Abstract
The first-dose pharmacokinetics of FK506 was studied in nine orthotopic liver transplant patients receiving continuous intravenous infusion of 0.15 mg/kg/day. Multiple blood samples were obtained during the infusion and plasma FK506 concentrations were measured hy enzyme-linked immunosorbent assay. The plasma clearance ranged from 0.47 to 5.8 L/minute, and the half-life ranged from 4.5 hours to 33.1 hours. These results indicate the pharmacokinetics of FK506 to be highly variable between patients. FK506 is extensively distributed outside the plasma compartment. FK506 is extensively metabolized in the body, with less than 1% of the administered dose being excreted in the urine as unchanged FK506. The large variability in FK506 kinetics during the immediate postoperative period is attributed to the variability in the functional status of the liver in the transplant patients. Because of the long half-life of FK506, it takes more than 45 hours to reach steady-state concentrations after continuous infusion. Based on the estimated kinetic parameters, it appears that a combination of a bolus or a rapid infusion of .02 mg/kg with a continuous infusion of 0.05 mg/kg/day will provide and maintain a concentration of more than 2 ng/mL from the beginning of the drug treatment.
FKC506 is a macrolide isolated from the fungus Streptomyces tsukubaensis.1 It is nearly 100 times more potent than cyclosporine (CsA) in inhibiting lymphocyte proliferation in mixed lymphocyte cultures.2 FK506 has been shown to prevent or reverse the rejection of heart, liver, kidney, pancreas, lung, intestine, and skin grafts in mice, rats, dogs, monkeys, and baboons.3 FK506 has been in clinical use since March 1989 at the University of Pittsburgh. It was used initially for rescuing livers that have failed under conventional immunosuppression and later as the primary immunosuppressant for liver, kidney, and heart transplantation.4-6 Current results indicate that FK506 provides better immunosuppression in liver and heart transplant recipients than does CsA.4,6-8
After transplantation, FK506 is administered intravenously as a continuous infusion at a dose of 0.15 mg/kg/day and then orally at a dose of 0.3 mg/kg/ day twice daily, when the patient can tolerate oral intake. Further dose alterations are normally made based on patient's liver function, episodes of rejection or toxicity, and trough plasma FK506 concentrations. The intravenous dosage form of FK506 is a 10-mg/mL FK506 solution with polyethoxylated hydrogenated castor oil (HCO-60, a nonionic surfactant) and alcohol. This solution is diluted in 100 mL of 5% dextrose and infused at a rate of 4 mL/hour over 24 hours. The pharmacokinetics of FK506 has been previously studied in liver transplant patients who received their daily dose as a 2- or 4-hour infusion.9-11 The purpose of the current study was to characterize the pharmacokinetics of FK506 in orthotopic liver transplant recipients receiving continuous intravenous administration of FK506 during the immediate postoperative period and to compare actual steady-state FK506 concentrations with concentrations predicted based on the 24-hour kinetic study.
MATERIALS AND METHODS
Patients
Nine consecutive adult patients (8 men and 1 woman) who had undergone orthotopic liver transplantation received a continuous intravenous infusion of FK506 (0.15 mg/kg/day) as their primary immunosuppressant 2 to 4 hours after surgery. FK506 was administered as a continuous intravenous infusion for a minimum of 3 to 6 days. They also received 20 mg of methylprednisone during the first 24 hours. Patients' ages ranged from 24 to 64 years, and the patients weighed between 40 and 114 kg, with a mean of 71 kg. Written informed consents were obtained from all patients, and the study protocol was approved by the University of Pittsburgh Institutional Review Board for Biomedical Studies.
Specimen Collection
Multiple blood samples (3 mL) were drawn at 0, 15, 30, 45 minutes, 1, 2, 3, 4, 5, 6, 8, 12,16, and 24 hours after starting FK506 infusion from arterial lines into heparinized vacutainers. Blood samples were incubated at 37°C for 30 minutes, and plasma was separated by centrifugation at 37°C and frozen at −70°C until analysis. Twenty-four-hour urine collections also were made, and aliquots of the samples were frozen for FK506 analysis.
Assay
FK506 concentration in plasma separated at 37°C was measured by a monoclonal-antibody–based enzyme-linked immunosorbent assay.12,13 The coefficient of variation of this assay is 19.8% at 0.4 ng/mL, 14% at 1.6 ng/mL, and 16% at 2.6 ng/mL.
Data Analysis
FK506 concentration–time data were analyzed by nonlinear curve fitting with MINSQ program (MINSQ, 1987) using a two-compartment model.
where Laml and Lam2 are the two exponents and the rest are as described by standard methods.14
Using the parameter estimates obtained from fitting the data (A, C. Laml. Lam2. K21,) kinetic parameters (half-life, clearance, volume of distribution, and steady state plasma concentration Css) were calculated according to standard techniques.14 In addition, the steady-state concentration that will be achieved on the infusion regimen was predicted for each patient based on the kinetic parameters (clearance) calculated from 24-hour plasma concentration–time data and the infusion rate (Css = infusion rate/clearance) and compared with the actual steady-state concentrations measured 3 to 6 days after the start of the infusion.
RESULTS
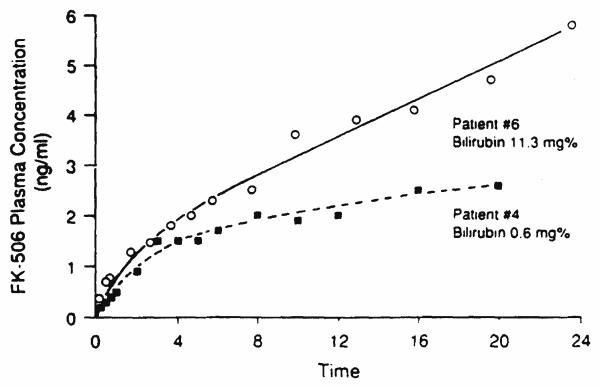
The characteristics of the patients who participated in the present study are summarized in Table I. The bilirubin and serum glutamic oxaloacetic transaminase and serum glutamic-pyruvic transaminase concentrations varied widely in these patients. A representative plasma concentration–time profile of FK506 in two patients (one with the lowest and one with the highest bilirubin) during continuous intravenous infusion is shown in Figure 1. Table II summarizes the pharmacokinetic parameters of FK506 in each of the patients studied. The total body clearance of FK506 is estimated to be 1.76 ± 1.75 L/min. The initial and terminal disposition rate constants are 1.23 ± 1.08/hour and .071 ± .05/hour, respectively. The mean disposition half-life is 15.5 hours. The steady-state volume of distribution, Vss, is 1252 ± 668 L.
TABLE I.
Biochemical Profiles in Patients*
| Patient # | Age yr |
B.W. kg |
Bilirubin mg/dL |
SGPT U/L |
SGOT UL |
SCR mg/dL |
BUN mg/dL |
|---|---|---|---|---|---|---|---|
| 1 | 37 | 75 | 5.5 | 893 | 1198 | 0.8 | 16 |
| 2 | 61 | 70 | 3.8 | 2244 | 2685 | 0.5 | 9 |
| 3 | 47 | 114 | 11.5 | 463 | 280 | 0.7 | 20 |
| 4 | 24 | 49 | 2.8 | 413 | 666 | 0.6 | 7 |
| 5 | 64 | 40 | 3.6 | 593 | 753 | 0.5 | 11 |
| 6 | 25 | 51 | 9.1 | 3100 | 4331 | 2.8 | 46 |
| 7 | 36 | 78 | 2.0 | 319 | 422 | 0.6 | 14 |
| 8 | 60 | 88 | 2.5 | 365 | 357 | 1.8 | 40 |
| 9 | 25 | 77 | 4.8 | 960 | 903 | 0.6 | 17 |
All biochemical parameters were measured on study date.
Figure 1.
Plasma concentration versus time curve after continuous intravenous infusion of FK 506 in patient 6 with the highest serum bilirubin and patient 2 with the lowest serum bilirubin concentrations.
TABLE II.
Pharmacokinetic Parameters of FK 506
| Patient # | Distribution Rate Constant (hr−1) |
Disposition Rate Constant (hr−1) |
Disposition Half-Life (hr) |
Volume of Distribution at Steady State (L) |
Clearance (L/min) |
|---|---|---|---|---|---|
| 1 | 0.469 | 0.0975 | 7.1 | 822 | 1.56 |
| 2 | 0.453 | 0.0939 | 7.4 | 406 | 0.62 |
| 3 | 0.469 | 0.0889 | 7.8 | 1355 | 3.38 |
| 4 | 0.770 | 0.0276 | 25.1 | 1857 | 1.06 |
| 5 | 0.521 | 0.0439 | 15.8 | 573 | 0.47 |
| 6 | 3.10 | 0.0210 | 33.1 | 960 | 0.36 |
| 7 | 2.61 | 0.0225 | 30.7 | 2441 | 1.00 |
| 8 | 0.456 | 0.0878 | 7.9 | 1058 | 1.63 |
| 9 | 2.18 | 0.1555 | 4.5 | 1796 | 5.75 |
| Mean ± SD | 1.23 ± 1.08 | 0.0710 | 15.5 ± 11.2 | 1252 ± 668 | 1.76 ± 1.75 |
| 0.05 |
Based on the pharmacokinetic parameters calculated in each patient, the steady-state concentration that will be reached eventually in these patients was calculated according to the following equation:
Table III summarizes the biochemical profile and observed and predicted FK506 plasma concentrations on the last day of intravenous therapy. Reasonable agreement (less than 30% difference) was observed between the calculated and the actual Css on days 3 to 6 in patients 4, 6, 7, and 8. In patients 2 and 5, where the liver function improved based on bilirubin concentrations, the actual steady-state concentration on day 4 was less than what was predicted based on the first 24-hour kinetics. In patients 1 and 3. the predicted steady-state levels were lower than actual Css reached on days 4 or 3, respectively. During this period, bilirubin concentrations increased in patient 3, indicating deterioration of liver function, but decreased in patient 1. There was no significant correlation (r*= .248) between the plasma clearance of FK506 and the serum bilirubin concentrations on day 1. The observed FK506 concentrations on days 3 to 6 also did not correlate well (r = 0.473; P = .234) with the total bilirubin concentrations on the corresponding days. Less than 1% of the administered dose was recovered in the urine as FK506 over a 24-hour period.
TABLE III.
Biochemical Profiles in Patients*
| Patient # | Last Day on IV Therapy |
Bilirubin mg/dL |
SGPT U/L |
SGOT UL |
SCr mg/dL |
BUN mg/dL |
Plasma FK 506 Concentration (ng/mL) |
|
|---|---|---|---|---|---|---|---|---|
| Observed | Predicted | |||||||
| 1 | 4 | 2.4 | 555 | 134 | 1.0 | 38 | 11.0 | 5.1 |
| 2 | 5 | 0.9 | 609 | 95 | 0.8 | 24 | 7.5 | 10.7 |
| 3 | 3 | 13.9 | 383 | 185 | 0.9 | 65 | 7.3 | 3.2 |
| 4 | 3 | 0.6 | 168 | 112 | 0.6 | 9 | 5.1 | 5.0 |
| 5 | 6 | 1.7 | 204 | 66 | 1.2 | 14 | 4.5 | 8.4 |
| 6 | 6 | 11.3 | 122 | 72 | 3.4 | 72 | 14.4 | 12.9 |
| 7 | 3 | 0.9 | 475 | 354 | 2.0 | 32 | 7.0 | 8.0 |
| 8 | 3 | 1.5 | 229 | 134 | 3.7 | 73 | 8.4 | 6.1 |
| 9 | 4 | 4.8 | 294 | 52 | 1.1 | 31 | — † | 1.5 |
Parameters estimated on last day of IV therapy.
Not available.
DISCUSSION
Since the introduction of CsA, the discovery of FK506 is the most important step in the advancement of immunosuppressive therapy for organ transplant patients. FK506 is a macrolide and is very different in its chemical structure from any other immunosuppressive drug used currently. In this study, we examined the plasma concentration–time profile in nine consecutive orthotopic liver transplant patients receiving a continuous infusion of FK506 as their primary immunosuppressant. The assay method that was used to measure FK506 in plasma is a sensitive monoclonal antibody–based enzyme immunoassay. Because metabolites of FK506 have not been completely characterized, it was not possible to determine the potential cross-reactivity of any FK506 metabolites with the antibody used in this assay. Preliminary studies, however, indicate that a fraction collected in HPLC (presumably a metabolite of FK506) does cross-react with the antibody used in the FK506 analysis (unpublished observation). In the absence of FK506 concentration measurement using a completely specific assay, the results of this study have to be interpreted with caution. The partitioning of FK506 between blood cells and plasma is temperature dependent.15 To be physiologically meaningful, blood was separated at 37°C in this study.
The overall behavior of the drug in the body could be adequately described by a biexponential equation. The plasma concentrations attained in 24 hours with the infusion rate of 0.15 mg/kg/day ranged between 2 and 7 ng/mL. The mean plasma clearance of FK506 was 1.76 L/minute and was variable between patients. Based on these data, one would classify FK506 as a high-clearance drug if the blood concentration of the drug were to be similar to the plasma concentration. Recent evidence, however, suggests that the drug is extensively distributed into red blood cells, with a blood-to-plasma ratio of nearly 12.15 This indicates that the clearance calculated from the blood data will be considerably small and the drug is actually a low clearance drug. In addition, it is possible that FK506 also may be metabolized extrahepatically; there is evidence of FK506 metabolism in rat small intestine.16
There was a large variation in FK506 clearance between patients. The differences in the functional status of the liver between patients is expected to have contributed to the observed variation in the clearance of FK506. Factors such as cold ischemic damage, reperfusion injury, and accumulated endogenous inhibitors of drug metabolism also may have contributed to the observed variation in the clearance of FK506 during the immediate postoperative period. Lack of correlation between bilirubin levels and FK506 concentrations may be related to the fact that bilirubin concentrations may not reflect the functional status of the liver during the immediate postoperative period. The highest half-life and the smallest clearance values, however, were observed in a patient who had the highest serum bilirubin concentrations (>9 mg/dL) during the entire first week after transplantation. Conversely, accumulation of FK506 metabolites in patients with poor liver function and the inability of the assay to distinguish between unchanged FK506 and some of its metabolites could presumably explain the lack of correlation between serum bilirubin concentration and FK506 clearance.
Analysis of the 12-hour trough concentrations of FK506 in a large group of patients (n = 49) with different qualities of liver graft function indicates high trough FK506 concentrations in patients with poorly functioning grafts.17 There also was a significant positive correlation between serum bilirubin and trough FK506 plasma concentrations despite a negative correlation between serum bilirubin and FK506 doses in these patients. It also has been shown that in patients with poor liver function, as indicated by bilirubin concentrations, the 24-hour trough concentrations after two or four infusions of FK506 (0.15 mg/kg/day) are higher than those observed in patients with normal liver function.11 The functional status of the liver does not remain constant during the immediate postoperative period in all liver transplant patients. It is not surprising, however, that steady-state concentrations could not be predicted in all patients based on the kinetic studies performed on day 1 after transplant. In four patients, where the liver function remained satisfactory or did not change significantly, the observed steady-state concentrations were within 30% of the predicted values. In two patients, where the liver function improved over the study period, the observed steady-state concentrations were lower than predicted. In patient 3, with a worsening of the liver status, the observed steady-state concentration was higher than the predicted value. Taken together, these observations indicate a dependency of FK506 concentrations on liver status and indicate a need for careful adjustments in FK506 dosing in such patients.
The large volume of distribution of FK506 suggests extensive distribution of the drug outside the plasma compartment. Recent studies have shown FK506 to bind preferentially to erythrocytes, with blood-to-plasma ratios of 10 to 1 (Unpublished observation). Based on studies in animals,9 high concentrations of FK506 are expected in lungs, kidneys, heart, and spleen. Because of the large volume of distribution of FK506, the fact that FK506 is extensively metabolized in the liver, and that a large amount of FK506 remains outside the plasma compartment, for a given period of dialysis, a significant fraction of the body load of FK506 is not likely to be removed.
The mean half-life of FK506 is 15.5 hours. This indicates that after continuous infusion it will take at least 45 hours to reach 90% of the steady-state levels. After a change in the dosing regimen, one has to wait for a minimum of 45 hours to attain a new steady-state FK506 plasma concentration. After continuous infusion of FK506 at a dosage of 0.15 mg/kg/day, on average it would take nearly 10 hours to reach a plasma concentration of 2 ng/mL. Conversely, based on computer simulations, after two or four infusions this concentration is achieved in about 1 hour, but with a peak plasma concentration of 6.7 and 5.1 ng/mL at the end of the corresponding infusion times. One approach to achieving a concentration of about 2 ng/mL immediately after beginning of treatment and to maintaining it at this concentration is to use a small bolus dose or a rapid infusion over 1 hour of FK506 (.02 mg/kg). The plasma concentration then can be maintained around 2 ng/mL by a continuous infusion at .05 mg/kg/day, started immediately.
The pharmacokinetic parameters calculated after continuous infusion of FK506 are similar to the values reported after shorter infusion.10,11 It takes more than 45 hours to reach a new steady state after any change in the dosing regimen. It is necessary to closely monitor the FK506 concentrations in patients, especially in the presence of abnormal liver function.
REFERENCES
- 1.Goto T, Kino T, Hatanaka H, Nishiyama M, Okuhara M, Kohsaka M, Aoki H, Imanaka H. Discovery of FK506, a novel immunosuppressant isolated from Streptomyces tsukubaensis. Transplant Proc. 1987;19(S-6):4–10. [PubMed] [Google Scholar]
- 2.Zeevi A, Duquesnoy R, Eiras G, Rabinowich H, Todo S, Makowka L, Starzl TE. Immunosuppressive effect of FK506 on in vitro lymphocyte alloactivation. Transplant Proc. 1987;19(S-6):40–44. [PMC free article] [PubMed] [Google Scholar]
- 3.Todo S, Fung JJ, Starzl TE. FK506: A potential breakthrough in immunosuppression. Transplant Proc. 1987;19(S-6) [PMC free article] [PubMed] [Google Scholar]
- 4.Todo S, Fund JJ, Starzl TE, Tzakis A, Demetris AJ, Kormos R, Iain A, Alessiani M, Takaya S. Liver, kidney, and thoracic organ transplantation under FK506. Ann Surg. 1990;212:295–307. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro R, Jordan M, Fung JJ, McCauley J, Johnson J, Iwaki J, Tzakis A, Hakala T, Todo S, Starzl TE. Kidney transplantation under FK506 immunosuppression. Transplant Proc. 1991;23:920–923. [PMC free article] [PubMed] [Google Scholar]
- 6.Armitage JM, Kormos RL, Griffith BP, Hardesty RL, Fricker FJ, Stuart RS, Marrone GC, Todo S, Fung JJ, Starzl TE. A clinical trial of FK506 as primary and rescue immunosuppression in cardiac transplantation. Transplant Proc. 1991;23:1149–1152. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK506 for human liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Abu-Elmagd K, Tzakis A, Fung JJ, Porter KA, Todo S. Selected topics on FK506. with special references to rescue of ex-trahepatic whole organ grafts, transplantation of “forbidden organs,” side effects, mechanisms, and practical pharmacokinetics. Transplant Proc. 1991;23:914–919. [PubMed] [Google Scholar]
- 9.Venkataramanan R, Jain A, Cadoff E, Warty V, Iwasaki K, Nagase K, Krajack A, Imventarza O, Todo S, Fung JJ, Starzl TE. Pharmacokinetics of FK506: Preclinical and clinical studies. Transplant Proc. 1990;22:52–56. [PMC free article] [PubMed] [Google Scholar]
- 10.Venkataramanan R, Jain A, Warty WV, Abu-Elmagd K, Furakawa H, Imventarza O, Fung J, Todo S, Starzl TE. Pharmacokinetics of FK506 following oral administration: A comparison of FK506and cyclosporine. Transplant Proc. 1991;23:931–933. [PMC free article] [PubMed] [Google Scholar]
- 11.Jain AB, Venkataramanan R, Cadoff E, Fung JJ, Todo S, Krajack A, Starzl TE. Effect of hepatic dysfunction and T tube clamping on FK506 pharmacokinetics and trough concentrations. Transplant Proc. 1990;22:57–59. [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura K, Kobayashi M, Hashimoto K, Kojima K, Nagase K, Iwasaki K, Kaizu T, Tanaka H, Niwa M. A highly sensitive method to assay FK506 levels in plasma. Transplant Proc. 1987;19(S-6):23–29. [PubMed] [Google Scholar]
- 13.Cadoff E, Venkataramanan R, Krajack A, Jain AS, Fung JJ, Todo S, Starzl TE. Assay of FK506 in plasma. Transplant Proc. 1990;22:50–51. [PMC free article] [PubMed] [Google Scholar]
- 14.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed Marcel Dekker; New York: 1985. [Google Scholar]
- 15.Venkataramanan R, Jain A, Warty VS, Abu-Elmagd K, Alessiani M, Lever J, Krajak A, Flowers J, Mehta S, Zuckerman S, Fung J, Todo S, Starzl TE. Pharmacokinetics of FK506 in Transplant Patients. Transplant Proc. 1991;23:2736–2740. [PMC free article] [PubMed] [Google Scholar]
- 16.Christians U, Kruse C, Kownatzki R, Schiebel HM, Schwinzer R, Sattler M, Schottman R, Linck A, Almeida VMF, Braun F, Sewing K-Fr. Measurement of FK506 by HPLC and isolation and characterization of its metabolites. Transplant Proc. 1991;23:940–941. [PubMed] [Google Scholar]
- 17.Abu-Elmagd K, Fung JJ, Alessiani M, Jain A, Venkataramanan R, Warty VS, Takaya S, Todo S, Shannon WD, Starzl TE. The effect of graft function on FK506 plasma levels, doses, and renal function: With particular reference to the liver. Transplantation. 1991;52:71–77. doi: 10.1097/00007890-199107000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]