) to evoke presynaptic release of serotonin. One sample was collected during high K+ stimulation, as well as a second sample containing the dialysate fraction during the 20 min period immediately after high K+ administration. All samples were frozen immediately at −80 °C sent to the University of Pittsburgh by overnight courier, also on dry ice. Samples from the SERT+/+ mouse were collected on “day one”, frozen at −80 °C, shipped on day 6, received on day 7, and analyzed with EC detection on day 9. SERT−/− samples were shipped on day 1, received and analyzed by EC detection on day 2, and by PFET on day 23. Samples were kept frozen at −80 °C when not being analyzed. Our tests of stability show that there is no loss of 5-HT in one day on dry ice, and no loss of 5-HT after 22 days at −80 °C. However, samples that are thawed and frozen do degrade.
3. Results and discussion
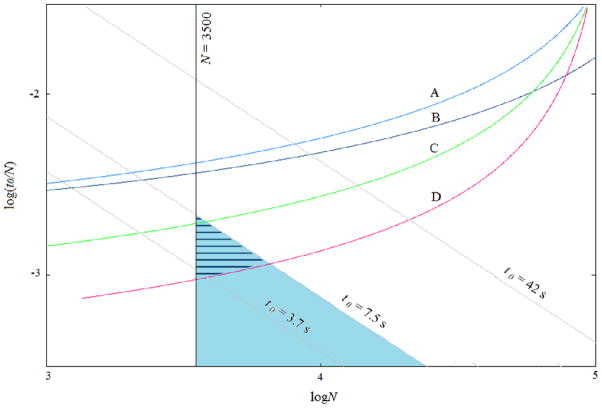
The Poppe plot provides a way to know whether or not a chromatographic system can provide a particular number of theoretical plates (N) in a particular time when full advantage17 of the maximum available pressure is taken. That pressure may be used with long columns to obtain the maximum N possible (with a given column at a particular temperature), or it may be used with short columns to achieve high speed with a sacrifice in N. Figure 1 shows Poppe plots for different HPLC configurations, e.g., stationary phase particle diameters, temperatures, and maximum column pressures, obtained according to a method previously described12b. Conceptually, the plate height, H, is determined by mobile phase velocity and parameters of the solute (diffusion coefficient, retention factor) and column (packing material diameter, porosity, etc.) as expressed in the well-known Knox or van Deemter equations. N depends on column length, L, and H. To arrive at a curve on this plot, first recall that the maximum available pressure, Pmax, is used. A point on the curve can be determined by choosing a value of L which, with pressure and column properties, gives a value of the mobile phase velocity. The velocity and length, along with some column properties, give the void time, t0, which is needed for the vertical axis. The velocity also gives H (measured or calculated, e.g, from the Knox equation). H and L give N, which is needed for both axes. Note that changes in temperature change viscosity. Changes in viscosity change the velocity for a given column and pressure as well as the diffusion coefficient of the solute. Thus changes in temperature affect H. Each curve in Fig. 1 represents the shortest time-per-theoretical-plate, t0/N, that can be achieved given a certain number of theoretical plates, N, using the given HPLC configuration. The desirable area of the plot is the lower right (large N, short time), however, the space below and to the right of each curve is inaccessible for the chosen conditions. Mass transfer resistance (C term in the plate height equation) dominates at the lower left of each curve, while the axial diffusion (B term) becomes dominant at the upper right. Each straight line with a slope of -1 consists of points with the same dead time, t0. A t0 of 7.5 s corresponds to a retention time of 1 min (k′ ~7 in preliminary work with a mobile phase containing 10% AN (v/v)) for serotonin. Hence, separation of serotonin within one min falls in the area to the left of the dead time line corresponding to 7.5 s. To obtain the needed separation, the column efficiency should be at least 3000–4000 plates. The shaded target area represents conditions that are sufficient (t0 less than 7.5 s and N>3500).
Figure 1. Poppe plots for various liquid chromatography configurations.
A: 2.6 μm particles, 4000 psi, 25 °C. Chromatograms from Jung, et al.9 and related unpublished work with 2.6 μm particles were used to establish an estimate of the “C term” which is the most important term for high-speed work. The dimensionless C term, which includes a contribution from the postcolumn reactor, was determined to be 0.22. Typical values of 2 and 1.5 for B and A respectively were used. These values represent data for 1.7 μm particles well. In order to determine Poppe plots with new conditions we used published equations and temperature dependence of viscosity20 B: 2.6 μm particles, 7000 psi, 25 °C, C: 1.7 μm particles, 7000 psi, 25 °C, D: 1.7 μm particles, 7000 psi, 70 °C. Here, N is the number of theoretical plates, t0 is the dead time for unretained analytes, and t0/N is plate time. Separation conditions that overlap with the desired separation time for serotonin<1 min, corresponding to t0<7.5 s, and that are accessible are denoted by the hatched blue area.
Curves A and B (Fig. 1) taken together show the effect of raising the maximum column pressure from 4000 psi (A) to 7000 psi (B) for a separation at 25 °C with 2.6 μm diameter particles. Condition B can produce a shorter time per theoretical plate than condition A, but there is no overlap between the accessible area (above the curves) and the target area, meaning that the desired separation cannot be achieved under these conditions. Replacing 2.6 μm particles with 1.7 μm particles (curve B vs. C) results in the accessible area moving down towards shorter plate times. This leads to a small amount of overlap with the target area. However, a much larger overlapping area, the hatched pseudo-triangle in Figure 1, is obtained by carrying out the separation at an increased temperature of 70°C (D). In recent years, others have reported experimental results that revealed similar effects of column pressure, temperature, and particle size on separation speed18.
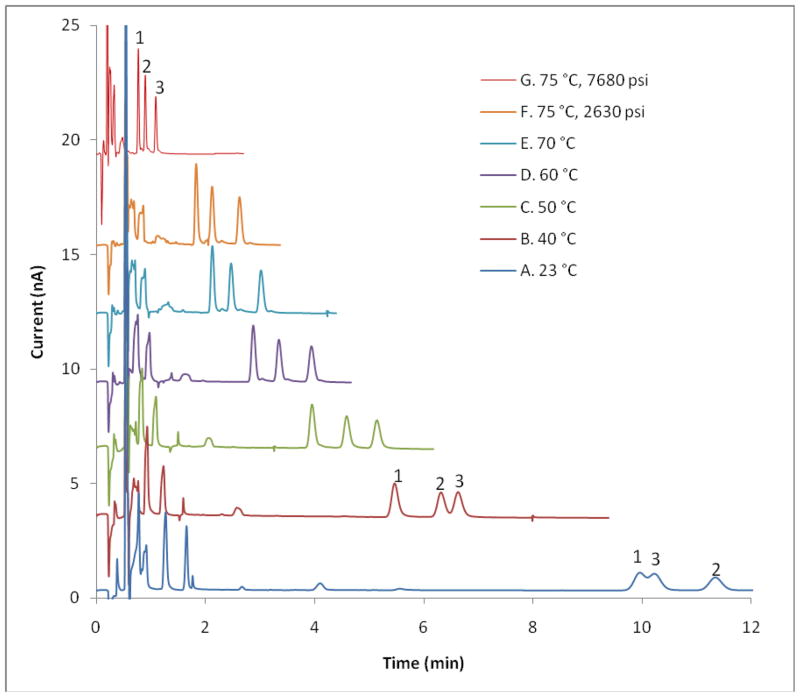
In anticipation of the decrease in k′ occurring on raising the column temperature, we adjusted the mobile phase to a composition containing only 4% AN (v/v, full details accompany the figures). Using the information derived from Figure 1, we conducted separations of standard mixtures of monoamine neurotransmitters, including serotonin, and their metabolites using several HPLC configurations. Figure 2 shows how the retention time and retention factor of serotonin were reduced when both higher pressures and temperatures were used. Ultimately, the changes caused a 13-fold decrease in the retention time of serotonin.
Figure 2. Effect of separation conditions on serotonin retention times.
Column: 100 μm i.d. × 5.5 cm length, 1.7 μm BEH C18 bonded stationary phase. Flow rates: A to F, 1.5 μL/min; G, 4.0 μL/min. Mobile phase: pH 4.0 aqueous buffer containing 100 mM sodium acetate, 0.15 mM disodium EDTA, and 10 mM SOS, with 4% (v/v) acetonitrile. Peaks: (1) Serotonin; (2) N-Me-5-HT; (3) 3-methoxytyramine. In separate experiments, we determined that norepinephrine elutes even earlier than dopamine. Thus, neither neurotransmitter elutes close to serotonin.
When analyzing trace amounts of serotonin, such as those found in basal microdialysis samples, another challenge appeared, namely interfering peaks from impurities in the aCSF. Further optimization of the separation conditions was necessary to avoid overlap of the serotonin peak and interfering peaks. At about 80 °C, the serotonin peak co-eluted with two unknown peaks (Figure S1). Because the relationship between retention factor and temperature for serotonin is different than for the interferents, lowering the operating temperature to 70 °C successfully shifted the serotonin peak to a position where there were no co-eluting species. With these conditions, serotonin was readily determined at concentrations as low as 200 pM. We expect that the elution conditions can be further optimized to obtain good separation within shorter run times. We note that a few peaks elute after 5-HT and thus may interefere with a subsequent chromatogram. These peaks elute in the subsequent chromatogram during the large “offscale” peak at low k′ attributable to polar electroactive substances, chiefly ascorbate. As a result, we do not see interferences from previous injections when making serial injections with one minute or less between injections.
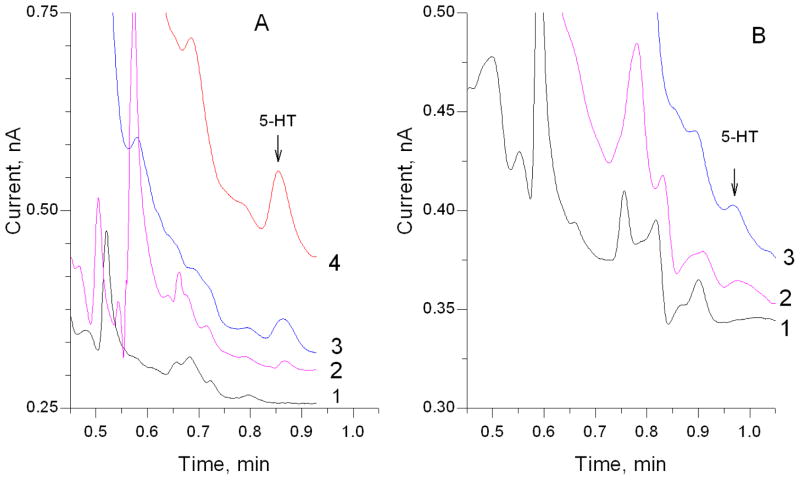
The limit of detection (LOD) was calculated based on three times the standard deviation of the noise level around the serotonin peak position (approximately 0.82 – 0.92 minutes when the retention time is 0.86 minutes), and was about 0.07 nM for the system with electrochemical detection, and 0.20 nM for the PFET detector. Figure 3 shows separations of serotonin in microdialysis samples obtained from the hippocampus of mice, both SERT−/− and SERT+/+. Peak identities were confirmed by spiking samples (2 nM spike concentration) and injecting again. Within-day retention reproducibility was excellent. For example, the serotonin peaks in standards and SERT−/− samples from one typical day had an average retention time of 51.68 s with a standard error of 0.08 s (38 injections). We have shown previously using microdialysis and conventional HPLC separation conditions that extracellular serotonin is elevated 6-fold in striatum and 10-fold in frontal cortex in SERT−/− mice14. A chromatogram from a SERT−/− mouse with PFET detection is shown as Fig. S2.
Figure 3. Fast analysis of microdialysis samples.
Left: Column: 100 μm i.d. X 5.1 cm, 1.7 μm BEH C18 bonded. Temperature: 70 °C. Flow rate: 4 μL/min. Mobile phase: pH 4.0 aqueous buffer containing 100 mM sodium acetate, 0.15 mM disodium EDTA, and 10.0 mM SOS, mixed with 4% (v/v) acetonitrile, electrochemical detection. 1. aCSF, 2. 500 pM 5-HT, 3. Representative baseline microdialysate collected from the hippocampus in a serotonin transporter deficient mouse (SERT−/−) at 3 μL/min, 4. Baseline microdialysate at 1 μL/min. Right: Column: 100 μm i.d. × 5.5 cm length, Flow rate: 3.7 μL/min, remainder of conditions same as above. Top trace, 200 pM 5-HT standard in aCSF; middle trace, representative hippocampal microdialysate sample from a wildtype SERT+/+ mouse; bottom trace, aCSF.
Table 1 shows that for both electrochemical and PFET detection, serotonin concentrations are significantly higher in samples collected at a perfusion flow rate of 1 μl/min than those measured in dialysate samples collected at a flow rate of 3 μl/min. This is due to the fact that relative recovery increases with decreasing dialysate flow rates19. Serotonin levels increased significantly after perfusion of high K+-containing aCSF and subsequently returned to lower levels in the dialysate samples immediately following perfusion with 120 mM K+. Together, these data demonstrate that the peak being monitored is sensitive to depolarization stimulus, which is further evidence, in conjunction with retention time and coelution with spiked standard, that this peak is serotonin14. The PFET detection experiments were carried out three weeks after the electrochemical detection experiments during which time the samples, which had been thawed for the electrochemical detection measurements for various times, were frozen at −80 °C, but without acid preservative. As mentioned in the experimental section, thawing and freezing results in some 5-HT loss. Thus, the concentrations determined by PFET are lower than those determined by electrochemical detection.
Table 1.
Serotonin concentrations (nM ± SEM (number of injections)) in dialysate samples from a SERT−/− mouse
| Sample | Electrochemical detection | PFET detection |
|---|---|---|
| Baseline aCSF perfused at 1 μL/min | 3.1 ± 0.2** (3) | 1.7 ± 0.4* (3) |
| Baseline aCSF perfused at 3 μL/min | 1.1 ± 0.01 (2) | 0.5 ± 0.1 (3) |
| 120 mM K+ aCSF at 3 μL/min | 5.5 ± 0.2††,*** (3) | 4.2 ± 0.5†,** (3) |
| Post 120 mM K+ aCSF at 3μL/min | 2.3 ± 0.2 (3) | 2.2 ± 0.1 (3) |
p<0.1,
p<0.01, and
p<0.001 vs. baseline aCSF perfused at 3 μL/min;
p<0.05 and
p<0.001 vs. post K+ aCSF at 3 μL/min. Comparisons are by unpaired two-tailed t-tests.
4. Conclusions
Equipped with sensitive electrochemical and PFET detectors, basal serotonin levels in microdialysis samples can be measured using capillary high pressure and temperature liquid chromatography in one minute or less. The high speed and improved mass sensitivity require the use of capillary columns, 1.7 μm particles (or similar sub-2 μm particles), elevated temperature, and elevated pressure. We envision this method, when coupled with microdialysis, will lead to the determination of serotonin levels in the central nervous system with a temporal resolution of one min or less in awake, behaving animals.
Supplementary Material
Acknowledgments
This project was supported by funding from the National Institute of Mental Health (MH083134 to SGW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. Thanks to Dr. Ed Bouvier of Waters Corp. for the kind donation of packing material.
Literature cited
- 1.Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch KP, Wendland JR. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–60. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luellen BA, Bianco LE, Schneider LM, Andrews AM. Reduced brain-derived neurotrophic factor is associated with a loss of serotonergic innervation in the hippocampus of aging mice. Genes, Brain and Behav. 2007;6:482–490. doi: 10.1111/j.1601-183X.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 3.Newton AP, Justice JB., Jr Temporal Response of Microdialysis Probes to Local Perfusion of Dopamine and Cocaine Followed with One-Minute Sampling. Anal Chem. 1994;66:1468–72. doi: 10.1021/ac00081a018. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Roman GT, Schultz K, Jennings C, Kennedy RT. Improved Temporal Resolution for in Vivo Microdialysis by Using Segmented Flow. Anal Chem (Washington, DC, United States) 2008;80:5607–5615. doi: 10.1021/ac800622s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Wu N, Clausen AM. Fundamental and practical aspects of ultrahigh pressure liquid chromatography for fast separations. J Sep Sci. 2007;30:1167–1182. doi: 10.1002/jssc.200700026. [DOI] [PubMed] [Google Scholar]; (b) McNeff CV, Yan B, Stoll DR, Henry RA. Practice and theory of high temperature liquid chromatography. J Sep Sci. 2007;30:1672–1685. doi: 10.1002/jssc.200600526. [DOI] [PubMed] [Google Scholar]; (c) Pursch M, Schweizer-Theobaldt A, Cortes H, Gratzfeld-Huesgen A, Schulenberg-Schell H, Hoffmann BW. Fast, Ultra-fast and High-resolution LC for Separation of Small Molecules, Oligomers and Polymers. LC-GC Europe. 2008;21:152–159. [Google Scholar]; (d) Xiang Y, Liu Y, Lee ML. Ultrahigh pressure liquid chromatography using elevated temperature. J Chromatogr A. 2006;1104:198–202. doi: 10.1016/j.chroma.2005.11.118. [DOI] [PubMed] [Google Scholar]
- 6.(a) Tobin VA, Leng G, Ludwig M, Douglas AJ. Increased sensitivity of monoamine release in the supraoptic nucleus in late pregnancy: region- and stimulus-dependent responses. J Neuroendocrinol. 2010;22:430–437. doi: 10.1111/j.1365-2826.2010.01957.x. [DOI] [PubMed] [Google Scholar]; (b) Nowak P, Kostrzewa RA, Skaba D, Kostrzewa RM. Acute L-DOPA effect on hydroxyl radical- and DOPAC-levels in striatal microdialysates of Parkinsonian rats. Neurotox Res. 2010;17:299–304. doi: 10.1007/s12640-009-9105-2. [DOI] [PubMed] [Google Scholar]; (c) Popa D, Cerdan J, Reperant C, Guiard BP, Guilloux JP, David DJ, Gardier AM. A longitudinal study of 5-HT outflow during chronic fluoxetine treatment using a new technique of chronic microdialysis in a highly emotional mouse strain. Eur J Pharmacol. 2010;628:83–90. doi: 10.1016/j.ejphar.2009.11.037. [DOI] [PubMed] [Google Scholar]; (d) Calcagno E, Invernizzi Roberto W. Strain-dependent serotonin neuron feedback control: role of serotonin 2C receptors. J Neurochem. 2010;114:1701–10. doi: 10.1111/j.1471-4159.2010.06880.x. [DOI] [PubMed] [Google Scholar]; (e) Navailles S, Benazzouz A, Bioulac B, Gross C, De Deurwaerdere P. High-frequency stimulation of the subthalamic nucleus and L-3,4-dihydroxyphenylalanine inhibit in vivo serotonin release in the prefrontal cortex and hippocampus in a rat model of Parkinson’s disease. J Neurosci. 2010;30:2356–2364. doi: 10.1523/JNEUROSCI.5031-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell MA, Reymann JM, Allain H, Leonard BE, Bentué-Ferrer D. Lisuride prevents learning and memory impairment and attenuates the increase in extracellular dopamine induced by transient global cerebral ischemia in rats. Brain Res. 1997;771:305–318. doi: 10.1016/s0006-8993(97)00817-2. [DOI] [PubMed] [Google Scholar]
- 8.Li N, Guo J, Liu B, Yu Y, Cui H, Mao L, Lin Y. Determination of monoamine neurotransmitters and their metabolites in a mouse brain microdialysate by coupling high-performance liquid chromatography with gold nanoparticle-initiated chemiluminescence. Anal Chim Acta. 2009;645:48–55. doi: 10.1016/j.aca.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 9.Jung MC, Shi G, Borland L, Michael AC, Weber SG. Simultaneous determination of biogenic monoamines in rat brain dialysates using capillary high-performance liquid chromatography with photoluminescence following electron transfer. Anal Chem. 2006;78:1755–1760. doi: 10.1021/ac051183g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dezaro DA, Dvorn D, Horn C, Hartwick RA. Time optimization for routine separations, using high-speed microbore HPLC. Chromatographia. 1985;20:87–96. [Google Scholar]
- 11.Ji C, Li W, Ren XD, El-Kattan AF, Kozak R, Fountain S, Lepsy C. Diethylation labeling combined with UPLC/MS/MS for simultaneous determination of a panel of monoamine neurotransmitters in rat prefrontal cortex microdialysates. Anal Chem. 2008;80:9195–9203. doi: 10.1021/ac801339z. [DOI] [PubMed] [Google Scholar]
- 12.(a) Poppe H. Some reflections on speed and efficiency of modern chromatographic methods. J Chromatogr A. 1997;778:3–21. [Google Scholar]; (b) Carr PW, Wang X, Stoll DR. Effect of Pressure, Particle Size, and Time on Optimizing Performance in Liquid Chromatography. Anal Chem. 2009;81:5342–5353. doi: 10.1021/ac9001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung MC, Munro N, Shi G, Michael AC, Weber SG. Use of tris(2,2′-bipyridine)osmium as a photoluminescence-following electron-transfer reagent for postcolumn detection in capillary high-performance liquid chromatography. Anal Chem. 2006;78:1761–1768. doi: 10.1021/ac051182o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Wu N, Lippert JA, Lee ML. Practical aspects of ultrahigh pressure capillary liquid chromatography. J Chromatogr A. 2001;911:1–12. doi: 10.1016/s0021-9673(00)01188-2. [DOI] [PubMed] [Google Scholar]
- 16.Sahlin E, Beisler AT, Woltman SJ, Weber SG. Fabrication of Microchannel Structures in Fluorinated Ethylene Propylene. Anal Chem. 2002;74:4566–4569. doi: 10.1021/ac025622c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giddings JC. Comparison of the theoretical limit of separating ability in gas and liquid chromatography. Anal Chem. 1964;36:1890–2. [Google Scholar]
- 18.(a) Zhang Y, Wang X, Mukherjee P, Petersson P. Critical comparison of performances of superficially porous particles and sub-2 [mu]m particles under optimized ultra-high pressure conditions. J Chromatogr A. 2009;1216:4597–4605. doi: 10.1016/j.chroma.2009.03.071. [DOI] [PubMed] [Google Scholar]; (b) de Villiers A, Lestremau F, Szucs R, Gélébart S, David F, Sandra P. Evaluation of ultra performance liquid chromatography: Part I. Possibilities and limitations. J Chromatogr A. 2006;1127:60–69. doi: 10.1016/j.chroma.2006.05.071. [DOI] [PubMed] [Google Scholar]; (c) Lestremau F, de Villiers A, Lynen F, Cooper A, Szucs R, Sandra P. High efficiency liquid chromatography on conventional columns and instrumentation by using temperature as a variable: Kinetic plots and experimental verification. J Chromatogr A. 2007;1138:120–131. doi: 10.1016/j.chroma.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 19.Benveniste H, Hüttemeier PC. Microdialysis--Theory and application. Prog Neurobiol. 1990;35:195–215. doi: 10.1016/0301-0082(90)90027-e. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Weber SG. Effect of an open tube in series with a packed capillary column on liquid chromatographic performance: The influence of particle diameter, temperature, and system pressure. J Chromatogr A. 2009;1216:1346–1352. doi: 10.1016/j.chroma.2008.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.