Summary
Mice that lack interleukin-23 (IL-23) are resistant to T cell-mediated autoimmunity. Although IL-23 is a maturation factor for T helper 17 (Th17) cells, a subset of γδ T cells expresses the IL-23 receptor (IL-23R) constitutively. Using IL-23R reporter mice, we showed that γδ T cells were the first cells to respond to IL-23 during experimental autoimmune encephalomyelitis (EAE). Although γδ T cells produced Th17 cell-associated cytokines in response to IL-23, their major function was to prevent the development of regulatory T (Treg) cell responses. IL-23-activated γδ T cells rendered αβ effector T cells refractory to the suppressive activity of Treg cells and also prevented the conversion of conventional T cells into Foxp3+ Treg cells in vivo. Thus, IL-23, which by itself has no direct effect on Treg cells, is able to disarm Treg cell responses and promote antigen specific effector T cell responses via activating γδ T cells.
Introduction
γδ T cells bear a T cell receptor (TCR) composed of rearranged γ and δ chains instead of the conventional αβ heterodimeric TCR. Whereas γδ T cells constitute the majority of immune cells in certain niches associated with epithelial surfaces, only about 1 percent of T cells in secondary lymphoid tissue are γδ T cells. Most of the γδ T cells in secondary lymphoid organs are positive for the non-canonical TCR-γ1 or TCR-γ4 chains (Hayday, 2000; Hayday and Pennington, 2007). Ligand recognition by γδ-TCRs appears to be dispensable for selection of γδ T cells in the adult thymus. Whether it is required for the selection of γδ T cells in embryonic thymus is not clear (Itohara et al., 1993; Lewis et al., 2006; Xiong et al., 2004). Little is known about the antigenic repertoire and restriction elements of the γδ-TCR. γδ T cell development is apparently neither affected in major histocompatibility complex (MHC) class II deficient nor in β2 microglobulin deficient animals (Bigby et al., 1993; Correa et al., 1992). In secondary and tertiary lymphoid tissue, γδ T cells are able to respond directly to pathogen associated molecular patterns and to cytokines in the local milieu in the absence of cognate TCR ligands. Thus, γδ T cells have many properties of cells of the innate immune system. γδ T cells appear early during immune responses and efficiently produce inflammatory cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor (TNF) (Duhindan et al., 1997), but have also been reported to secrete interleukin-10 (IL-10) (Rhodes et al., 2008). The ability of γδ T cells to produce IL-17 has been appreciated only recently and the role of IL-17 producing γδ T cells has been evaluated in various models of infection and autoimmunity (Jensen et al., 2008; Martin et al., 2009; Ribot et al., 2009; Sutton et al., 2009).
IL-23 is a major pathogenic factor in organ specific autoimmunity and chronic inflammation (Cua et al., 2003; Murphy et al., 2003; Yen et al., 2006) since IL-23 is a maturation and potentially also a growth factor for pathogenic IL-17 producing αβ T cells, so-called Th17 cells (Korn et al., 2009). Yet, other αβ T cell extrinsic functions of IL-23 have been proposed because first, the IL-23 receptor (IL-23R) is expressed on a variety of non-αβ T cells (Awasthi et al., 2009) and second, IL-23 is also relevant in models of chronic inflammation that were independent of αβ T cells (Buonocore et al., 2010; Uhlig et al., 2006). However, the function of IL-23 in vivo is still not completely understood. To identify the cell types responding to IL-23 during experimental autoimmune encephalomyelitis (EAE) in vivo, we generated a mutant mouse, in which an internal ribosome entry site (IRES)-green fluorescent protein (GFP)-cassette was introduced into the endogenous Il23r gene (Awasthi et al., 2009). As a result of this “knock-in” strategy, heterozygous Il23r+/− animals can be used as reporter mice to identify IL-23R positive cells on the basis of GFP expression while still fully responsive to IL-23 due to one intact Il23r allele. Homozygous Il23r−/− mice are unresponsive to IL-23 due to two mutated Il23r alleles.
Here, we found that in the steady state, γδ T cells express constitutively high amounts of IL-23R and respond early and vigorously to IL-23. IL-23 drives the expansion and accumulation of IL23R+ γδ T cells and the secretion of IL-17, IL-21, and IL-22 by γδ T cells. Yet, the most crucial effector function of IL-23-activated γδ T cells in an inflammatory environment is not the production of Th17 cell-associated cytokines, but their ability to antagonize Treg cell-mediated suppression of αβ T cells. This offers an explanation why the presence of Treg cells at sites of inflammation is not sufficient to suppress αβ T cell-mediated immunopathology during highly inflammatory stages of organ specific autoimmunity.
Results
IL-23R expressing γδ T cells are a functionally distinct subset of γδ T cells
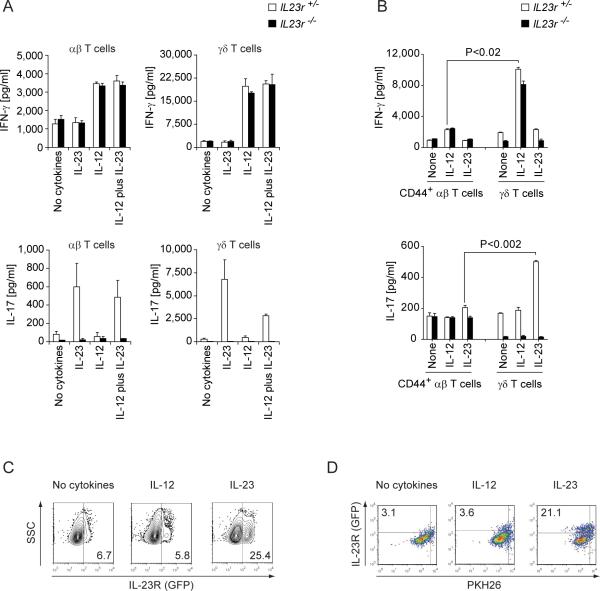
Naïve αβ T cells lack the receptor for IL-23 (Mangan et al., 2006) whereas the IL-23R is highly expressed in marked fractions of γδ T cells in lymph nodes and spleen of unmanipulated mice (Awasthi et al., 2009). Therefore, we first tested the responsiveness of purified CD4−CD8− γδ T cells from Il23r−/− and Il23r+/− littermates to IL-12 and IL-23, respectively. Both αβ T cells and γδ T cells produced IFN-γ in response to IL-12 and as expected, their IFN-γ production was not affected by lack of IL-23R expression (Figure 1A). In contrast, IL-23 induced the secretion of IL-17 in bulk Il23r+/− CD4+ αβ T cells and purified γδ T cells (Figure 1A). γδ T cells responded to IL-23 even in the absence of TCR stimulation (data not shown). Neither αβ T cells nor γδ T cells from Il23r−/− mice produced IL-17 in response to IL-23 (Figure 1A). As γδ T cells have the phenotype of activated lymphocytes, we compared their cytokine secretion capacity with the cytokine response of antigen experienced CD4+CD44+ αβ T cells. On a per cell basis, γδ T cells were more potent producers of IFN-γ and IL-17 than CD44+ αβ T cells in response to IL-12 and IL-23, respectively (Figure 1B). Unlike IL-12, IL-23 had an additional effect on its target population in that it also increased the fraction of IL-23R expressing γδ T cells by promoting the proliferation of IL-23R+ but not IL-23R− γδ T cells (Figure 1C, D). In order to correlate the production of IFN-γ and IL-17 with the IL-23R status in individual γδ T cells, we purified γδ T cells from the lymph nodes of Il23r+/− mice and determined the production of IFN-γ and IL-17 upon ex vivo stimulation with PMA plus ionomycin. All IFN-γ producing γδ T cells were IL-23R− while IL-17 was exclusively produced by IL-23R+ γδ T cells. The IL-23R−IFN-γ+ and IL-23R+IL-17+ phenotypes segregated with the expression of NK1.1 and C-C motif receptor (CCR)6, respectively (Figure S1). Thus, CCR6 defines the population of IL-23R+IL-17+ γδ T cells whereas NK1.1 is a marker for IL-23R−IFN-γ+ γδ T cells (see also (Haas et al., 2009)).
Figure 1. γδ T cells produce high amounts of IFN-γ and IL-17 in response to IL-12 and IL-23, respectively.
CD4−CD8− γδ T cells and bulk CD4+ αβ T cells (A) or flow cytometrically purified CD4+CD44+ αβ T cells (B) were isolated from Il23r−/− mice or Il23r+/− littermates. Cells were stimulated for 72hr with anti-CD3 plus anti-CD28 coated beads (A) or for 24 hr with plate-bound monoclonal antibodies to CD3 (0.5 μg/ml) and CD28 (0.25 μg/ml) (B) in the presence of the indicated cytokines. IFN-γ and IL-17 production were determined by ELISA. Amount of cytokines produced in triplicate wells ± SD. (C) After in vitro stimulation with anti-CD3 plus anti-CD28 coated beads, Il23r+/−γδ T cells were analyzed for IL-23R (GFP) expression. Numbers indicate percentages within the γδ-TCR gate. (D) Il23r+/− γδ T cells isolated from naïve Il23r+/− reporter mice were labelled with PKH26 and stimulated with anti-CD3 plus anti-CD28 beads in the presence of the indicated cytokines. After 3 days, dilution of PKH26 was measured in the gate of blasting γδ T cells by flow cytometry. Numbers indicate percentages of proliferating IL-23R+ γδ T cells. See also Figure S1.
The frequency of Th17 cells in the CD44+ compartment of activated or memory T cells is diminished in Il6−/− mice because IL-6 together with transforming growth factor-β (TGF-β) is essential in generating Th17 cell precursors. TGF-β and IL-6 are also required to induce retinoic acid-related orphan receptor-γt (ROR-γt) and IL-23R in αβ T cells (Zhou et al., 2007). Thus, we wondered how IL-6 deficiency affected the frequency of γδ T cells in secondary lymphoid tissues. While the overall fraction of γδ T cells in Il6−/− mice was not diminished and was even slightly higher in spleen (Figure S1), CCR6+ γδ T cells were significantly decreased and NK1.1+ γδ T cells were increased in IL-6 deficient mice as compared with wild type animals. The decreased fraction of CCR6+ γδ T cells in Il6−/− mice correlated with a lower percentage of IL-23R+ γδ T cells and IL-17 producers both in lymph node and splenic Il6−/− γδ T cells (Figure S1). Together these data suggest that the development and homeostasis of IL-23R+ γδ T cells may be modulated by IL-6.
IL-23R+ γδ T cells expand upon immunization with MOG35–55 plus CFA and accumulate in the central nervous system (CNS)
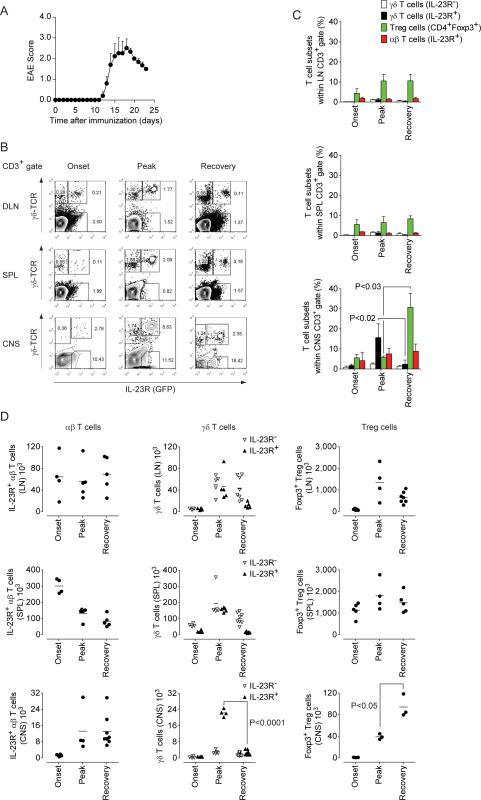
Next, we wanted to investigate how IL-23R+ γδ T cells responded during an autoimmune reaction in vivo (Figure 2A). Although γδ T cells constitute the minority of T cells in secondary lymphoid tissues, 7 percent of γδ T cells as compared to only 0.3 percent of αβ T cells in lymph nodes of naïve mice were positive for IL-23R (Figure S2). Upon sensitization with MOG35–55 plus CFA, a new population of IL-23RlowCD44+CD25low cells emerged within the αβ T cells compartment likely representing Th17 cells that were primed in an antigen specific manner (Figure S2). Notably, the fraction of γδ T cells in draining lymph nodes also expanded and now, one fifth or more of these γδ T cells expressed IL-23R. As a result, IL-23R+ γδ T cells (which were also CCR6+TCR-γ4+) were significantly enriched in the draining lymph nodes of immunized animals (Figure S2).
Figure 2. IL-23R+ γδ T cells accumulate in the CNS at the peak of EAE and rapidly contract during recovery.
Il23r+/− and Foxp3gfp.KI mice were immunized with MOG35–55 plus CFA. Both strains behave like wild type C57BL/6 mice and a representative disease course is shown (Mean clinical score + SEM, n=5) (A). Draining lymph node (LN) cells, splenocytes, and mononuclear cells from the CNS were prepared at different stages of EAE. (B) IL-23R (GFP) expression in γδ T cells (γδ-TCR+) and αβ T cells (CD3+γδ-TCR−) was analyzed by flow cytometry at the onset and peak of disease and during recovery. (C) Percentages of IL-23R− γδ T cells, IL-23R+ γδ T cells, Treg cells (CD4+Foxp3+), and IL-23R+ αβ T cells within the CD3+ T cell gate in various lymphoid compartments and the CNS. Mean percentages ± SD. (D) Absolute numbers of IL-23R+ αβ T cells, IL-23R− and IL-23R+ γδ T cells as well as CD4+Foxp3+ Treg cells were calculated in individual mice based on percentages obtained in the flow cytometric analysis and assessment of total live mononuclear cells in a trypan blue exclusion analysis. Note that IL-23R+ αβ T cells in the CNS correspond to Th17 cells. See also Figure S2.
In order to evaluate the relative contribution of IL-23R− and IL-23R+ γδ T cells to disease development, we followed their dynamics in various lymphoid compartments and in the CNS and compared them with the population dynamics of Th17 cells and Foxp3+ Treg cells. Upon immunization with MOG35–55 plus CFA, Th17 cells (IL-23R+ αβ T cells) expanded in the draining lymph nodes and accumulated in the spleen prior to clinical signs of disease (Figures 2B–D). When the animals became sick, Th17 cells appeared in the CNS. The inverse population dynamics of Th17 cells in spleen vs CNS suggested that the splenic pool of Th17 cells drained into the target tissue. IL-23R+ γδ T cells but also IL-23R− γδ T cells in the spleen of immunized mice increased until the peak of disease and declined during recovery from EAE. However, IL-23R+ γδ T cells never outnumbered IL-23R− γδ T cells in the peripheral immune compartment (Figures 2B–D). In contrast in the CNS, IL-23R+ γδ T cells accumulated in an almost exclusive manner. Here, the fraction of IL-23R+ γδ T cells within the CD3+ T cell infiltrate increased about 8-fold (Figure 2C) and the absolute number of IL-23R+ γδ T cells expanded 20-fold during development of clinical signs of disease (Figure 2D). Consistent with the preponderance of IL-23R+ over IL-23R− γδ T cells, γδ T cells contributed substantially to the IL-17-production, but not the IFN-γ-production in the CNS (Figure S2). During recovery, the IL-23R+ γδ T cells population in the CNS contracted to numbers observed in naïve mice. Thus, changes of the IL-23R+ γδ T cells population in the CNS reflected closely the clinical course of EAE and the state of inflammation in the target tissue. Interestingly, Foxp3+ Treg cells were present in the CNS at the onset of clinical signs of disease, but expanded to high numbers only after the attrition of IL-23R+ γδ T cells (Figures 2C, D). Moreover, Foxp3+ Treg cells appeared to control effector functions of αβ T cells only after the disappearance of γδ T cells. Thus, these data suggested a functional relationship between the dynamics of γδ T cells and Foxp3+ Treg cells in inflamed tissue.
γδ T cells prevent the induction of Foxp3 in CD4+ T cells in an IL-23 dependent manner
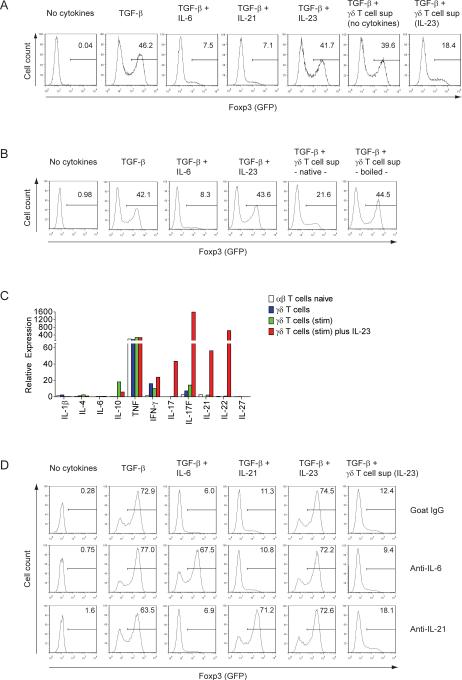
The massive accumulation of IL-23R+ γδ T cells in the CNS at the peak of inflammation suggested that IL-23 was a dominant factor in the local milieu. We wondered whether IL-23 had any additional effect on local γδ T cells in addition to the induction of IL-17, because in this disease phase, large amounts of IL-17 are already produced by Th17. Furthermore, we noticed an accelerated increase in the Foxp3+ Treg cell population in the CNS only after the disappearance of IL-23R+ γδ T cells. Thus, in an initial in vitro screening approach, we activated γδ T cells with IL-23 and tested the effect of their cell culture supernatant on the conversion of conventional CD4+ T cells into Foxp3+ Treg cells. Naïve Foxp3− T cells (CD4+CD44−) were purified from Foxp3gfp.KI mice and polyclonally stimulated in the presence of TGF-β. Similarly to recombinant IL-6 (Bettelli et al., 2006) and IL-21 (Korn et al., 2007a) native but not boiled supernatant from IL-23-activated γδ T cells inhibited the TGF-β-driven conversion of naïve Foxp3− αβ T cells into Foxp3 expressing T cells (Figures 3A, B). The most efficient (heat-sensitive) factors that suppress TGF-β-driven induction of Foxp3 in naïve T cells are IL-4, IL-6, IL-21, and IL-27 (Awasthi et al., 2007; Bettelli et al., 2006; Dardalhon et al., 2008; Korn et al., 2007a; Stumhofer et al., 2007). However, IL-4, IL-6, and IL-27 were not produced by IL-23-activated γδ T cells. In contrast, IL-21 was produced by IL-23-activated γδ T cells (Figure 3C). Yet, neutralizing IL-21 or IL-6 with blocking antibodies did not diminish the capacity of supernatant from IL-23-activated γδ T cells to suppress TGF-β-driven expression of Foxp3 (Figure 3D). In addition, IL-6 or IL-21 together with TGF-β are strong inducers of IL-17 in naïve αβ T cells (Korn et al., 2007a) whereas IL-23-activated γδ T cell supernatant together with TGF-β failed to induce IL-17 in naïve responder T cells (Figure S3). The gene expression program that was induced in αβ T cells in the presence of TGF-β plus IL-23-activated γδ T cell supernatant included T-bet and IL-12Rβ2, as well as IL-22 (Figure S3). Thus, we conclude that exposure of γδ T cells to IL-23 creates a cytokine milieu that directly acts on the intrinsic development of αβ T cells by inhibiting their conversion into Foxp3+ Treg cells.
Figure 3. Cell free supernatant from IL-23-activated γδ T cells abrogates the TGF-β driven induction of Foxp3 in conventional T cells independently of IL-6 and IL-21.
Flow cytometrically purified naïve conventional T cells (CD4+CD44−Foxp3−) from Foxp3gfp.KI mice were activated with antibodies to CD3 and CD28 in the presence of different cytokines or γδ T cell culture supernatants as indicated. After 3 days, Foxp3 (GFP) expression was determined by flow cytometry (histograms in A and B; numbers indicate percentages). (C) Cytokine profile of γδ T cells. γδ T cells were purified by magnetic bead sorting and directly used for preparation of RNA or further stimulated with anti-CD3 plus anti-CD28 coated beads for 4 days without or in the presence of IL-23 followed by isolation of RNA. Expression of cytokines was determined by quantitative PCR as described in Supplemental Experimental Procedures. Flow cytometrically sorted naïve conventional T cells (CD4+CD44−Foxp3−) were analyzed for comparison. (D) Neutralization of IL-6 or IL-21: Naïve conventional T cells were stimulated with anti-CD3 plus anti-CD28 and the indicated cytokines or supernatants from γδ T cells in the presence of goat IgG or blocking antibodies to IL-6 or IL-21. After 3 days, induction of Foxp3 (GFP) was determined by flow cytometry (histograms in D; numbers indicate percentages). See also Figure S3.
γδ T cells inhibit Treg cell responses in vivo and promote inflammation
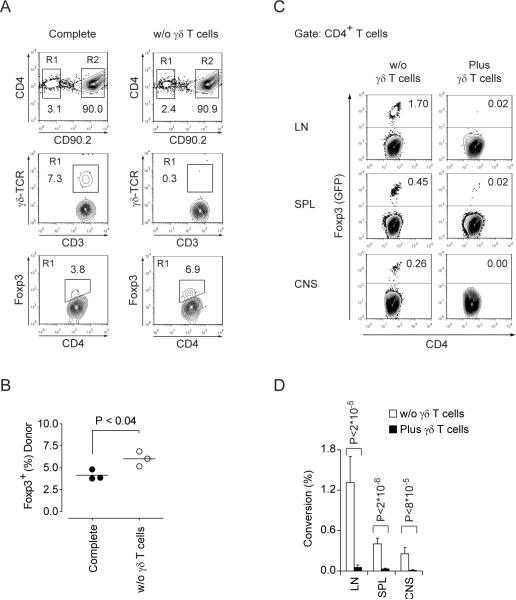
To evaluate the impact of IL-23-driven functions of γδ T cells in vivo, we adoptively transferred CD3+ T cells derived from wild type mice (CD90.1) that were left untreated or depleted of γδ T cells into Il23r−/− hosts. The recipient mice were then immunized with MOG35–55 plus CFA and the activation status of the donor T cells was investigated after two weeks. In this scenario, sensitization of donor CD4+ T cells must have occurred either in the absence or in the presence of (donor derived) IL-23 responsive γδ T cells in vivo. When priming of T cells occurred in the presence of IL-23R+ γδ T cells, the fraction of Foxp3+ T cells in the donor T cell population was always reduced as compared to priming without IL-23 responsive γδ T cells (Figure 4A, B). Thus, the presence of IL-23 responsive γδ T cells appeared to restrain Treg cell expansion. In order to test whether γδ T cells also inhibited the conversion of conventional T cells into Foxp3+ Treg cells in vivo (as we found in vitro), we injected Foxp3− MOG35–55 specific TCR transgenic (2D2) indicator T cells purified from 2D2 × Foxp3gfp.KI mice into Rag1−/− host mice in the absence of or with co-transfer of wild type γδ T cells and immunized the recipient mice with MOG35–55 plus CFA. Since CFA induces high amounts of IL-6 which would disguise any γδ T cell-mediated effects on inhibiting the conversion of conventional T cells into Foxp3+ Treg cells, we administered a monoclonal antibody to IL-6R. Under these conditions, we observed a measurable MOG35–55-induced conversion of conventional 2D2 cells into Foxp3+ 2D2 T cells (Figure 4C). However, this conversion was abrogated by γδ T cells (Figure 4C, D) confirming our in vitro-finding that activated γδ T cells are efficient in suppressing the conversion of conventional T cells into Foxp3+ Treg cells independently of IL-6. Taken together, these data suggest that γδ T cells can modulate the commitment of αβ T cells to effector vs regulatory T cell lineages in the peripheral immune compartment.
Figure 4. γδ T cells inhibit Treg cell responses in vivo.
(A, B) CD3+ T cells derived from congenic wild type mice (CD90.1) were left untreated or depleted of γδ T cells followed by intravenous transfer (5×106) into Il23r−/− hosts and immunization with MOG35–55 plus CFA one day later. (A) After in vivo sensitization, donor cells were re-isolated from draining lymph nodes and analyzed for expression of γδ-TCR and Foxp3 by flow cytometry. (B) Fraction of Foxp3+ Treg cells within the CD4+ T cell compartment of substituted cells (CD90.1). (C, D) γδ T cells inhibit the conversion of conventional T cells into Foxp3+ Treg cells in vivo: Foxp3− 2D2 responder T cells were sorted by flow cytometry from 2D2 × Foxp3gfp.KI mice and transferred iv into Rag1−/− recipients without or with co-transfer of γδ T cells from wild type donors. Host mice were immunized with MOG35–55 plus CFA and treated with a monoclonal antibody to IL-6R ip to prevent CFA-induced IL-6 from overriding γδ T cell effects on the conversion of conventional T cells into Foxp3+ Treg cells. On day 15 after immunization, 2D2 cells were re-isolated from lymph nodes, spleen, and CNS, and the frequency of MOG35–55 specific Foxp3 (GFP)+ Treg cells was measured by flow cytometry (C). The rate of 2D2 T cells converted into antigen specific Foxp3+ Treg cells is depicted for the various lymphoid compartments and the CNS. Mean + SD, n=3 to 4 (D).
γδ T cells contribute to the development of EAE by restraining Treg cell responses
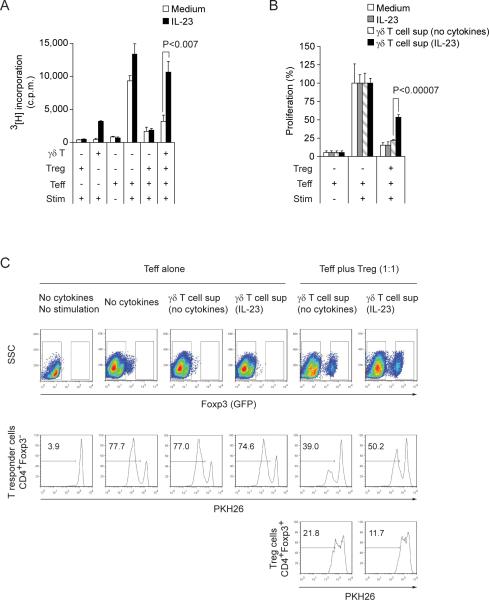
Treg cells do not express IL-23R (Figure S4) and IL-23 alone had no impact on the efficacy of naturally occurring Foxp3+ Treg cells to suppress polyclonal αβ T cell responses in vitro (Figure 5A). Only in the presence of γδ T cells was IL-23 able to reverse the suppression of effector T cells by Treg cells (Figure 5A). Notably, cell free supernatant of IL-23-activated γδ T cells was equally efficient in abrogating Treg cell-mediated suppression of polyclonal αβ T cell responses (Figure 5B). Since in an antigen presenting cell (APC) free system, γδ T cell-derived supernatant abrogated the proliferative arrest of effector T cells, but did not enhance proliferation of Foxp3+ Treg cells (Figure 5C), we hypothesize that the biological activity of γδ T cell supernatant might render responder T cells resistant to Treg cell-mediated suppression, reminiscent of the effect of IL-6 (Pasare and Medzhitov, 2003).
Figure 5. IL-23 reverses Treg cell-mediated suppression of αβ T cells only in the presence of γδ T cells.
(A) CD4+Foxp3+ Treg cells isolated by flow cytometry sorting from the spleens and lymph nodes of naïve Foxp3gfp.KI mice were tested for their suppressive capacity in the presence of γδ T cells in vitro. A fixed number of naïve responder T cells (CD4+Foxp3−) was cultured 1:1 with Treg cells, γδ T cells and a monoclonal antibody to CD3 in the presence of syngeneic irradiated and γδ T cell-depleted splenocytes as APCs. IL-23 was added at a concentration of 25 ng/ml as indicated. (B) In an analogous assay, Treg cell-mediated suppression of responder T cells was tested in the presence of IL-23 or cell culture supernatants of γδ T cells that had been stimulated for 4 days with anti-CD3/anti-CD28 coated beads without or in the presence of IL-23. Proliferation was determined by 3[H]thymidine incorporation (Mean + SD of triplicate cultures). In B, the proliferation was normalized to the unsuppressed condition (absence of Treg cells) within each condition. The unsuppressed proliferation of responder T cells was 10,600±1,410 cpm and 20,300±1,330 cpm in the presence of regular and IL-23-activated γδ T cell supernatant, respectively. (C) Dye dilution assay for differential assessment of responder cell and Treg cell proliferation. CD4+CD44− Foxp3-responder T cells and CD4+Foxp3+ Treg cells were FACS-sorted from Foxp3gfp.KI mice, loaded with the red dye PKH26, and cultured in the presence of anti-CD3 plus anti-CD28-coated beads and γδ T cell-derived supernatants as indicated. After 3 days, the percentages of PKH26dim cells were determined separately within the Foxp3− and Foxp3 (GFP)+ T cell gates as a measure of the proliferative response. Representative histograms out of 3 independent experiments. See also Figure S4.
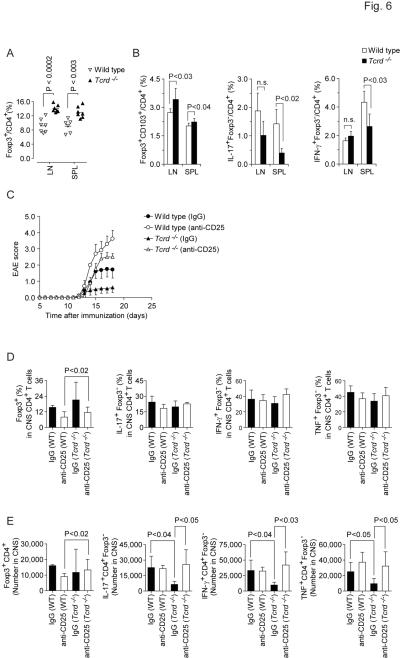
To determine the functional relevance of γδ T cell mediated inhibition of both Treg cell conversion and suppression of αβ T cell responses by naturally occurring Treg cells in a disease model, we revisited the Treg cell compartment in Tcrd−/− mice. Unmanipulated Tcrd−/− mice harbored higher fractions of Foxp3+ Treg cells in secondary lymphoid organs as compared to age-matched controls housed in the same facility (Figure 6A). It has previously been shown that CD103+ Treg cells accumulate in inflamed tissues (Huehn et al., 2004) and in the CNS of EAE mice, the vast majority of MOG35–55-IAb tetramer+ Foxp3+ Treg cells are CD103+ (Korn et al., 2007b). Upon immunization with MOG35–55 plus CFA, the percentage of CD103+ Treg cells in draining lymph nodes and spleen was increased in Tcrd−/− mice as compared with wild type controls (Figure 6B). In contrast, the CD4+ T cell compartment - in particular in the spleen - contained lower fractions of IL-17 and IFN-γ producers in MOG35–55-sensitized Tcrd−/− mice than in wild type controls (Figure 6B). Together, these data suggested that antigen driven Treg cell responses were enhanced in Tcrd−/− mice. The development of EAE in Tcrd−/− mice is attenuated (Figure 6C) and we assumed that the relative protection from disease in Tcrd−/− mice is due to the very strong Treg cell response in these animals. In order to test this hypothesis, we depleted Treg cells in Tcrd−/− mice with a monoclonal antibody to CD25 and studied whether this restored full susceptibility to EAE. In contrast to control treated Tcrd−/− animals, Treg cell-depleted Tcrd−/− mice were no longer protected from EAE and developed severe EAE identical to wild type mice (Figure 6C and Table 1). At the peak of disease, Tcrd−/− mice tended to have higher fractions of Treg cells in the inflamed CNS than wild type mice (Figure 6D). While the percentages of IL-17-producers, IFN-γ-producers, and TNF-producers among CD4+Foxp3− T cells that were recovered from the CNS parenchyma were not different between wild type and Tcrd−/− mice, the latter had significantly decreased absolute numbers of cytokine producing CD4+Foxp3− effector T cells in the CNS (Figure 6E). Treg cell depletion restored the absolute amount of cytokine producing effector T cells in the CNS of Tcrd−/− mice to wild type numbers (Figure 6E). Accordingly, the disease phenotype in Treg cell-depleted Tcrd−/− was similar to wild type animals. However, Foxp3+ Treg cells recovered faster in Tregcell-depleted Tcrd−/− mice than in Treg cell-depleted wild type animals which developed a fulminant disease with marked mortality (Figure 6E and Table 1). Together, these data confirm that the priming of autoantigen specific αβ T cells is not impaired in a cell intrinsic manner in Tcrd−/− mice. Rather, the relative protection from EAE in these animals is mediated by an enhanced Treg cell response. Thus, reminiscent of IL-6 deficient mice, the absence of γδ T cells appears to favor the generation and expansion of Treg cells which then successfully control antigen specific activation of αβ T cells.
Figure 6. γδ T cells promote EAE by antagonizing Treg cell function.
(A) Tcrd−/− mice have increased frequencies of Foxp3+ Treg cells. Lymph node cells and splenocytes were isolated from naïve wild type mice and age-matched Tcrd−/− animals bred in the same facility. The percentage of Foxp3+ Treg cells in the compartment of CD4+ T cells was determined by FACS staining. (B) On day 6 after sensitization with MOG35–55 plus CFA, draining lymph node cells and splenocytes were isolated and stained for Foxp3 or intracellular cytokines. The percentages of Foxp3+CD103+ Treg cells, IL-17+Foxp3−, and IFN-γ+Foxp3− effector T cells within the compartment of conventional CD4+ T cells are depicted (Mean + SD, n=4). (C–E) Treg cell depletion in Tcrd−/− mice restores full susceptibility to EAE. Wild type or Tcrd−/− mice were treated with control IgG1 or with a monoclonal antibody to CD25 (PC61) to deplete Treg cells followed by immunization with MOG35–55 plus CFA. Means of clinical scores (+SEM, n=5) are presented (C). Pooled EAE scores from two independent experiments. (D) At the peak of disease (d18), mononuclear cells from the CNS were recovered and tested for expression of Foxp3 and intracellular cytokines within the population of CD4+ αβ T cells. Fractions of Foxp3+ cells and cytokine+ cells within the CNS CD4+ T cell population (D, Mean + SD, n ≥ 4) and absolute numbers (E, Mean + SD, n ≥ 4) of Foxp3+ cells or IL-17+Foxp3−, IFN-γ+Foxp3−, and TNF+Foxp3− CD4+ effector T cells are depicted.
Table 1.
Summary of EAE clinical parameters†
| Wild type (IgG) | Wild type (anti-CD25) | Tcrd−/− (IgG) | Tcrd−/− (anti-CD25) | |
|---|---|---|---|---|
| Incidence | 7/9 = 78% | 5/5 = 100% | 3/9 = 33% | 11/11 = 100% |
| Mortality | 0/9 = 0% | 1/5 = 20% | 0/9 = 0% | 0/11 = 0% |
| Day of onset | 14.3 ± 0.70 | 13.8 ± 0.20 | 14.0 ± 1.0 | 14.9 ± 0.4 |
| Maximum EAE score | 2.2 ± 0.46 | 3.6 ± 0.40 | 0.7 ± 0.34a) | 2.7 ± 0.22 b) |
Cumulative results from 2 independent experiments
Compared to Wild type (IgG): P < 0.03; calculated by Mann-Whitney U rank sum test.
Compared to Tcrd−/− (IgG): P < 0.0009; calculated by Mann-Whitney U rank sum test.
Discussion
In this study, we have identified a mechanism by which γδ T cells enable the initiation of adaptive immune responses: γδ T cells suppressed early Treg cell responses in an IL-23 dependent manner. γδ T cells expressed high amounts of IL-23R and responded to IL-23 by secretion of IL-17, IL-21, and IL-22. However, IL-23-activated γδ T cells also exerted a marked inhibitory effect both on Treg cell-mediated suppression of effector T cell responses and on conversion of conventional T cells into Foxp3 expressing Treg cells. While IL-23 had no direct impact on the generation and function of antigen specific Treg cells, products of IL-23-activated γδ T cells restrained the generation and function of Treg cells independently of Th17 cell-associated cytokines. By this mechanism, IL-23-activated γδ T cells shifted the balance between Treg cells and conventional αβ T cells in favor of effector T helper cells. These results may explain in part why IL-23 holds a key position in initiating autoreactive αβ T cell responses in vivo.
Hypotheses on the pathogenic mechanism of IL-23 include expansion of Th17 memory cells (Aggarwal et al., 2003; McGeachy et al., 2009) and maturation of Th17 cells by increasing their capacity to secrete IL-17 (Veldhoen et al., 2006) and by shutting down other cytokine genes such as Il10 (McGeachy et al., 2007). IL-23 might endow αβ T cells with encephalotropism although it remains to be determined whether this includes the induction of molecular addressins (Gyulveszi et al., 2009). Finally, IL-23 was proposed to exert functions that are not targeted to T cells but are independent of conventional T cells (Buonocore et al., 2010; Uhlig et al., 2006). Blockade of IL-23 is effective in early phases of autoimmune reactions. However, IL-23 has also been suggested to play a role in the late plasticity of committed Th17 cells in that it resurrects the transcription of IFN-γ in the absence of TGF-β (Lee et al., 2009). Moreover, EAE is transient and self-limiting when the availability of IL-23 is not sustained (Veldhoen et al., 2006). Together, these observations are consistent with the idea that IL-23 acts as a major pathogenic factor in autoimmunity. Yet, it is not just a theoretical concern that every single step in the pathogenic cascade of αβ T cell-driven autoimmunity is feasible in the absence of IL-23 (Jager et al., 2009), but still Il23p19−/− mice and Il23r−/− mice are completely resistant to actively induced EAE (Awasthi et al., 2009; Cua et al., 2003).
Upon exposure to IL-23, γδ T cells produce IL-17 irrespective of further γδ-TCR stimuli. IL-17 production by γδ T cells is dependent on expression of IL-23R. In contrast to naïve αβ T cells where TGF-β plus IL-6 is required to induce IL-17 production, γδ T cells respond directly to IL-23. Consistent with previous results, IL-6 is no longer necessary to induce the production of IL-17 in those γδ T cells that already express ROR-γt (Lochner et al., 2008). However, precursor frequencies of CCR6+IL-23R+ γδ T cells are reduced in lymph nodes and spleen of Il6−/− mice suggesting that IL-6 is still required for the commitment of γδ T cells to express IL-23R. This concept is not mutually exclusive with the finding that the ability of IL-17-production by γδ T cells may already be acquired in the thymus triggered by either lack of TCR ligand engagement (Jensen et al., 2008) or failure to upregulate CD27 (Ribot et al., 2009).
The property of swift production of IL-17 and IL-22 by IL-23R+ γδ T cells fits in the concept that these effector cytokines are associated with immunity at epithelial surfaces where γδ T cells are abundant and where IL-23 may be playing an important part in guiding immune responses (Aujla et al., 2008; Becher et al., 2003; Ouyang et al., 2008; Ye et al., 2001). However, the importance of IL-17 and IL-22 has been questioned for autoimmune neuroinflammation since Il17a−/−, Il21−/−, and Il22−/− mice are not protected from EAE (Haak et al., 2009; Kreymborg et al., 2007; Sonderegger et al., 2008). Thus, individual effector cytokines might not play a non-redundant role in the pathogenic potential of Th17 cells.
Our data suggest that IL-23R+ γδ T cells provide a more general means to enhance immune responses in that they suppress Treg cell responses. It has recently been shown that a high frequency of γδ T cells precedes the generation of MOG35–55 specific αβ T cells in the draining lymph nodes until day 4 after immunization with MOG35–55 plus CFA (Wohler et al., 2009). Indeed, we here demonstrate that IL-23R+ γδ T cells also accumulate in the CNS simultaneously to the full display of αβ T cell-driven immunopathology. More importantly, the IL-23R+ γδ T cells population in the CNS rapidly contracts prior to clinical recovery. Only then the Treg cell response is able to control inflammation in the CNS. We propose that γδ T cells, when activated by IL-23, create a cytokine milieu in the vicinity of αβ T cells and Treg cells that initiates inflammation by restraining the generation and function of Treg cells. It remains to be determined whether a putative soluble factor produced by activated γδ T cells directly inhibits Treg cells or strengthens the resistance of αβ T effector cells to Treg cell-mediated suppression (e. g. by promoting the production of IL-2). Paracrine factor(s) from IL-23-activated γδ T cells offer an explanation why Treg cells – even though present in abundance – fail to curtail tissue damage at early stages of an immune response. It has always been a major question why Treg cells at various sites of inflammation (CNS, synovial tissue, and intestines) fail to be effective in the local milieu at the onset of inflammation (Korn et al., 2007b). IL-6 inhibits Treg cell-mediated suppression (Pasare and Medzhitov, 2003) and the de novo induction of Foxp3 in conventional T cells while – together with TGF-β – IL-6 promotes the proliferation of Th17 cells. However, IL-23-activated γδ T cells did not produce IL-6. Cell-free supernatant from IL-23-activated γδ T cells inhibited both Treg cell-mediated suppression of conventional αβ T cells and TGF-β-driven induction of Foxp3 in naïve CD4+ T cells in APC-free systems. A number of cytokines including IL-4, IL-21, and IL-27 (but not IL-17 and IL-22) have been identified to inhibit TGF-β-driven conversion of conventional T cells into Foxp3+ Treg cells (Awasthi et al., 2007; Dardalhon et al., 2008; Korn et al., 2007a). Unlike IL-4 and IL-27, which are not produced by IL-23-stimulated γδ T cells, IL-21 is strongly induced in γδ T cells upon exposure with IL-23. Yet, neutralization of IL-21 did not reverse the suppression of Foxp3-induction by γδ T cell supernatants. We conclude that presumably by inducing a heat-sensitive mediator or a panel of humoral factors with paracrine activity, IL-23 prompts γδ T cells to block TGF-β-driven Foxp3 induction in αβ T cells and to restrain Treg cell function in vitro and in vivo.
γδ T cells responses can be essential and non-redundant in host defense at epithelial surfaces. This has been shown for infection with Nocardia and Listeria whose early control most likely involves direct effector functions of γδ T cells (King et al., 1999; Riol-Blanco et al., 2010; Skeen and Ziegler, 1993). γδ T cells have also been implicated in the pathogenesis of autoimmune tissue inflammation (Odyniec et al., 2004; Rajan et al., 1998). For example, γδ T cells were detected in multiple sclerosis (MS) lesions and appeared clonally expanded based on restricted usage of TCR variable chain segment (V) γ9 (Wucherpfennig et al., 1992). Furthermore, the fraction of γδ T cells in circulating blood, most of which are Vγ9 positive (Poggi et al., 2007), is a predictor of disease activity in MS patients as measured by magnetic resonance imaging (Rinaldi et al., 2006). Thus, it is likely that non-canonical γδ T cells from secondary lymphoid organs are involved in promoting tissue inflammation in human autoimmunity. Various effector functions of γδ T cells have been proposed. For example, γδ T cells interact directly with B cells and promote Ig class switching (Wen et al., 1996). γδ T cells also interact with αβ T cells, to which they can present antigen but which can also be a target of direct killing by γδ T cells (Brandes et al., 2005; Vincent et al., 1996). The role of IL-23 for γδ T cell-mediated effects in vivo has only been discussed in terms of inducing Th17-associated cytokines in γδ T cells, which might license APCs to activate αβ T cells (Sutton et al., 2009). However, we did not observe a priming defect of encephalitogenic αβ T cells in Tcrd−/− mice per se while we clearly identified enhanced Treg cell responses in the absence of γδ T cells.
Thus, we propose an unexpected mechanism to explain how non-canonical Vγ4+ γδ T cells that express IL-23R might enhance autoimmune tissue inflammation: IL-23 that is exclusively expressed under inflammatory conditions, can reverse Treg cell-mediated suppression by activating γδ T cells. IL-23 arms γδ T cells to restrain Treg cell responses in a locally constrained manner, releasing nascent adaptive αβ T cell responses from inhibition. As a result, those body compartments that contain high frequencies of γδ T cells or are easily accessible to γδ T cells with non-canonical γδ-TCR receptors, like neuroectodermal tissue, might be particularly susceptible to IL-23-driven inflammation.
Experimental procedures
Animals and induction of EAE
Foxp3gfp.KI-mice and IL-23Rgfp.KI mice have been described previously (Awasthi et al., 2009; Bettelli et al., 2006; Korn et al., 2007b). CD90.1 congenic C57BL/6 mice, Tcrd−/− mice, and IL-6 deficient mice were obtained from Jackson Laboratories. EAE was induced by subcutaneous immunization of mice with 100 μ1 of an emulsion containing 100 μg of MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) and 250 μg of M. tuberculosis H37Ra (Difco, Detroit, MI) in Freund's adjuvant oil (CFA) plus intraperitoneal (ip) injection of 200 ng pertussis toxin (Fluka) on day 0 and day 2. For Treg cell depletion, animals were injected ip with 500 μg of monoclonal antibody to CD25 (PC61) on day -5 and day -3 prior to immunization. For adoptive transfer experiments, 5×106 purified CD3+ T cells or γδ T cell-depleted CD3+ T cells from congenic mice (CD90.1) were transferred intravenously (iv) into Il23r−/− recipients 1 day prior to immunization. In some experiments, we injected 3.5×106 naïve (CD4+CD44−Foxp3−) T cells purified from 2D2 × Foxp3gfp.KI mice into Rag1−/− host recipients in the absence of or with co-transfer of 0.5×106 wild type γδ T cells 2 days prior to immunization with MOG35−55 plus CFA. Recipient mice received ip injections of a monoclonal antibody to IL-6R (15A7, 0.5 mg) on days 1, 3, 5, and 9 after cell transfer. Clinical signs of EAE were assessed as reported (Korn et al., 2007b). Animals were kept in a specific pathogen-free facility at the Technical University Munich, and all experiments were carried out in accordance with the guidelines prescribed by the Bavarian state authorities (Az 55.2-1-54-2531-88-08).
T cell differentiation
Cells were cultured in Dulbecco's Modified Eagle medium (DMEM) with 10% fetal calf serum (FCS) supplemented with 5×10−5 M β-mercaptoethanol, 1 mM sodium pyruvate, non-essential amino acids, L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. For in vitro-T cell differentiation, CD4+ T cells were purified from naive splenocytes and lymph node cells with anti-CD4+ magnetic beads (Miltenyi), and γδ T cells were purified from CD4− flow through by using the γδ T cell two-step magnetic bead-purification kit from Miltenyi. To obtain naïve T cells, CD4+ T cells were sorted into CD4+CD44−Foxp3− T cells by fluorescence activated cell sorting. For some experiments γδ T cells or CD4+CD44+ activated or memory T cells were purified by fluorescence activated cell sorting. T cells were stimulated for 3 days with plate-bound antibody to CD3 (145-2C11, 0.5 μg/ml) and antibody to CD28 (PV-1, 0.25 μg/ml) or by anti-CD3 plus anti-CD28 coated beads (Miltenyi). Recombinant cytokines were added to the differentiation cultures as indicated: human TGF-β1 (3 ng/ml), mouse IL-6 (30 ng/ml), mouse IL-21 (100 ng/ml), mouse IL-22 (200 ng/ml), mouse IL-27 (50 ng/ml), mouse IL-12 (25 ng/ml), or mouse IL-23 (25 ng/ml, all R&D systems).
Treg cell suppression and conversion assays
25.000 CD4+Foxp3− T cells were cocultured with 25.000 Treg cells and 200.000 irradiated (3.000 rad) syngeneic splenic γδ T cell-depleted APCs per well in the presence of an antibody to mouse CD3 (1 μg/ml) for 72hr. During the last 16hr, cells were pulsed with 1 μCi of 3[H]thymidine (PerkinElmer) followed by harvesting on glass fiber filters and analysis of incorporated 3[H]thymidine in a β-counter (1450 Microbeta, Trilux, PerkinElmer). Where indicated, 25.000 γδ T cells were co-cultured in addition to the responder plus Treg cell mixture. Where indicated, IL-23 (R&D systems) was added to the cultures at a concentration of 25 ng/ml. In some experiments, supernatants from γδ T cells were used. Supernatants were collected from γδ T cells that were stimulated for 3 days with anti-CD3 plus anti-CD28 coated beads in the absence or presence of IL-23 (25 ng/ml). Besides measuring proliferation by 3[H]thymidine incorporation, a dye dilution approach was used. Here, responder T cells and Treg cells were labeled with PKH26 (Sigma-Aldrich) according to the manufacturer's instructions.
In order to determine conversion of conventional T cells into Foxp3+ Treg cells, naïve T cells from Foxp3gfp.KI mice were purified by flow cytometric sorting (CD4+CD44−Foxp3−). Naïve T cells were stimulated with plate-bound monoclonal antibodies to mouse CD3 (145-2C11, 2 μg/ml) and CD28 (PV-1, 4 μg/ml) for 3 days in the presence of the indicated cytokines: human TGF-β1 (5 ng/ml), mouse IL-6 (37,5 ng/ml), mouse IL-21 (100 ng/ml), mouse IL-23 (25 ng/ml, all R&D systems), or γδ T cell-derived supernatants as described. Where indicated, control goat IgG or blocking antibodies to IL-6 or IL-21 (R&D systems) were added at a concentration of 12.5 μg/ml. Foxp3 (GFP) induction and - after additional stimulation with PMA plus ionomycin in the presence of monensin - intracellular cytokines were assessed by flow cytometry.
Statistical analysis
Statistical evaluations of cell frequency measurements and proliferation data were performed using the unpaired Student's t-test. For comparison of clinical EAE scores, the Mann-Whitney U rank sum test was used. Two-tailed P values <0.05 were considered significant.
Highlights.
-
-
IL-23R is constitutively expressed in a subset of γδ T cells.
-
-
IL-23R+ γδ T cells accumulate in the CNS during EAE.
-
-
IL-23-activated γδ T cells suppress conversion of naive T cells into Treg cells.
-
-
IL-23-activated γδ T cells inhibit Treg cell responses in vivo.
Supplementary Material
Acknowledgments
We would like to thank S. Woeste for skillful technical assistance. VR receives funding from the Gemeinnützige Hertie-Stiftung. MCC is funded by intramural grants from the Technical University Munich (KKF). TK is recipient of a Heisenberg grant and other grants from the Deutsche Forschungsgemeinschaft (KO 2964/3-1, KO 2964/4-1, and KO 2964/5-1). This work was also supported by grants from the National Institutes of Health (R01NS 30843 to VKK and RO1AI 073542-03 to MO).
References
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J. Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Durell BG, Noelle RJ. IL-23 produced by CNS-resident cells controls T cell encephalitogenicity during the effector phase of experimental autoimmune encephalomyelitis. J. Clin. Invest. 2003;112:1186–1191. doi: 10.1172/JCI19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bigby M, Markowitz JS, Bleicher PA, Grusby MJ, Simha S, Siebrecht M, Wagner M, Nagler-Anderson C, Glimcher LH. Most gamma delta T cells develop normally in the absence of MHC class II molecules. J. Immunol. 1993;151:4465–4475. [PubMed] [Google Scholar]
- Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa I, Bix M, Liao NS, Zijlstra M, Jaenisch R, Raulet D. Most gamma delta T cells develop normally in beta 2-microglobulin-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1992;89:653–657. doi: 10.1073/pnas.89.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhindan N, Farley AJ, Humphreys S, Parker C, Rossiter B, Brooks CG. Patterns of lymphokine secretion amongst mouse gamma delta T cell clones. Eur. J. Immunol. 1997;27:1704–1712. doi: 10.1002/eji.1830270717. [DOI] [PubMed] [Google Scholar]
- Gyulveszi G, Haak S, Becher B. IL-23-driven encephalo-tropism and Th17 polarization during CNS-inflammation in vivo. Eur. J. Immunol. 2009;39:1864–1869. doi: 10.1002/eji.200939305. [DOI] [PubMed] [Google Scholar]
- Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JD, Gonzalez FH, Schmitz S, Chennupati V, Fohse L, Kremmer E, Forster R, Prinz I. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur. J. Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- Hayday AC, Pennington DJ. Key factors in the organized chaos of early T cell development. Nat. Immunol. 2007;8:137–144. doi: 10.1038/ni1436. [DOI] [PubMed] [Google Scholar]
- Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Hyde DM, Jackson KA, Novosad DM, Ellis TN, Putney L, Stovall MY, Van Winkle LS, Beaman BL, Ferrick DA. Cutting edge: protective response to pulmonary injury requires gamma delta T lymphocytes. J. Immunol. 1999;162:5033–5036. [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007a;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007b;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC, Becher B. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JM, Girardi M, Roberts SJ, Barbee SD, Hayday AC, Tigelaar RE. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat. Immunol. 2006;7:843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J. Exp. Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odyniec A, Szczepanik M, Mycko MP, Stasiolek M, Raine CS, Selmaj KW. Gammadelta T cells enhance the expression of experimental autoimmune encephalomyelitis by promoting antigen presentation and IL-12 production. J. Immunol. 2004;173:682–694. doi: 10.4049/jimmunol.173.1.682. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Poggi A, Catellani S, Fenoglio D, Borsellino G, Battistini L, Zocchi MR. Adhesion molecules and kinases involved in gammadelta T cells migratory pathways: implications for viral and autoimmune diseases. Curr. Med. Chem. 2007;14:3166–3170. doi: 10.2174/092986707782793835. [DOI] [PubMed] [Google Scholar]
- Rajan AJ, Klein JD, Brosnan CF. The effect of gammadelta T cell depletion on cytokine gene expression in experimental allergic encephalomyelitis. J. Immunol. 1998;160:5955–5962. [PubMed] [Google Scholar]
- Rhodes KA, Andrew EM, Newton DJ, Tramonti D, Carding SR. A subset of IL-10-producing gammadelta T cells protect the liver from Listeria-elicited, CD8(+) T cell-mediated injury. Eur. J. Immunol. 2008;38:2274–2283. doi: 10.1002/eji.200838354. [DOI] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi L, Gallo P, Calabrese M, Ranzato F, Luise D, Colavito D, Motta M, Guglielmo A, Del Giudice E, Romualdi C, et al. Longitudinal analysis of immune cell phenotypes in early stage multiple sclerosis: distinctive patterns characterize MRI-active patients. Brain. 2006;129:1993–2007. doi: 10.1093/brain/awl179. [DOI] [PubMed] [Google Scholar]
- Riol-Blanco L, Lazarevic V, Awasthi A, Mitsdoerffer M, Wilson BS, Croxford A, Waisman A, Kuchroo VK, Glimcher LH, Oukka M. IL-23 receptor regulates unconventional IL-17-producing T cells that control bacterial infections. J. Immunol. 2010;184:1710–1720. doi: 10.4049/jimmunol.0902796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeen MJ, Ziegler HK. Induction of murine peritoneal gamma/delta T cells and their role in resistance to bacterial infection. J. Exp. Med. 1993;178:971–984. doi: 10.1084/jem.178.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger I, Kisielow J, Meier R, King C, Kopf M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur. J. Immunol. 2008;38:1833–1838. doi: 10.1002/eji.200838511. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- Vincent MS, Roessner K, Lynch D, Wilson D, Cooper SM, Tschopp J, Sigal LH, Budd RC. Apoptosis of Fashigh CD4+ synovial T cells by borrelia-reactive Fas-ligand(high) gamma delta T cells in Lyme arthritis. J. Exp. Med. 1996;184:2109–2117. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Pao W, Wong FS, Peng Q, Craft J, Zheng B, Kelsoe G, Dianda L, Owen MJ, Hayday AC. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non alpha/beta” T cells. J. Exp. Med. 1996;183:2271–2282. doi: 10.1084/jem.183.5.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohler JE, Smith SS, Zinn KR, Bullard DC, Barnum SR. Gammadelta T cells in EAE: early trafficking events and cytokine requirements. Eur. J. Immunol. 2009;39:1516–1526. doi: 10.1002/eji.200839176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. Gamma delta T-cell receptor repertoire in acute multiple sclerosis lesions. Proc. Natl. Acad. Sci. U. S. A. 1992;89:4588–4592. doi: 10.1073/pnas.89.10.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong N, Kang C, Raulet DH. Positive selection of dendritic epidermal gammadelta T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. 2004;21:121–131. doi: 10.1016/j.immuni.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.