Abstract
The present study compares rat liver preservation for 9, 12, and 24 h in the standard Eurocollins solution with preservation for the same time periods in the new UW-lactobionate solution. Pharmacologic manipulation with a potent platelet-activating factor antagonist, SRI 63–441, was also evaluated. After cold storage in each of the test solutions, the livers underwent 90 min of warm, oxygenated, sanguinous perfusion. A significant increase in liver weight was noted in Eurocollins-stored versus UW-lactobionate-stored livers. After 90 min of perfusion, livers preserved in UW-lactobionate produced significantly more bile and liberated significantly less glucose and transaminases when compared with Eurocollins-stored livers. Significant augmentation of bile production was observed when donor animals were pretreated with SRI 63–441 and the livers were then stored in UW-lactctbionate for 24 h. Eurocollins-stored livers demonstrated increased hepatocyte vacuolization and endothelial disruption when compared with UW-lactobionate-stored livers after 12 and 24 h of preservation. This study demonstrates the superiority of UW-lactobionate solution in liver preservation and suggests that SRI 63–441 may be beneficial in the further reduction of cold ischemic injury.
The development of a technique with the capability of preserving livers for at least 24–36 h, with consistent and good postimplantation function, would maximize donor use and ensure better organ recipient matching and sharing. Recently, Jamieson et al. reported successful transplantation of canine livers preserved for >20 h using simple, static hypothermic storage in a novel solution, known as the UW-lactobionate (UW) solution (1–3).
In the present study we have further evaluated the efficacy of static hypothermic liver preservation in UW solution, by comparing it with preservation in standard Eurocollins (EC) solution after 9, 12, and 24 h of cold storage. Liver function was assessed on an isolated perfused rat liver apparatus, modified from Miller et al. (4). The results of these studies revealed evidence of improved hepatic status in those livers preserved in UW solution, with the greatest difference in treatment groups being exhibited after 24 h of preservation.
Platelet-activating factor (PAF) is a key inflammatory mediator (5–7) implicated in the microcirculatory failure that ensues after ischemic organ injury. Previously we reported the protective effect of a platelet-activating factor antagonist, SRI 63–441 (Sandoz Pharmaceuticals, North Hanover, N.J.), on postischemic hepatic function after warm ischemic injury (8).
In the present study we have investigated the role of PAF antagonism on hepatic function after a cold ischemic insult. The results of this study suggest that protection of the microvasculature by a combined approach utilizing UW solution and SRI 63–441 can significantly reduce cold ischemic injury to the liver and result in reliable and prolonged extension of the preservation period.
Materials and Methods
Animals
Male Lewis rats (Charles River Breeding Laboratories, Wilmington, Mass.) weighing 225–300 g were used as liver donors, and male Lewis rat retired breeders were used as blood donors. Animals were acclimatized for 1 wk before experimentation, housed in a standard animal facility at the University of Pittsburgh, and fed standard laboratory diet for rats and water ad libitum.
Operative Procedure
Inhalational anesthesia was induced and maintained with methoxyflurane. All animals received 300 U of heparin intravenously via the penile vein 5 min before cannulation of the bile duct and portal vein. The bile duct and portal vein were cannulated and, after the vena cava was vented, the liver was flushed via the portal vein with 20 ml of cooled (4°C) preservation solution from a height of 20 cm. The liver was excised during the flushing period, weighed, immersed in preservation solution, and stored at 4°C. Before placement on the perfusion apparatus, livers were flushed with 8 ml of lactated Ringer’s solution to remove the preservation solution.
Isolated Perfusion
The perfusion apparatus was a recirculating system (4, 9, 10) maintained at 37°C by a circulating water bath and oxygenated with a 95% O2–5% CO2 mixture. The liver was perfused via the portal vein from a height of 18 cm (11). The perfusate consisted of a dilute sanguinous solution prepared from 2 parts by volume of heparinized fresh whole rat blood and 1 part Krebs’ bicarbonate buffer (12) at a hematocrit of 25 (13) and pH = 7.4.
Experimental Protocol
Experimental groups are described in Table 1. After storage at 4°C, all livers were flushed with Ringer’s lactate and placed on the perfusion apparatus for 90 min.
Table 1.
Experimental Groups
| Group | n | Storage time (h) | Solution | SRI 63–441 (20 mg/kg) |
|---|---|---|---|---|
| 1a | 7 | No storage | – | – |
| 2 | 5 | 9 | EC | – |
| 3 | 6 | 9 | UW | – |
| 4 | 5 | 12 | EC | – |
| 5 | 6 | 12 | UW | – |
| 6 | 6 | 24 | EC | – |
| 7 | 5 | 24 | UW | – |
| 8b | 6 | 24 | UW | + |
EC, Eurocollins; UW, UW-lactobionate.
Livers were flushed with lactated Ringer's solution and immediately placed on the perfusion apparatus.
Animals were intravenously pretreated with 20 mg/kg of SRI 63–441 5 min before harvesting the liver.
Platelet-Activating Factor Antagonist
SRI 63–441, a PAF receptor antagonist, was supplied by Sandoz Pharmaceuticals. It was supplied in a powdered form and a 15-mg/ml solution was prepared daily in 0.9% sodium chloride. The solution was warmed to 37°C to ensure that the SRI 63–441 was completely solubilized before injection.
Assessment of Liver Status
Livers were weighed immediately after harvesting and after the preservation period. Baseline perfusate levels of serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), lactic dehydrogenase (LDH), and glucose were measured before placing the liver on the perfusion apparatus. Liver function during perfusion was determined by measuring bile production and SGOT, SGPT, LDH, and glucose levels in the perfusate every 30 min during perfusion. Serum glutamic oxaloacetic transaminase, SGPT, LDH, and glucose levels were determined using the Technicon RA 500 (Technicon Instruments Corp., Tarrytown, N.Y.).
After 90 min of warm sanguinous perfusion, the livers were perfuse-fixed via the portal vein with 2.0% glutaraldehyde in 0.125 M cacodylate buffer, minced to 1 × 1 × 1-mm cubes, and placed in 2.0% glutaraldehyde for 2 h (14). The livers were post-fixed in buffered 2.0% osmium tetroxide, dehydrated in ethanol, and embedded in Epon 812. Blocks were cut using a Reichert microtome. Semi-thin sections were stained with toluidine blue for light microscopy. Ultrathin sections were collected on grids, stained with uranyl acetate and lead citrate, and observed with a Phillips 300 electron microscope (Phillips Electronic Instruments, Mahwah, N.J.) (15).
Statistics
All values were corrected to baseline by subtracting the values obtained before placing the liver on the perfusion apparatus from subsequent measurements after 30, 60, and 90 min of perfusion. Statistical evaluation was made using analysis of variance and Student’s t-test (16). All values are expressed as mean ± SEM. Probability values of <0.05 were considered statistically significant.
Results
Liver Weight
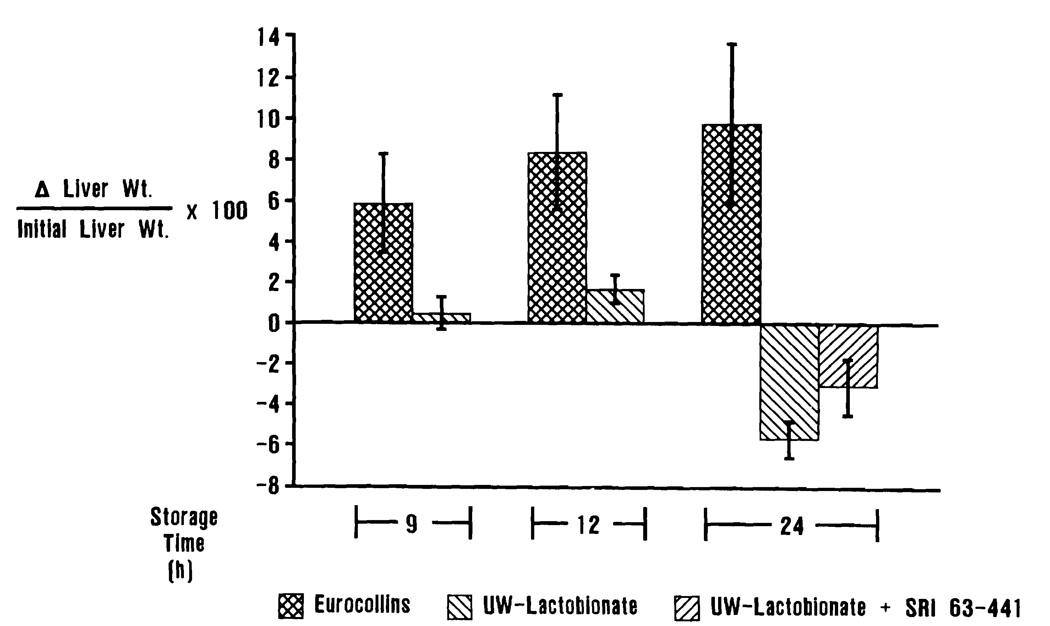
There was a significant increase in liver weight for those organs stored in EC solution when compared with UW-stored livers (p < 0.05). There were no significant differences in the weights of livers stored in UW solution for 24 h regardless of pretreatment with SRI 63–441 (Figure 1).
Figure 1.
Percentage change in liver weight during the preservation period. Livers preserved in EC solution gained significantly more weight than those preserved in UW solution. The addition of SRI 63–441 did not significantly alter weight change during the storage period.
Transaminase Release
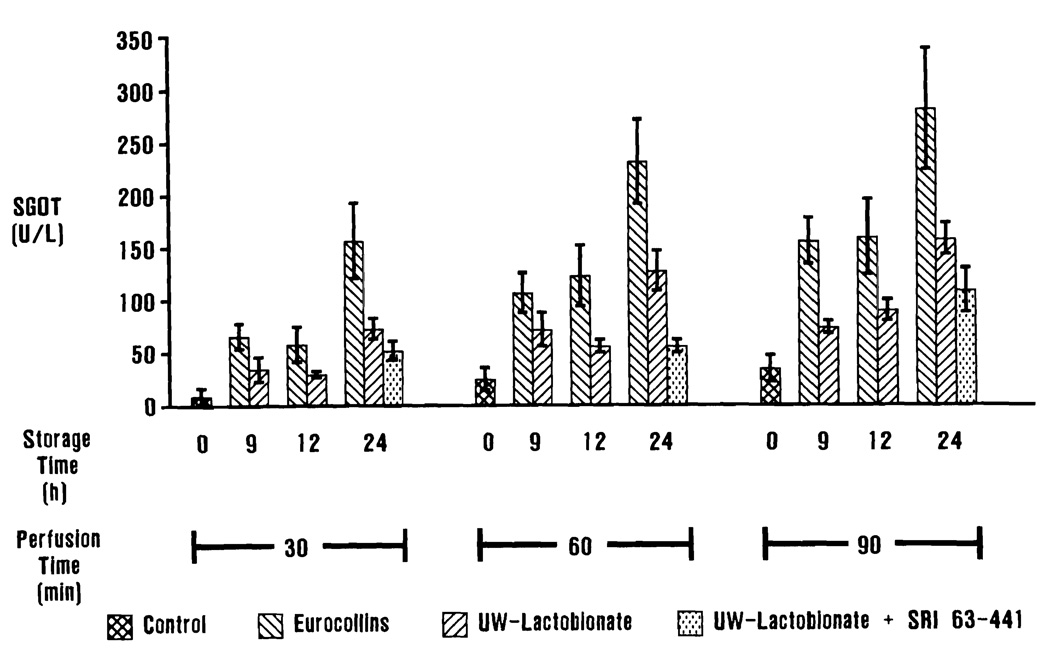
Serum glutamic oxaloacetic transaminase values are presented in Figure 2. Serum glutamic oxaloacetic transaminase and SGPT liberation into the perfusate was significantly higher (p < 0.01) in livers preserved in EC solution when compared with controls that did not undergo preservation. The livers stored in UW solution did not release significantly more SGOT into the perfusate than controls until after 60 min of perfusion (p < 0.05). Similarly, livers preserved in UW solution did not liberate significantly more SGPT into the perfusate than controls, except after 24 h of storage and 90 min of perfusion (p < 0.05). The 24-h, UW-stored, SRI 63–441-pretreated livers did not release significantly more SGOT than controls until after 90 min of perfusion (p < 0.05), and did not release significantly more SGPT than controls.
Figure 2.
Perfusate SGOT levels. Differences in SGOT liberation into the perfusate are most dramatically demonstrated after 24 h of preservation. Serum glutamic oxaloacetic transaminase release from livers stored for 24 h in UW solution is comparable to the values at 9 and 12 h for livers stored in EC solution. Perfusate levels of SGPT, LDH, and glucose, and bile production followed a similar pattern.
After 30 min of perfusion, there was no significant difference in SGOT release between UW-stored and EC-stored livers until after 24 h of preservation (p < 0.05). Perfusate SCOT levels became significantly different between UW-stored and EC-stored livers after 60 min of perfusion for those livers preserved for 12 and 24 h (p < 0.01). After 90 min of perfusion, perfusate SGOT levels were significantly lower for UW-stored livers when compared with EC-stored livers (p < 0.01). Serum glutamic pyruvic transaminase liberation by livers stored in UW solution was significantly decreased when compared with livers stored in EC solution (p < 0.01).
Although there was a trend of decreased transaminase release in the SRI 63–441-pretreated, 24-h UW-stored livers when compared with 24-h UW-stored livers alone, these differences were not statistically significant.
Lactic Dehydrogenase Release
There was a significant increase in LDH liberation by both UW-stored and EC-stored livers when compared with nonpreserved controls (p < 0.01). Livers from animals pretreated with SRI 63–441, stored in UW solution for 24 h, similarly released significantly more LDH than controls (p < 0.01).
No significant difference was found in perfusate LDH levels between livers stored in UW and livers stored in EC solution after 9 and 12 h; however, after 24 h of preservation, there was a significant increase in LDH release by livers preserved in EC solution versus those preserved in UW solution (p < 0.01). The addition of SRI 63–441 to the protocol did not further significantly reduce LDH release in livers stored for 24 h in UW solution.
Perfusate Glucose Levels
There was a significant increase in perfusate glucose levels for those livers stored in EC solution when compared with controls (p < 0.01). There was no significant increase in perfusate glucose levels for livers preserved in UW solution when compared with controls until after 24 h of storage (p < 0.01) and only after 30 and 60 min of perfusion. There was no significant difference in perfusate glucose levels between UW-stored livers and controls after 90 min of perfusion for all periods of preservation. Although there was a significant increase in perfusate glucose levels for the livers from animals pretreated with SRI 63–441 and stored in UW solution for 24 h after 30 min of reperfusion when compared with controls (p < 0.01), this difference was no longer statistically significant after 60 and 90 min of perfusion.
There was a significant decrease in perfusate glucose levels for livers stored in UW solution when compared with EC-stored livers (p < 0.05), except for those livers stored in UW solution for 24 h and only after the 30- and 60-min perfusion periods. SRI 63–441 pretreatment resulted in no further significant reduction in perfusate glucose levels over livers stored in UW solution alone.
Bile Production
Bile production results followed a pattern similar to that seen for transaminase release. Livers preserved in EC solution produced significantly less bile than immediately perfused nonpreserved controls (p < 0.01). Bile production by UW-stored livers was not significantly reduced from nonpreserved controls until the 24-h preservation period (p < 0.01). There was a significant increase in bile production by the livers preserved in UW solution when compared with EC-preserved livers (p < 0.01). Livers harvested from rats pretreated with 20 mg/kg of SRI 63–441 intravenously and stored in UW solution for 24 h produced significantly more bile than livers preserved in the UW solution alone (p < 0.05).
Morphologic Evaluation
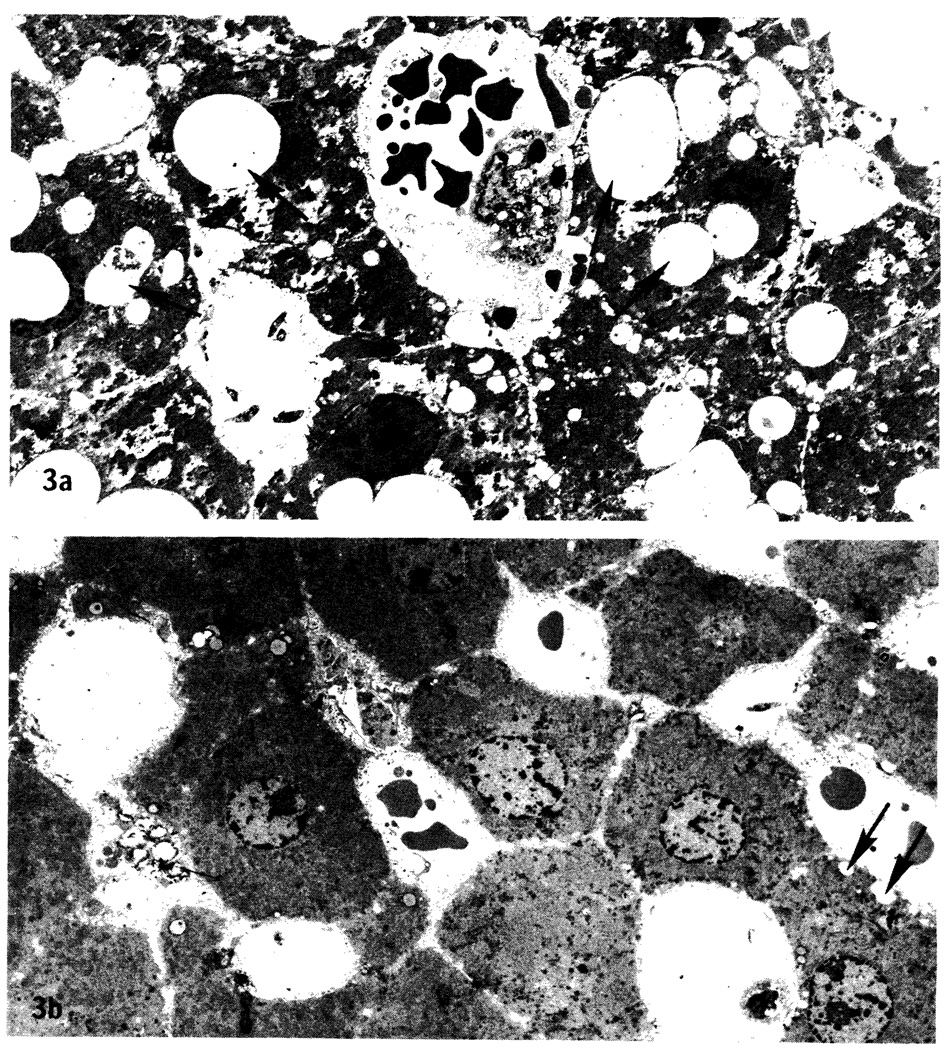
Light and low-magnification electron microscopy revealed extensive vacuolization of hepatocytes in the 24-h EC-preserved livers (Figure 3a) when compared with controls and with livers preserved in UW for 24 h (Figure 3b) regardless of pretreatment with SRI 63–441. Vacuolization was present to a minor extent in the 9- and 12-h EC-preserved livers and rarely present in the 9- and 12-h-UW-preserved livers (not shown).
Figure 3.
Low-magnification electron micrographs (×1700). a. Liver preserved in EC solution for 24 h. Note extensive vacuolization of hepatocytes (arrows). b. Liver preserved in UW solution for 24 h. A few small vacuoles are present (arrows).
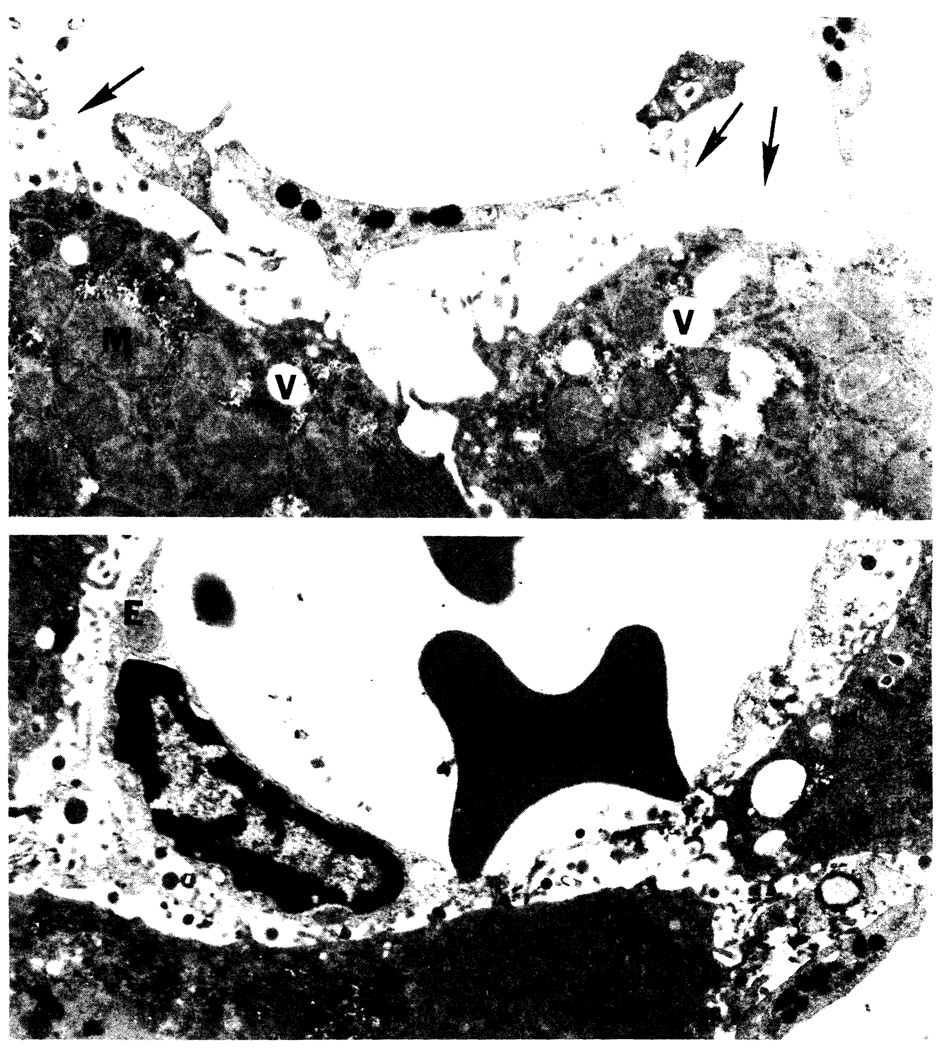
Electron microscopy revealed extensive sinusoidal endothelial disruption in livers stored in EC for 24 h (Figure 4a). Livers preserved in UW solution for 24 h also exhibited some endothelial damage in the sinusoids, but to a lesser extent than EC-stored livers (Figure 4b). The sinusoidal endothelium of livers preserved in UW for 9 h could not be distinguished from the sinusoidal endothelium of nonpreserved control livers; however, 9-h EC-preserved livers manifested a minor degree of endothelial damage (not shown). After 12 h of preservation, both EC- and UW-stored livers exhibited areas of sinusoidal endothelial damage interspersed with areas containing intact sinusoidal endothelium when compared with controls; however, the endothelium appeared to be better preserved in the livers stored in UW solution (not shown). Livers that were pretreated with SRI 63–441 and stored in UW solution for 24 h did not demonstrate any discernable histologic improvement over the livers stored in UW alone for 24 h (not shown).
Figure 4.
Electron micrographs after 24-h preservation (×9700). a. Liver preserved in EC solution for 24 h. Note swollen mitochondria (M), vacuoles (V), and endothelial disruption (arrows). b. Liver preserved in UW solution for 24 h. Note areas of intact endothelium (E). Areas of endothelial injury were also present (not shown).
Discussion
Before the development of UW solution, there had been a resurgence of interest and enthusiasm in hypothermic perfusion as the method best suited for prolonged hepatic preservation (17–19). Simple hypothermic preservation of the rat liver using isotonic citrate was much less successful than that obtained by continuous hypothermic perfusion (20,21). The consistently successful transplantation of canine livers after 24–30 h of cold storage in UW solution reported by Jamieson et al. (1) is a landmark event in liver preservation. In the present study we have evaluated hepatic function after 9, 12, and 24 h of preservation with UW solution using the isolated perfused rat liver.
A substantial improvement in hepatic function, compared with livers stored in conventional EC solution, was demonstrated in UW-stored organs after 90 min of perfusion, as manifested by increased bile production, decreased transaminase release, and decreased glucose release into the perfusate. A significant difference in perfusate LDH levels between EC- and UW-stored livers only became evident after 24 h of preservation.
Tamaki et al. (22) observed that livers that gained the least weight during preservation resumed a normal color and consistency more rapidly, did not become congested or bleed, and had the best histologic appearance after revascularization and transplantation. In agreement with the experiments of Tamaki et al., the UW-stored livers gained less weight than the EC-stored livers and demonstrated the best function after preservation.
Preliminary results from our laboratory, comparing liver preservation in the standard EC solution with the new UW solution using orthotopic liver transplantation in the rat, demonstrate improved graft survival in those rats receiving livers preserved in UW solution after 9 and 24 h of preservation (Liu T, personal communication). These initial transplantation findings confirm our observations of the improved status of the isolated perfused rat livers that were stored in UW solution.
Controls that did not undergo any period of preservation, but underwent the same method of fixation, exhibited completely intact endothelium in both the sinusoids and larger vessels, indicating that the changes seen in the experimental groups were not artifactual. The endothelial disruption seen most dramatically in the sinusoids of the livers stored for 24 h in EC solution may be the result of (a) true endothelial denudation during the reperfusion period or (b) a deterioration of the endothelium’s ability to adhere due to ischemia/reperfusion injury, thus allowing its disruption during the process of fixation. The degree of endothelial injury observed in the preserved livers correlated well with the degree of hepatic dysfunction manifested by the 24-h EC-stored livers. A correlation between liver dysfunction and severe endothelial disruption has been reported by McKeown et al. (23). The importance of endothelial injury after ischemia/reperfusion has also been reported in other organs (24, 25).
The livers preserved in EC solution for 24 h exhibited severe hepatocytic vacuolar degeneration, which is characteristic of hypoxic injury (26,27). The severe hepatocellular injury was evident only in the 24-h EC-stored livers, whereas endothelial injury was present to some extent even after 12 h of EC preservation. It appears that the injury to the sinusoidal endothelium is an early event in ischemia/reperfusion injury and that the ensuing microcirculatory failure then results in parenchymal hypoxia and vacuolar degeneration.
Attempts to prevent or diminish ischemic injury associated with organ procurement and preservation have involved the utilization of several pharmacologic agents (28–31). The PAF antagonist SRI 63–441 has been shown to have a protective effect on post-ischemic hepatic function after warm ischemic injury (8). In addition to its effects on platelets, PAF induces neutrophil aggregation and activation and superoxide anion release, and acts directly on vascular endothelium to increase permeability (32). Rat platelets are thought to be unresponsive to PAF (33, 34), therefore PAF-mediated ischemic liver injury may be neutrophil-dependent.
The 24-h UW-stored livers from animals pretreated with 20 mg/kg of SRI 63–441 produced significantly more bile than livers similarly stored from untreated animals. This is a notable improvement in hepatic synthetic function. Although the other parameters of liver function evaluated in this study did not reach statistical significance, a sustained general trend toward improvement was evident for all variables.
In summary, the results of this study provide objective data to further substantiate the superiority of UW solution over EC solution in liver preservation. Pretreatment of donors with SRI 63–441 is also beneficial in the further reduction of cold ischemic injury, resulting in improved postischemic hepatic function. The isolated perfused rat liver has proved to be an excellent model for the study of hepatic function after preservation, as it correlates well with the technically more difficult in vivo studies.
Acknowledgments
This work was supported by research grants from the Veterans Administration, The Children’s Liver Foundation, The Competitive Medical Research Fund of Presbyterian Hospital. Pittsburgh, Pennsylvania, and project grant AM-29961 from the National Institutes of Health, Bethesda, Maryland.
Sheryl J. Ontell is an Allen Scholar, Elizabeth Severance Prentiss Foundation, Department of Surgery, Case Western Reserve University, Cleveland, Ohio.
The authors thank Gloria DiLuiso and Mary Watash for excellent technical assistance, Dr. Floyd Taylor of the Department of Community Medicine for assistance with the statistical analysis, and Donna Ross for typing the manuscript.
Abbreviations used in this paper
- EC
Eurocollins
- PAF
platelet-activating factor
- UW
UW-lactobionate
References
- 1.Jamieson NV, Sundberg R, Lindell S, et al. Successful 24–30 hour preservation of the canine liver: a preliminary report. Transplant Proc. 1988;20:945–947. [Google Scholar]
- 2.Wahlberg JA, Southard JH, Belzer FO. Development of a cold storage solution for pancreas preservation. Cryobiology. 1986;23:477–482. doi: 10.1016/0011-2240(86)90056-8. [DOI] [PubMed] [Google Scholar]
- 3.Wahlberg JA, Love R, Landegaard L, Southard JH, Belzer FO. 72-Hour preservation of the canine pancreas. Transplantation. 1987;43:5–8. doi: 10.1097/00007890-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Miller LL, Bly CG, Watson ML, et al. The dominant role of the liver in plasma protein synthesis. J Exp Med. 1951;94:431–453. doi: 10.1084/jem.94.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder F. Chemical and biochemical aspects of platelet activating factor: a novel class of acetylated ether-linked choline-phospholipids. Med Res Rev. 1985;5:107–140. doi: 10.1002/med.2610050105. [DOI] [PubMed] [Google Scholar]
- 6.Vargaftig BB, Chignard M, Benveniste J, Lefort J, Wal F. Background and present status of research on platelet activating factor (PAF-acether) Ann NY Acad Sci. 1981;370:119–137. doi: 10.1111/j.1749-6632.1981.tb29727.x. [DOI] [PubMed] [Google Scholar]
- 7.Bussolino F, Biffignandi P, Arese P. Platelet activating factor–a powerful lipid autocoid possibly involved in microangiopathy. Acta Haematol. 1986;75:129–140. doi: 10.1159/000206106. [DOI] [PubMed] [Google Scholar]
- 8.Ontell SJ, Makowka L, Ove P, Starzl TE. The effects of a platelet activating factor antagonist on post ischemic hepatic function in the rat. Surg Forum. 1987;38:388–389. [Google Scholar]
- 9.Hamilton RL, Berry MN, Williams MC. A simple and inexpensive membrane lung for small organ perfusion. J Lipid Res. 1974;15:182–185. [PubMed] [Google Scholar]
- 10.Ontell SJ, Colella MS, Horowitz J, Makowka L, Trager J, Starzl TE. Applications of the isolated perfused rat liver in transplantation research. J Invest Surg. 1988;1:25–27. doi: 10.3109/08941938809141072. [DOI] [PubMed] [Google Scholar]
- 11.Levine RA, Pesh LA, Klatskin G, Giarman NJ. Effect of serotonin on glycogen metabolism in isolated rat liver. J Clin Invest. 1964;43:797–809. doi: 10.1172/JCI104966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krebs HA, Henseleit K. Untersuchungen uber die Harnstoffbildung im Tierkorper. Hoppe-Seyler’s Z Physiol Chem. 1932;210:33–66. [Google Scholar]
- 13.Riedel GL, Scholle JL, Shepherd AP, Ward WF. Effects of hematocrit on oxygenation of the isolated perfused rat liver. Am Physiol Soc. 1983;345:G769–G774. doi: 10.1152/ajpgi.1983.245.6.G769. [DOI] [PubMed] [Google Scholar]
- 14.Fahimi HD. Perfusion and immersion fixation of rat liver with glutaraldehyde. Lab Invest. 1967;16:736–751. [PubMed] [Google Scholar]
- 15.Reynolds ES. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol. 1963;17:208–211. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 2nd ed. San Francisco: WH Freeman; 1981. [Google Scholar]
- 17.Petrie CR, Woods JE. Successful 24 hour preservation of the canine liver. Arch Surg. 1973;107:461–464. doi: 10.1001/archsurg.1973.01350210091023. [DOI] [PubMed] [Google Scholar]
- 18.Sung DTW, Woods JE. 48 Hour preservation of the canine liver. Ann Surg. 1974;179:422–426. doi: 10.1097/00000658-197404000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toledo-Pereyra LH. Twenty-four hour liver preservation by hypothermic intermittent perfusion. Transplantation. 1979;24:291–293. [PubMed] [Google Scholar]
- 20.Tamaki T, Kamada N, Wight DG, Pegg DE. Hypothermic preservation of the rat liver assessed by orthotopic transplantation. II. Evaluation of citrate solutions. Transplantation. 1987;43:357–361. doi: 10.1097/00007890-198703000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Tamaki T, Kamada N, Wight DG, Pegg DE. Successful 48-hour preservation of the rat liver by continuous hypothermic perfusion with Haemaccel-isotonic citrate solution. Transplantation. 1987;43:468–471. doi: 10.1097/00007890-198704000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Tamaki T, Kamada N, Pegg DE. Hypothermic preservation of the rat liver assessed by orthotopic transplantation. A comparison of flush solutions. Transplantation. 1986;4:396–397. [PubMed] [Google Scholar]
- 23.McKeown CMB, Edwards V, Phillips MJ, Harvey PRC, Petrunka CN, Strasberg SM. The critical injury in cold preservation of liver allografts in the rat is sinusoidal lining cell damage. Transplantation. 1988 (in press) [PubMed] [Google Scholar]
- 24.Suval WD, Duran WN, Boric MP, et al. Microvascular transport and endothelial cell alterations preceding skeletal muscle damage in ischemia and reperfusion injury. Am J Surg. 1987;154:211–218. doi: 10.1016/0002-9610(87)90181-4. [DOI] [PubMed] [Google Scholar]
- 25.Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashford TP, Burdette WJ. Response of the isolated perfused hepatic parenchyma to hypoxia. Ann Surg. 1965;162:191–207. doi: 10.1097/00000658-196508000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakata K, Fukumoto O, Fujimoto K, Fujikawa Y. Development of hypoxic change of the liver cells as revealed by the isolated perfused rat liver. Acta Pathol Jpn. 1971;21:313–328. doi: 10.1111/j.1440-1827.1971.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 28.Monden M, Fortner JG. 24 and 48 hour canine liver preservation by simple hypothermia with prostacyclin. Ann Surg. 1982;196:38–42. doi: 10.1097/00000658-198207000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atalla SL, Toledo-Pereyra LH, Mackenzie GH, Cederna JP. Influence of oxygen derived free radical scavengers on ischemic livers. Transplantation. 1985;40:584–589. doi: 10.1097/00007890-198512000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Arthur MJP, Bently IS, Tanner AR, et al. Oxygen derived free radicals promote hepatic injury in the rat. Gastroenterology. 1985;89:1114–1122. doi: 10.1016/0016-5085(85)90218-5. [DOI] [PubMed] [Google Scholar]
- 31.Rizzuti RP, Cunningham PR, Easley D, Fushiki T, Thomas FT. A new method of hepatic preservation using pulsatile perfusion and allopurinol. Curr Surg. 1986 November–December;:492–494. [PubMed] [Google Scholar]
- 32.Pinckard RN. Platelet-activating factor. Hosp Pract. 1983;18:67–76. doi: 10.1080/21548331.1983.11702680. [DOI] [PubMed] [Google Scholar]
- 33.Saunders RN, Handley DA. Platelet-activating factor antagonists. Ann Rev Pharmacol Toxicol. 1987;27:237–255. doi: 10.1146/annurev.pa.27.040187.001321. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Crespo M, Alonso F, Inarrea P, Vlvarez V, Egido J. Vascular actions of synthetic PAF-acether (a synthetic platelet-activating factor) in the rat: evidence for a platelet independent mechanism. Immunopharmacology. 1982;4:173–185. doi: 10.1016/0162-3109(82)90019-4. [DOI] [PubMed] [Google Scholar]