Abstract
Objective
Given the fact that Mullerian Inhibiting Substance (MIS) causes complex remodeling of the urogenital ridge and regression of the Mullerian ducts during male embryonic development, we examined whether MIS could affect similar cell properties such as migration and invasion that could contribute ultimately to micro-metastasis of cancers arising from Mullerian tissues. MIS receptor expressing cell lines found to be invasive and migratory in vivo are examined in an in vivo assay that is cost effective.
Methods
We designed in vitro and in vivo experiments to determine if MIS inhibited the movement of cancer lines IGROV-1, HEp3, MDA-MB-231, and HT1080 in cell culture invasion/migration chamber assays and in chick embryo metastasis assays.
Results
. MIS, at concentrations below those that inhibit cell proliferation, blocked in vitro invasion and in vivo migration of epithelial cancer cells that express the MIS receptor.
Conclusions
While our laboratory has previously established MIS as an inhibitor of cancer cell proliferation using in vitro assays and in vivo xenografts, we now show that MIS can also inhibit in vivo tumor migration.
Keywords: Mullerian Inhibiting Substance, Anti-Mullerian hormone, Cell migration, Cell invasion
Introduction
For most cancers, recurrence and metastases are the characteristics largely responsible for patient mortality. Although many conventional chemo-therapeutics are known to target cell proliferation, their effects on other aspects of malignancies that contribute to their morbidity and mortality, such as migration, invasion, and metastases, are not as well studied. Developing therapeutics capable of both inhibiting tumor growth and preventing invasion, contributing to migration and metastases, could offer significant benefits.
Our laboratory has shown that Mullerian Inhibiting Substance (MIS, also known as Anti-Mullerian hormone) is an effective anticancer agent in vitro and in vivo both as a single agent and in combination with traditional chemotherapeutics for a variety of cancer types [1–4]. MIS is a member of the transforming growth factor-beta (TGF – β) family of proteins and is responsible for regression of the Mullerian duct in male embryos during development [5] involving a complex process of remodeling, apoptosis [6], and cell migration [5–9]. Given the profound effect that MIS has on tissue remodeling during normal embryonic ductal regression, we sought to examine whether, in addition to its effect on cancer cell proliferation, MIS could affect the invasion and migration of aggressive epithelial cancer cell lines, particularly those which express the MIS type II receptor (MIS RII) and show an inhibitory response to MIS in other assays [1–4]. The biological activity of MIS is receptor dependent and is initiated by binding to a heterodimeric complex of two very similar single membrane spanning serine/threonine kinase receptors known as types I and II. The ligand binding type II receptor was cloned in several species [10–13], and the human gene is located on chromosome 12q13 [14].
The MIS type II receptor is expressed in Mullerian duct mesenchymal cells surrounding the adjacent ductal epithelium [15], and mutations in the gene have consequences. For example, Persistent Mullerian Duct Syndrome (PMDS) can be caused by mutations in the MIS type II receptor or the MIS molecule itself (for a review, see Ref. 16). More recently, our group identified a splicing MIS type II receptor mutant that plays a role in this syndrome as well [17]. MIS RII is also expressed in fetal and adult Leydig and granulosa cells [10–12] where the ligand regulates testosterone [18–21], estradiol, and progesterone synthesis [22], respectively. Functional MIS type II receptor is in adult rodent uterus [12,23], human endometrium [23], breast [24,25], and prostate tissues [24] and, unexpectedly, in motor neurons in the mouse brain [26]. It is not yet understood what receptor mediated actions MIS has on these tissues in normal adults, but MIS inhibits the proliferation of MISRII expressing tumor samples and established cell lines derived from them. Importantly, between 50% and 70% of samples from over 300 human ovarian cancer cases express the Type II receptor as detected by immunohistochemistry, in situ hybridization, PCR, hormone binding, or growth inhibition assays [1,27,28]. In addition purified human MIS inhibited human ovarian carcinoma cell lines including OVCAR 3, 5, and 8, and IGROV-1 in vitro [1,29] and for IGROV-1, OVCAR3, and OVCAR8 in vivo [29,30]. Several different type I receptors have been identified as interacting with the type II receptor (Alk3, Alk2, and Alk6 [31–36]). How each type I receptor interacts with the type II receptor in human tissues is not yet known although all are expressed in the human cancer cell lines studied, thus far.
In the model systems employed in the present studies, invasion and migration are considered key elements contributing to metastasis.
Materials and Methods
Cell Culture
IGROV-1 and OVCAR-5 (ovarian epithelial carcinoma cell lines) were grown in DMEM (Invitrogen, Carlsbad, CA) with 1% penicillin/streptomycin/fungizone (Invitrogen, Carlsbad, CA; P/S/F), 1% L-glutamine and 5% fetal calf serum (FCS). HEp3 cells (epithelioid head and neck carcinoma) were grown in DMEM with 10% FCS and 1% P/S/F. MDA-MB-231 cells (breast epithelial carcinoma) were grown in alpha-MEM with 10% FCS and 1% P/S/F. HT1080 (highly metastatic fibrosarcoma), as a positive control for the in vitro invasion assays, and the non-metastatic cell line, WI-38 (embryonal fibroblasts) as a negative control for the in vivo migration assays, were grown in EMEM with 5% FCS and 1% P/S/F. All cells were incubated humidified at 37 °C and 5% CO2. Cells were passed using 1% EDTA-trypsin when approximately 80% confluent, a maximum of 5 times before fresh cultures were restarted.
Quantitative PCR
Primate Alu detection
Quantitative polymerase chain reaction was performed to detect the primate specific Alu sequences or MIS RII mRNA using a Cepheid SmartCycler (Cepheid, Sunnyvale, CA) with iTaq polymerase SYBR Green Supermix (BioRad, Hercules, CA). Complementary DNA was made using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) from RNA extracted from each cell line by Trizol (Invitrogen, Carlsbad, CA) reagent using manufacturer's protocol.
For the detection of repeat Alu sequences in the chick CAM assay (see below), 50 ng of DNA was assayed using primer sequences sense 5′; ACG CCT GTA ATC CCA GCA CTT 3′ and antisense 5′; TCG CCC AGG CTG GAG TGC A 3′. PCR conditions used were 95 °C for 120 s, then 40 cycles of 95 °C for 30 s, 62 °C for 30 s, and 72 °C for 45 s, with a 72 °C hold for 300 s.
Chick GAPDH (glyceraldehyde 3-phosphate dehydrogenase) primers of sense 5′; GAG GAA AGG TCG CCT GGT GGA TCG-3′ and antisense 5′; GGT GAG GAC AAG CAG TGA GGA ACG 3′ were run using the conditions described above as reagent control and an indicator of cDNA integrity, respectively. Negative controls for Alu sequence detection, which produced background signals for all reagents in the PCR reactions, included no-template reactions and the use of lung and CAM tissue from non-tumor bearing cell bearing embryos. Because Ct values are inversely related to mRNA levels, Ct values were subtracted from 40 (the maximum number of cycles for PCR runs) to obtain a value that increases with increasing mRNA levels and, thus, increased migration (e.g., a Ct of 5 converts to 35 and indicates more mRNA than a Ct of 35 whose corrected value is 5).
Human MISRII detection
For the detection of human MISRII mRNA in cell lines, 1 ug of cDNA was made from RNA; the primer sequences used were sense 5′; TCC CAA GGC CAA TAT AAA CC 3′ and antisense 5′; TTA TCC AGA GAA CTC ACT TCC A 3′ [1]. PCR conditions used were 95 °C for 120 s, then 40 cycles of 95 °C for 30 s, 56 °C for 60 s, and 72 °C for 60 s, followed by a hold for 300 s at 72 °C. The human GAPDH primers were 5′ TCA CCA GGG CTG CTT TTA AC 3′ and antisense: 5′ GAC AAG CTT CCC GTT CTC AG 3′ were run using the conditions described above. The resulting PCR products were run on a 1% agarose TAE (Tris-acetate-EDTA) gel.
Methylthiazoletetrazolium (MTT) growth inhibition assay
Cells were harvested at 80% confluency and plated in 96-well plates at 1000 cells/well in 200 uL media. After 24 hours, sets of 10 wells were treated with MIS or paclitaxel at increasing doses, or the buffer control. After three or seven days of incubation, the remaining living or surviving cells were quantified as a correlative measure of proliferation by adding methylthiazole-tetrazolium (MTT) reagent to the wells as described for MIS and paclitaxel by our group earlier [4]. Three- and seven day time points were chosen to mirror the incubation times for the in vitro invasion (3 days) and in vivo migration (7 days) assays (see below). The resulting formazan precipitate formed by the action of mitochondrial enzymes on MTT was dissolved in DMSO, and the plates were read reader and absorbance at 550 nm recorded. Data are presented as Percent Growth relative to control cultures ((test OD/control OD) × 100). Values less than 100% reflect fewer viable cells than the control. All of the cell types used in this study proliferated in control media during the incubation periods tested.
In vitro extracellular matrix invasion assay
In vitro cell invasiveness was assayed using an extracellular matrix (ECM) invasion assay kit (ECMatrix Cell Invasion Assay (catalog # ECM550, Millipore, Billerica, MA) according to the manufacturer's protocols. In brief, cells incubated for 1 hour in serum-free media were resuspended at 3 × 105 cells in 300 ul of serum-free media and plated on the top of an ECM coated membrane insert. The insert was then incubated for 72 hours in serum-containing media with 7 ug/ml MIS (50 nM), 1 nM paclitaxel, or an equal volume buffer control. Cells that invaded to the underside of the ECM membrane were quantified using the manufacturer provided cell dye and digital imaging software. Digital images of the migrated cells were analyzed using ImageJ (NIH, Bethesda, MD) software after red/green/blue color splitting. Using the green channel image, black-white thresholds were set from 0 to 100. Particle analysis was then performed using settings of circularity: 0 – 1 and size: 5 – 200 pixels.
Chick chorioallantoic membrane migration assay
The in vivo chick chorioallantoic membrane (CAM) migration assay (see Fig. 4A for an illustration) was performed generally as described in Zijlstra et al. [37] and as previously used in our laboratory [38] but adapted to study migration of cells after treatment with paclitaxel or MIS. Approximately 0.5 to 1.0 ml of air was removed by syringe from E7 fertilized chick egg natural air sack. A 10 mm window was then made through the shell on the top of the egg, and the intact anterior CAM was then gently pushed from the underlying soft shell with a cotton-tipped applicator. IGROV-1 ovarian cancer cells, which were incubated for 2 hours in 7 ug/ml (50 nM) of MIS, or paclitaxel (1 nM), or equal volume buffer control, were inoculated on the exposed CAM at 5 × 105 cells in 25 ul of media. The non-metastatic embryonic fibroblast cell line WI-38 was used as a negative control, and the HT1080 highly metastatic fibrosarcoma cell line was used as a positive control. After 7 days of incubation, the chick embryos were dissected with collection of the posterior CAM, away from the site of original implantation, and the chick lungs and liver. Genomic DNA was extracted from each of the dissected tissues using the Gentra DNA extraction kit (Qiagen, Valencia, CA) according to manufacturer's protocols. Quantitative polymerase chain reaction (q-PCR) was then used to detect and measure human Alu sequences. Relative migration was expressed in terms of the number of cycle counts (Ct) required to reach an arbitrary optical threshold, reflecting the flat portion of the curve, which was then subtracted from the maximum number of cycles (relative migration = 40 – Ct; see above). We chose to use molecular methods to detect cells that have moved after only seven days from the anterior CAM to the lungs before the cells could form a significant tumor mass that could be dissected and examined by histological methods.
Fig. 4.
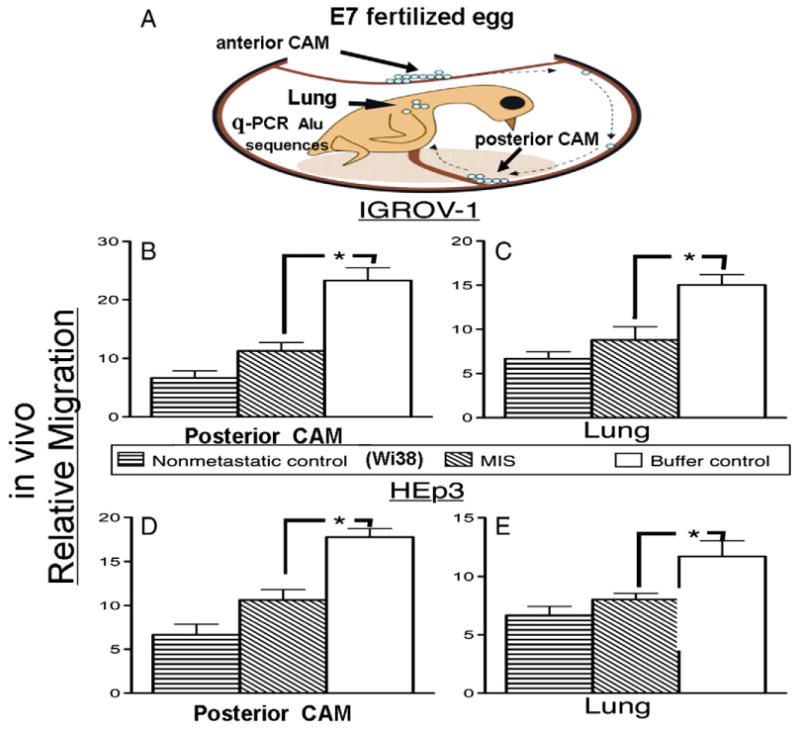
Chick chorioallantoic membrane (CAM) assay for cell migration (A). Pretreatment with 7 ug/ml (50 nM) MIS of IGROV-1 (B, C) and HEp3 cells (D, E) significantly reduces relative migration to the posterior CAM (B, D) and lung (C, E) when compared to buffer controls ([B] 11.44 ± 1.43, n = 5 vs. 23.32 ± 2.19, n = 6, p < 0.002; [C] 8.87 ± 1.58, n = 4 vs. 15.04 ± 1.15, n = 6, p < 0.02; [D] 10.62 ± 1.18, n = 8 vs. 17.76 ± 0.97, n = 5, p < 0.002; [E] 8.04 ± 0.52, n = 9 vs. 11.49 ± 1.37, n = 11, p < 0.045). Relative migration levels were not statically different from the non-metastatic negative control cell line WI-38. Longer bars indicate more migration. Error bars represent the standard error of the mean. Asterisks indicate p < 0.05. Ns = not significant.
Results
MISRII is detected by quantitative PCR in the epithelial cell lines tested
MISRII was detected by quantitative PCR in the epithelial cancer cell lines IGROV-1 (ovarian), MDA-MB-231 (breast), and HEp3 (head and neck), but not in fibrosarcoma cell line HT1080. IGROV-1 showed the highest relative expression of MISRII, with MDA-MB-231 showing moderate, HEp3 showing less, and with HT1080 showing no expression (Fig. 1A). Electrophoretic gel examination of the PCR products confirms an appropriate band of the predicted length of approximately 200 bp for MISRII (Fig. 1B). GAPDH was detected in all samples attesting to the integrity of the DNA.
Fig. 1.
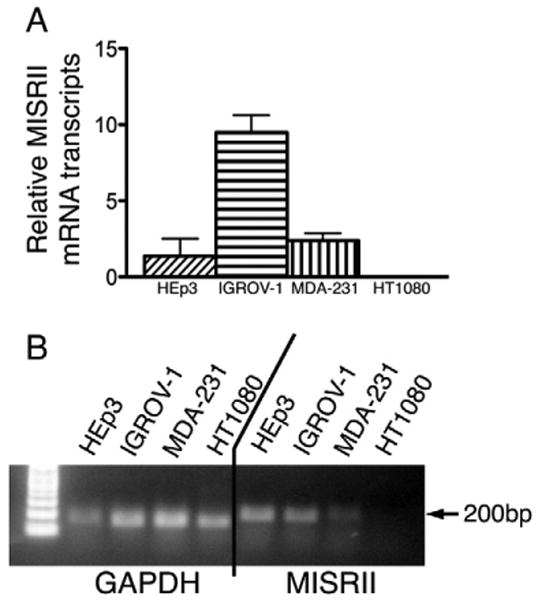
The presence of the MISRII is detected by quantitative PCR. (A) The relative amount of mRNA transcript (Ct) for MISRII expression for each cell line is shown. (B) Agarose gel of the PCR products confirms the appropriate length bands for both GAPDH and MISRII.
Growth inhibitory concentrations for MIS and paclitaxel determined by MTT
In order to ensure that any inhibition of invasion and migration by MIS is distinct from its ability to inhibit cell proliferation, dose-response curves were determined using MTT growth inhibition assays. Dose responses of MIS ranging 0 to 60 ug/ml (0 to 420 nM) and paclitaxel from 0 to 8 nM were performed on four cell lines (MDA-MB-231, HEp3, IGROV-1, and HT1080) at 3 and 7 days. MIS did not inhibit HT1080 at any dose or time tested and only nominally inhibited HEp3 at 60 ug/ml after 7 days (Figs. 2A and B). MDA-MB-231 and IGROV-1 were significantly inhibited by this preparation of MIS, but only at concentrations above 20 ug/ml (140 nM), regardless of the time of incubation (Figs. 2A and B).
Fig. 2.
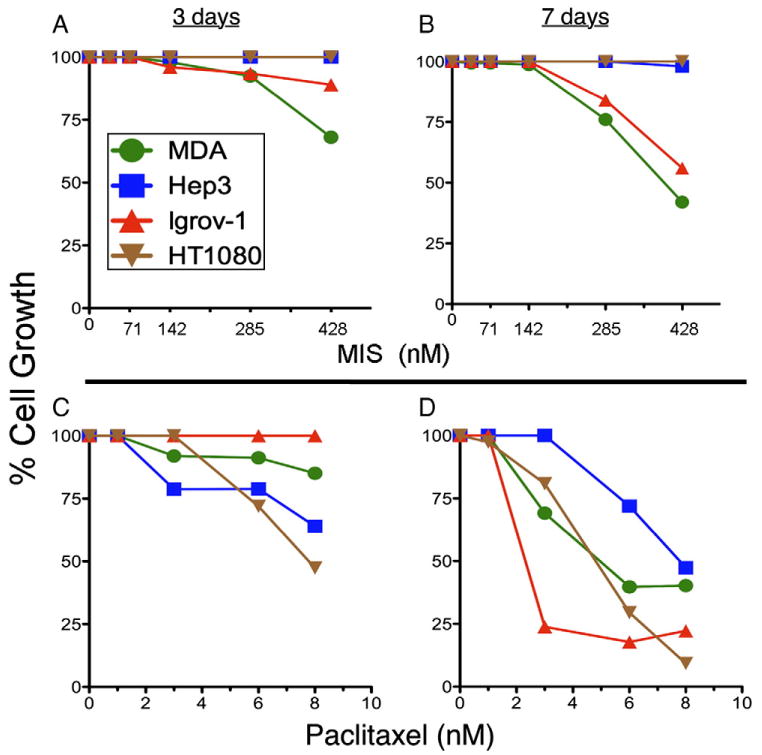
MIS dose responses for inhibition of cell proliferation (% Cell Growth) at concentrations between 0 and 60 ug/ml for MDA-MB-231, HEp3, IGROV-1, and HT1080 cells at either (A) 3 days or (B) 7 days. Paclitaxel cell proliferation dose responses between 0 and 8 nM are also shown for the same cell lines at 3 (C) and 7 (D) days of incubation.
With the exception of IGROV-1 cells at 3 days of incubation (no inhibition, Fig. 2C), and HEp3 cells at 7 days (only above 4 nM, Fig. 2D), paclitaxel significantly inhibited all cell lines at doses above 2 nM and for either length of exposure (Figs. 2C and D). Based on these results, concentrations well below those showing significant growth inhibition, namely, 7 ug/ml (50 nM) for MIS and 1 nM for paclitaxel, were chosen for invasion and migration assays to ensure that differences in cell invasion assays due to these agents would not be confounded by inhibition of cell proliferation.
MIS reduces invasiveness of epithelial cancer cell lines in vitro
To evaluate the ability of MIS to inhibit cell invasion of IGROV-1, MDA-MB-231, HEp3, and HT1080, each cell line was tested in a Matrigel dual-chamber invasion assay in the presence of 50 nM MIS, a dose below that required to inhibit cell proliferation, or an equal volume buffer control. While there was significant inhibition of cell invasion by MIS when compared to buffer controls in the epithelial cancer cell lines, IGROV-1 (Fig. 3A, 60.50 ± 8.9, n = 8 vs. 371.1 ± 70.9, n = 7, p < 0.0006), MDA-MB-231 (Fig. 3B, 134.8 ± 33.5, n = 8 vs. 640.7 ± 117.4, n = 9, p < 0.0015), and HEp3 (Fig. 3C, 114.4 ± 18.2, n = 8 vs. 406.4 ± 44.6 n = 10, p < 0.0001), MIS had no effect on the fibrosarcoma-derived HT 1080 (Fig. 3D, 1282 ± 91.7 n = 6 vs. 1171 ± 140.1, n = 9, p = 0.57) which does not express MISRII.
Fig. 3.
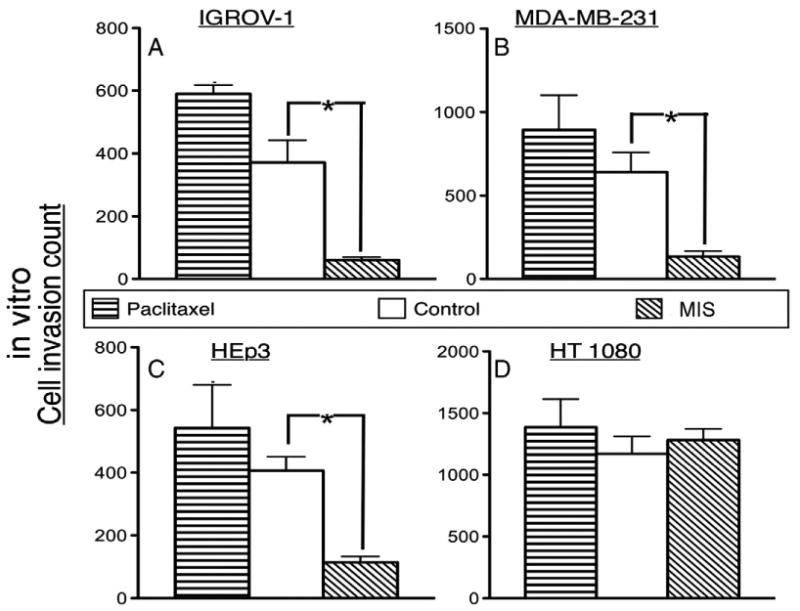
50nM MIS inhibits the invasion in vitro (Cell invasion count) of the epithelial cell lines IGROV-1 (A, 60.50 ± 8.99, n = 8 vs. control 371.1 ± 70.99, n = 7, p < 0.0006), MDA-MB-231 (B, 134.8 ± 33.51, n = 8 vs. control 640.7 ± 117.4, n = 9, p < 0.0015), and HEp3 (C, 114.4 ± 18.24, n = 8 vs. control 406.4 ± 44.64, n = 10, p < 0.0001), but not the fibrosarcoma cell line HT 1080 (D, 1282 ± 91.67 n = 6 vs. control 1171 ± 140.1, n = 9, p = 0.57). Longer bars indicate more invasion. 1nM paclitaxel did not inhibit invasion. Error bars show the standard error of the mean. Asterisks indicate treatment outcomes significantly different from controls (p < 0.05).
To ensure that the inhibition of invasion was specific to MIS, we performed the invasion assay in the presence of sub-therapeutic levels of the chemotherapeutic paclitaxel (1 nM). There was no significant difference in cell invasion with the paclitaxel when compared to buffer control for IGROV-1 (Fig. 3A, control 371.1 ± 70.99, n = 7, paclitaxel 589.7 ± 28.1, n = 3, p = 0.09 vs. control), MDA-MB-231 (Fig. 3B, control 640.7 ± 117.4, n = 9, paclitaxel 892.5 ± 207.5, n = 3, p = 0.38 vs. control), HEp3 (Fig. 3C, control 406.4 ± 44.6, n = 10, paclitaxel 543.3 ± 137.2, n = 3, p = 0.23 vs. control), and HT 1080 (Fig. 3D, control 1171 ± 140.1, n = 9, paclitaxel 1386 ± 228.4, n = 3, p = 0.45 vs. control). These results suggest that MIS inhibits the invasion of epithelial cancer cells expressing MISRII while having no effect on the mesenchyme-derived fibrosarcoma without MISRII expression.
MIS inhibits migration of IGROV-1 and HEp3 cells in vivo
The ability of MIS to inhibit migration in vivo was further explored in vivo by using a unique application of the chick CAM metastasis assay. Of the four cell lines tested in the in vitro experiments (Fig. 2), only IGROV-1 and HEp3 showed spontaneous invasion and migration in the chick CAM assay (data not shown); these two cell lines were, therefore, chosen for further evaluation. The non-metastatic embryonic fibroblast cell line WI-38 was used as a negative control for migration [39]. After treatment for 2 hours with 7 ug/ml (50 nM) of MIS, migration of IGROV-1 to the posterior CAM (Fig. 4B) was significantly less than that observed after treatment with buffer (MIS 11.44 ± 1.4, n = 5, vs. buffer control 23.32 ± 2.2, n = 6, p < 0.002) and were not significantly different from the non-metastatic control (MIS 11.44 ± 1.4, n = 5, vs. non-metastatic control, WI-38, 6.67 ± 1.2, n = 3, p = 0.51). MIS treatment of IGROV-1 also significantly reduced migration to the chick lung compared to that observed after treatment with buffer (Fig. 4C, MIS 8.87 ± 1.6, n = 4 vs. buffer control 15.04 ± 1.2, n = 6, p < 0.02) to levels comparable to the non-metastatic control (WI-38) (Fig. 4C, MIS 8.87 ± 1.6, n = 4 vs. the non-metastatic control 6.68 ± 0.8, n = 3, p = 0.26). MIS also inhibited HEp3 migration to the CAM as compared to buffer controls (Fig. 4D, MIS 10.62 ± 1.2, n = 8 vs. buffer control 17.76 ± 0.9, n = 5, p < 0.002) and resulted in levels of HEp3 migration comparable to the non-metastatic control cell line (WI-38) (Fig. 4D MIS 10.62 ± 1.2, n = 8 vs. non-metastatic control 6.67 ± 1.2, n = 3, p = 0.093). MIS-treated HEp3 cells also showed reduced migration to the chick lung compared to buffer control (Fig. 4E, MIS 8.04 ± 0.5, n = 9 vs. buffer control 11.49 ± 1.4 n = 11, p < 0.045), resulting in levels similar to non-metastatic control (Fig. 4E, MIS 8.04 ± 0.5, n = 9 vs. non-metastatic control 6.68 ± 0.7, n = 4, p = 0.17).
Discussion
In the present study, we show for the first time that MIS inhibits invasion in vitro and spontaneous migration to lung or posterior CAM in vivo of aggressive and invasive cancer cell lines which express MISRII and that this inhibition can occur at doses below those required to cause growth inhibition. The specificity of the response to MIS was confirmed by showing that paclitaxel, a treatment for patients with ovarian cancer, has no such effect. The time of exposure required for MIS to initiate inhibition of the Mullerian duct in regression assays is 12 to 24 hours [39]; the exposure time required for MIS to inhibit cell survival in MTT assays has been measured as 1 hour (Pieretti-Vanmarcke, MacLaughlin, and Donahoe, unpublished data). Therefore, the 2-hour exposure used in current experiments is appropriate. Incubating the cells before placing them on the allantoic membrane makes systemic treatment of the chick egg unnecessary. Selecting a dose of MIS that does not inhibit cell proliferation allows the results to be interpreted in terms of blocking moving of cells, i.e., migration, rather than reducing the number of cells available to move.
MIS, a member of the TGF- b family of growth factors, is best known for causing Mullerian duct regression in developing male embryos [5]. Like other members of the TGB-β family, MIS functions by binding to its specific type II receptor (MISRII) and activating type I receptors Alk-2, Alk-3, or Alk6 [34,36,40], which are also employed by the bone morphogenesis protein (BMP) Type II receptor [41], to initiate its downstream signaling activity [5]. Though MISRII is specific for MIS the type I receptors implicated in MIS signaling are more ubiquitous. Alk2 is also responsible for selected downstream signaling for TGF – β [32]. While knowledge of the downstream genes regulated by MIS is incomplete, our laboratory showed that signaling is mediated by phosphorylation of Smads 1, 5, and 8, with upregulation of total Smad 8 and downregulation of Smad 5 [8]. Whether the Smads direct the inhibition of metastasis has yet to be determined.
The ability of MIS to affect cell movement of MIS expressing and responsive tumor cells is also predicted by its normal developmental role in causing Mullerian duct regression. However, in the embryo, MIS appears to enhance migration of the coelomic and Mullerian duct epithelium and to induce migration of mesonephric cells into the female embryonic gonad [42]. The process of Mullerian duct regression initiated by MIS involves a complex combination of apoptosis, migration, and mesenchymal transition of ductal epithelial cells [5–9]. Most recently, our laboratory reported that early formation of the Mullerian ducts prior to regression requires a combination of a series of independent processes including cell proliferation and migration [9]. Thereafter, MIS causes migration of Mullerian duct epithelial cells, which do not express MISRII before their transition to mesenchyme, and Mullerian duct mesenchyme that does express MISRII, to the mesonephros [9]. In the present study, we show that MIS affected the migration of the MISRII tumor expressing cells, indicating that the MIS effect on invasion and migration of cancer cells was correlated with MIS RII expression, indicating that differences exist in the context of the cancer cell compared to the normal embryonic Mullerian duct epithelium.
While unexpected, the detection of MISRII in the head and neck cancer cell line HEp3 may explain its response to MIS in migration assays. Despite expression of MISRII, HEp3 did not show growth inhibition by MIS treatment for 3-days (Fig. 3A) or 7-days (Fig. 3B). Quantitative PCR, however, revealed that HEp3 expressed low levels of MISRII, suggesting that doses well above those required to inhibit migration may be needed to illicit a growth inhibitory response to MIS.
Our laboratory has previously shown that MIS is effective in inhibiting the growth of ovarian [1,4] and breast [2] cancer cell lines in vivo and in vitro, and prostate [3] cancer cells in vitro. We have also shown that MIS, as an adjuvant, reduces the dose of conventional chemotherapeutics needed to inhibit survival of ovarian cancer cell lines in MTT assays [4] and that MIS is effective against subpopulations of ovarian cancer cell lines [43] enriched for stem/progenitor cell properties of invasion and growth in vivo [44]. These ovarian cancer subpopulations are resistant to conventional chemotherapeutics [43,44].
Though, as stated above, it is not surprising that MIS inhibits cell migration, this study presents experimental evidence that, as an anti-cancer agent, MIS may be as effective an anti-metastatic agent as it is an anti-proliferation agent. This ability to target more than one aspect of cancer biology is predicted to make MIS an advantageous anticancer agent in the clinical setting.
Acknowledgments
Funding Sources: H.L.C. was supported by NIH/MGH T32 in Cancer Biology T32CA071345-11 and was the recipient of the Judah Folkman Award of the American Pediatric Surgical Association for this work. X.L. received the Hoopes Prize from Harvard University for work contributing to this manuscript; she was funded by the Scott Foundation. D.T.M. was supported by NIH CA17393, the Ovarian Cancer Research Fund, the McBride Family Fund, and the Commons Development Group, Inc. P.K.D. was funded by a Brigham and Women's SPORE Grant 5P50CA105009-03, a Harvard Stem Cell Institute Grant DP-0010-07-00, the NIH/NCI Grant 5R01CA017393, the McBride Family Fund, and the Gerald Austen Fund.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- 1.Masiakos PT, MacLaughlin DT, Maheswaran S, Teixeira J, Fuller AF, Jr, Shah PC, et al. Human ovarian cancer, cell lines, and primary ascites cells express the human Mullerian Inhibiting Substance (MIS) type II receptor, bind, and are responsive to MIS. Clin Cancer Res. 1999;7:3488–99. [PubMed] [Google Scholar]
- 2.Segev DL, Ha TU, Tran TT, Kennealy M, Harkin P, Jung M, et al. Mullerian Inhibiting Substance inhibits breast cancer cell growth through an NFkappa B-mediated pathway. J Biol Chem. 2000;275:28371–9. doi: 10.1074/jbc.M004554200. [DOI] [PubMed] [Google Scholar]
- 3.Segev DL, Hoshiya Y, Hoshiya M, Tran TT, Carey JL, Stephen AE, et al. Mullerian Inhibiting Substance regulates NF-kappa B signaling in the prostate in vitro and in vivo. Proc Natl Acad Sci. 2002;99:239–44. doi: 10.1073/pnas.221599298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pieretti-Vanmarcke R, Donahoe PK, Pearsall LA, Dinulescu DM, Connelly DC, Halpern EF, et al. Mullerian Inhibiting Substance enhances subclinical doses of chemotherapeutic agents to inhibit human and mouse ovarian cancer. Proc Natl Acad Sci. 2006;103:17426–31. doi: 10.1073/pnas.0607959103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teixeira J, Maheswaran S, Donahoe PK. Mullerian Inhibiting Substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001;22:657–74. doi: 10.1210/edrv.22.5.0445. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi A, Donahoe PK, Budzik GP, Trelstad RL. Periductal and matrix glycosaminoglycans in rat Mullerian duct development and regression. Dev Biol. 1982;92:16–26. doi: 10.1016/0012-1606(82)90146-4. [DOI] [PubMed] [Google Scholar]
- 7.Austin HB. DiI analysis of cell migration during Mullerian duct regression. Dev Biol. 1995;169:29–36. doi: 10.1006/dbio.1995.1123. [DOI] [PubMed] [Google Scholar]
- 8.Zhan Y, Fujino A, MacLaughlin DT, Manganaro TF, Szotek PP, Arango NA, et al. Mullerian Inhibiting Substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Mullerian duct regression. Development. 2006;133:2359–69. doi: 10.1242/dev.02383. [DOI] [PubMed] [Google Scholar]
- 9.Fujino A, Arango NA, Zhan Y, Manganaro TF, Li X, MacLaughlin DT, et al. Cell migration and activated PI3K/AKT-directed elongation in the developing rat Müllerian duct. Dev Biol. 2009 Jan 15;325(2):351–62. doi: 10.1016/j.ydbio.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baarends WM, van Helmond JM, Post M, van der School PJ, Hoogerbrugge JW, de Winter JP, et al. A Novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the Mullerian duct. Development. 1994;120:189–97. doi: 10.1242/dev.120.1.189. [DOI] [PubMed] [Google Scholar]
- 11.DiClemente N, Wilson C, Faure E, Boussin L, Carmillo P, Tizard R, et al. Cloning, expression, and alternative splicing of the receptor for anit-Mullerian hormone. Mol Endocrinol. 1994;8:1006–20. doi: 10.1210/mend.8.8.7997230. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira J, He WW, Shah PC, Morikawa N, Lee MM, Catlin EA, et al. Developmental expression of a candidate Mullerian Inhibiting Substance type II receptor. Endocrinology. 1996;137:160–5. doi: 10.1210/endo.137.1.8536608. [DOI] [PubMed] [Google Scholar]
- 13.Mishina Y, Tizard R, Deng JM, Pathak BG, Copeland NG, Jenkins NA, et al. Sequence, genomic organization and chromosomal location of the mouse Mullerian Inhibiting substance type II receptor gene. Biochem Res Commun. 1997;237:741–6. doi: 10.1006/bbrc.1997.7224. [DOI] [PubMed] [Google Scholar]
- 14.Imbeaud S, Faure E, Lamarre I, Mattei MG, di Clemente N, Tizard R, et al. Insensitivity to anti-mullerian hormone due to a mutation in the human anti-mullerian hormone receptor. Nat Genet. 1995;11:382–8. doi: 10.1038/ng1295-382. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji M, Shima H, Yonemura CY, Brody J, Donahoe PK, Cunha GR. Effect of human recombinant Mullerian inhibiting substance on isolated epithelial and mesenchymal cells during mullerian duct regression in the rat. Endocrinology. 1992;131:1481–8. doi: 10.1210/endo.131.3.1505479. [DOI] [PubMed] [Google Scholar]
- 16.Josso N, Belville C, di Clemente N, Picard YP. AMH and AMH receptor defects in persistent Mullerian duct syndrome. Hum Reprod Update. 2005;11:351–6. doi: 10.1093/humupd/dmi014. Review. [DOI] [PubMed] [Google Scholar]
- 17.Hoshiya M, Christian B, Cromie W, Zhan Y, MacLaughlin DT, Donahoe PK. Persistent Mullerian duct syndrome with a deletion and a novel splicing Mutation in the MIS type II receptor. Birth Defects Res. 2003;67:868–74. doi: 10.1002/bdra.10091. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira J, Fynn-Thompson E, Payne A, Donahoe PK. Müllerian-inhibiting substance regulates androgen synthesis at the transcriptional level. Endocrinology. 1999;140:4732–8. doi: 10.1210/endo.140.10.7075. [DOI] [PubMed] [Google Scholar]
- 19.Sriraman V, Niu E, Matias JR, Donahoe PK, MacLaughlin DT, Hardy MP, et al. Müllerian inhibiting substance inhibits testosterone synthesis in adult rats. J Androl. 2001;22:750–8. [PubMed] [Google Scholar]
- 20.Trbovich AM, Sluss PM, Laurich VM, O'Neill FH, MacLaughlin DT, Donahoe PK, et al. Mullerian Inhibiting Substance lowers testosterone in luteinizing hormone-stimulated rodents. Proc Natl Acad Sci USA. 2001;98:3393–7. doi: 10.1073/pnas.051632298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MM, Seah CC, Masiakos PT, Sottas CM, Preffer F, Donahoe PK, et al. Mullerian Inhibiting Substance type II receptor expression and function in purified rat Leydig cells. Endocrinology. 1999;140:2819–27. doi: 10.1210/endo.140.6.6786. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Seibel MM, MacLaughlin DT, Donahoe PK, Ransil BJ, Hametz PA, et al. The inhibitory effects of Müllerian Inhibiting Substance on epidermal growth factor induced proliferation and progesterone production of human granulosa-luteal cells. J Clin Endocrinol Metab. 1992;75:911–7. doi: 10.1210/jcem.75.3.1517385. [DOI] [PubMed] [Google Scholar]
- 23.Renaud EJ, MacLaughlin DT, Oliva E, Rueda BR, Donahoe PK. Endometrial Cancer is a receptor Mediated Target for Mullerian Inhibiting Substance. Proc Natl Acad Sci USA. 2005;102:111–6. doi: 10.1073/pnas.0407772101. Erratum in: Proc Natl Acad Sci USA 2005;102:6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoshiya Y, Gupta V, Segev DL, Hoshiya M, Carey JL, Sasur LM, et al. Mullerian Substance induces NFkB signaling in breast and prostate cancer cells. Mol Cell Endocrinol. 2003;211:43–9. doi: 10.1016/j.mce.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Gupta V, Carey JL, Kawakubo H, Muzikansky A, Green JE, Donahoe PK, et al. Mullerian inhibiting substance suppresses tumor growth in the C3(1)T antigen transgenic mouse mammary carcinoma model. Proc Natl Acad Sci USA. 2005;102:3219–24. doi: 10.1073/pnas.0409709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang PY, Koishi K, McGeachie AB, Kimber M, MacLaughlin DT, Donahoe PK, et al. Mullerian Inhibiting Substance acts as a motor neuron survival factor in vitro. Proc Natl Acad Sci USA. 2005;102:16421–5. doi: 10.1073/pnas.0508304102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakkum-Gamez JN, Aletti G, Lewis KA, Keeney GL, Thomas BM, Navarro-Teulon I, et al. Müllerian Inhibiting substance type II receptor (MISIIR): a novel, tissue-specific target expressed by gynecologic cancers. Gynecol Oncol. 2008 Jan;108(1):141–8. doi: 10.1016/j.ygyno.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Song JY, Chen KY, Kim SY, Kim MR, Ryu KS, Cha JH, et al. The expression of Müllerian Inhibiting substance/anti-Müllerian hormone type II Receptor protein and mRNA in benign, borderline and malignant ovarian neoplasia. Int J Oncol. 2009 Jun;34(6):1583–91. doi: 10.3892/ijo_00000288. [DOI] [PubMed] [Google Scholar]
- 29.Chin T, Parry RL, Donahoe PK. Human Müllerian Inhibiting Substance inhibits tumor growth in vitro and in vivo. Can Res. 1991;51:2101–6. [PubMed] [Google Scholar]
- 30.Stephen AE, Pearsall LA, Christian BP, Donahoe PK, Vacanti JP, MacLaughlin DT. Highly purified Mullerian Inhibiting Substance inhibits ovarian cancer in vivo. Clin Can Res. 2002;8:2640–6. [PubMed] [Google Scholar]
- 31.He WW, Gustafson ML, Hirobe S, Donahoe PK. The developmental expression of four novel serine/threonine kinase receptors homologous to the activin/TGF-b II receptor family. Dev Dyn. 1993;196:133–42. doi: 10.1002/aja.1001960207. [DOI] [PubMed] [Google Scholar]
- 32.Gu Z, Reynolds EM, Song J, Lei H, Yu L, He W, et al. The Type I Serine/threonine Kinase Receptor ActRIA (ALK 2) is required for Gastrulation of the mouse Embryo. Development. 1999;26:2551–61. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- 33.Gouedard L, Chen YG, Thevenet L, Racine C, Borie S, Lamarre I, et al. Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Mullerian hormone and its type II receptor. J Biol Chem. 2000;275:27973–78. doi: 10.1074/jbc.M002704200. [DOI] [PubMed] [Google Scholar]
- 34.Visser JA, Olaso R, Verhoef-Post M, Kramer P, Themmen APN, Ingraham HA. The serine/threonine transmembrane receptor ALK2 mediates Mullerian Inhibiting Substance signaling. Mol Endocrinol. 2001;15:936–45. doi: 10.1210/mend.15.6.0645. [DOI] [PubMed] [Google Scholar]
- 35.Jamin S, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of BMPR1a for Mullerian duct Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, and Donahoe PK. Müllerian Inhibiting Substance Signaling uses a BMP-like pathway mediated by ALK2 and induces Smad6 expression. Mol Endocrinol. 2001;15:946–95. doi: 10.1210/mend.15.6.0664. [DOI] [PubMed] [Google Scholar]
- 36.Clarke TR, Hoshiya Y, Yi SE, Liu K, Lyons KM, Donahoe PK. Mullerian Inhibiting Substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol Endocrinol. 2001;15:946–59. doi: 10.1210/mend.15.6.0664. [DOI] [PubMed] [Google Scholar]
- 37.Zijlstra A, Mellor R, Panzarella G, Aimes RT, Hooper JD, Marchenko ND, et al. A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Res. 2002;62:7083–92. [PubMed] [Google Scholar]
- 38.Loscertales M, Mikels A, Kuang-Hsein HuJ, Donahoe PK, Roberts DJ. Chick pulmonary Wnt5a directs airway and vascular tubulogenesis. Development. 2008;135(7):1365–76. doi: 10.1242/dev.010504. [DOI] [PubMed] [Google Scholar]
- 39.Donahoe PK, Ito Y, Hendren WH., 3rd A graded organ culture assay for the detection of Mullerian inhibiting substance. J Surg Res. 1977;23(2):141–8. doi: 10.1016/0022-4804(77)90202-5. [DOI] [PubMed] [Google Scholar]
- 40.Gouédard L, Chen Y, Thevenet L, Racine C, Borie S, Lamarre I, et al. Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Müllerian hormone and its type II receptor. J Biol Chem. 2000;275:27973–8. doi: 10.1074/jbc.M002704200. [DOI] [PubMed] [Google Scholar]
- 41.Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci STKE. 2002:pe40. doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- 42.Ross AJ, Tilman C, Yao H, MacLaughlin DT, Capel B. AMH induces mesonephric cell migration in XX gonads. Mol Cell Endocrinol. 2003;211:1–7. doi: 10.1016/j.mce.2003.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci. 2006;103:11154–9. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei X, Dombkowski David, Meirelles K, Pieretti-Vanmarcke R, Szotek PP, Chang HL, et al. Mullerian Inhibiting Substance preferentially inhibits stem/cell progenitors in human ovarian cancer cell lines compared to chemotherapeutic agents. PNAS. doi: 10.1073/pnas.1012667107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


