Abstract
An intramural hematoma is an accumulation of blood between the internal and external elastic membranes within the medial space, whereas an extramural hematoma is a dilution and/or dissemination of blood throughout the adventitia. Intra- and extra-hematomas are observed by intravascular ultrasound during percutaneous coronary intervention (PCI). The patient described herein presented with angina pectoris. Her coronary angiogram showed diffuse narrowing of the mid-left anterior descending artery and total occlusion of the distal right coronary artery (RCA). Intra- and extra-mural hematomas developed during PCI of the RCA; however, the lesions were covered successfully using long drug-eluting stents.
Keywords: Hematoma; Ultrasonography, interventional
Introduction
Intra- and extra-mural hematomas are known complications after percutaneous coronary intervention (PCI).1-4) Based on an intravascular ultrasound (IVUS) examination, an intramural hematoma represents an accumulation of blood between the internal (IEM) and external elastic membranes (EEM) within the medial space, whereas dilution and/or dissemination of blood throughout the adventitia are observed in extramural hematomas.5),6) Although one-third of patients with chronic total occlusion (CTO) have intramural hematomas during successful recanalization,7) no cases of combined intramural and extramural hematomas have been reported. We report a case of combined intramural and extramural hematomas during successful recanalization of a CTO lesion demonstrated by IVUS, which was treated with long drug-eluting stents (DESs).
Case
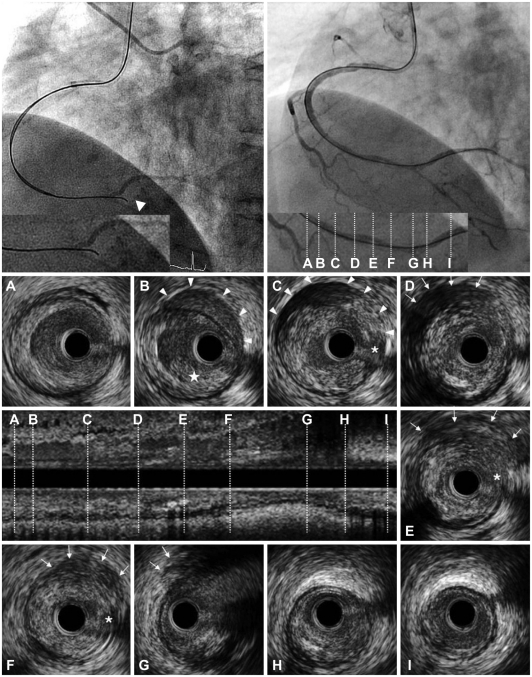
A 51-year-old woman was admitted with effort-induced chest pain. She had hypertension, diabetes, and hypercholesterolemia for 7 years. The physical examination and laboratory findings showed no abnormalities. A baseline electrocardiogram showed left ventricular hypertrophy by criteria and inverted T waves in the inferior leads and leads V4-V6. Gated myocardial scintigraphy demonstrated reversible perfusion defects in the anterior and inferior myocardial walls. An echocardiogram showed no regional wall motion abnormalities with preservation of the left ventricular ejection fraction. A 64 multi-slice computerized tomography showed significant stenosis in the mid-portion of the left anterior descending artery (LAD) and total occlusion in the distal portion of the right coronary artery (RCA). Similarly, a diagnostic coronary angiogram revealed diffuse narrowing of the mid-LAD (70%) and total occlusion of the distal RCA. There was grade 2 collateral flow from the LAD to the RCA via the septal branch (Fig. 1). The patient was initially scheduled to undergo PCI for recanalization of the RCA. The right coronary ostium was engaged with a 7-Fr Amplatz 4.0 guiding catheter and the left coronary ostium was cannulated with a 5-Fr Judkin catheter for the contralateral angiogram. With the support of a 1.5-mm over-the-wire balloon system (Boston Scientific, Natick, MA, USA), a PT ll (Boston Scientific) 0.014-inch guidewire was advanced. After failing the first guidewire passage, a Miracle 3 g, 6 g, and 12 g guidewires (Asahi, Seto, Japan) were sequentially attempted, but could not be passed into the distal true lumen. Therefore, a parallel wire technique using Miracle 12 g and Conquest Pro guidewires (Asahi) was attempted. By this parallel wire technique, a Conquest Pro guidewire was advanced to the totally occluded lesion. Following the angiogram, however, the tip of the Conqest Pro guidewire was shown to be placed outside of the vessel beyond the distal RCA bifurcation without extravasation of contrast media (Fig. 2). After repeated attempts to manipulate the guidewire, the Conquest Pro guidewire successfully entered the true lumen of the posterolateral (PL) branch and pre-dilatation with a Voyager balloon (2.5×20 mm; Abbott, Santa Clara, CA, USA) was performed. At this point, IVUS (Atlantis SR Pro 40 MHz; Boston Scientific) was performed to obtain vessel information as well as identification of PCI complications. The qualitative IVUS finding showed the following: 1) a hypo-echogenic, inhomogeneous, lobulated mass within the lumen in the distal RCA, suggesting an intraluminal thrombi (Fig. 2A and B), 2) a crescent-shaped, hypo-echogenic, accumulation of contrast media with displacement of the IEM into the lumen in the distal RCA, suggesting an intramural hematoma (Fig. 2B and C), 3) an eccentric inhomogeneous echogenecity, consistent with accumulated blood, in the proximal portion of the PL branch to the distal bifurcation site, and a probable communication site between the lumen and adventitia, suggesting an extramural hematoma (Fig. 2D-G), and 4) an intimal dissection to the media from 3 to 6 o'clock in the mid-portion of the PL branch (Fig. 2H). A quantitative IVUS measurement showed that a total lesion length from the PL branch to the mid-RCA was 50 mm and the distal reference diameter was 2.8 mm. For entire coverage of the occluded lesion, as well as intra- and extra-mural hematomas, 2 Taxus stents (3.0×28 mm and 2.75×28 mm; Boston Scientific) were implanted with an overlapping technique. The final coronary angiogram demonstrated no residual lumen narrowing with thrombolysis in myocardial infarction 3 flow (Fig. 3). A post-stenting IVUS revealed well-opposed stent struts to the vessel wall and a 4.66 mm2 minimal stent area (MSA) (Fig. 3C). Because the MSA was located at the distal bifurcation site with co-existing intra- and extra-mural hematomas and there was a risk of coronary rupture, no further intervention, such as adjunctive balloon dilatation, was performed. The next day, 2 additional Taxus stents (3.0×28 mm and 2.75×28 mm) were deployed with an overlapping technique for the mid-LAD lesion.
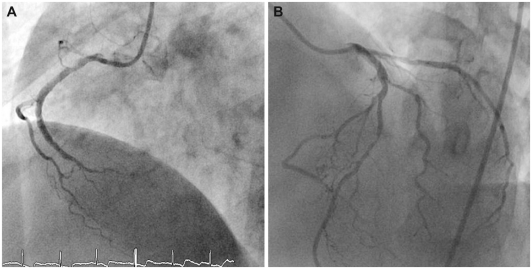
Fig. 1.
Diagnostic coronary angiogram revealed total occlusion of the distal RCA (A) and collateral flow from the left coronary artery system to the distal RCA (B). RCA: right coronary artery.
Fig. 2.
After advancement of a Conquest Pro guidewire (Asahi, Seto, Japan) using a parallel guidewire technique to the RCA, a coronary angiogram showed that the tip of the guidewire was placed outside of the vessel (arrowhead; upper left). After several attempts to cross the CTO lesions, a Conquest Pro guidewire was succeeded to enter into the true lumen of the PL branch (upper right). The lower panel shows cross-sectional images, as well as a longitudinal image of the IVUS from the PL branch to the distal RCA. A and B: at the proximal reference (in the distal RCA) of the CTO, an intraluminal thrombus (★) and intramural hematoma (arrowheads) located in the normal arc of the arterial wall were identified. C: at the proximal end of the CTO, there were intramural hematomas (arrowheads) included with the accumulation of blood and contrast media at the suspected entry site (*). D, E and F: at the middle of the CTO, an extramural hematoma represented as an echo-dim pattern throughout an echogenic adventitia (arrows) and the suspected entry site was detected (*). G: at the distal end of the CTO (in the distal RCA bifurcation), an extramural hematoma was identified (arrows). H: at the proximal PL branch, an intimal dissection to the media from 3 to 6 o'clock was noted. I: at the distal reference in the PL branch. RCA: right coronary artery, CTO: chronic total occlusion, PL: posterolateral.
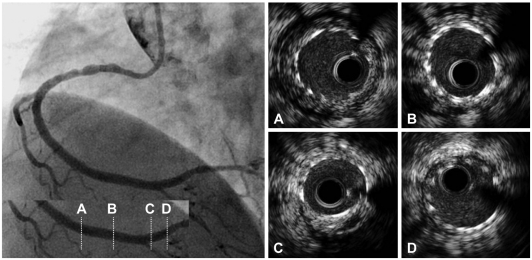
Fig. 3.
Final coronary angiogram demonstrated no residual lumen narrowing with thrombolysis in myocardial infarction 3 flow. Post-stenting intravascular ultrasound images revealed well-opposed stent struts to the vessel wall (A-D) and a 4.66 mm2 minimal stent area (C).
For antiplatelet therapy, the patient received a triple regimen, including aspirin (100 mg once daily), clopidogrel (75 mg once daily), and cilostazol (100 mg twice daily). The fourth day of admission, she discharged without any procedure-related complications. During the 3-year clinical follow-up, the patient remained stable and had no evidence of a cardiovascular event.
Discussion
After coronary intervention, several complications, including dissection, vasospasm, and hematomas may occur within the target vessel.3),8),9) Among the complications, intra- or extra-mural hematomas have been diagnosed infrequently because of the absence of definitive angiographic findings. Because IVUS can provide high-quality, morphologic information regarding the coronary arteries before and after coronary intervention, IVUS permits detection of intra- or extra-mural hematomas during an interventional procedure. An intramural hematoma after PCI is defined as an accumulation of blood within the medial space displacing the IEM inward and the EEM outward, with or without identifiable entry and exit points.6) In contrast, an extramural hematoma is defined as an accumulation of blood outside the arterial wall in the adventitia tissue and presents with an echo-dim pattern due to the dilution of the red blood cells and dissemination throughout an echogenic adventitia.5)
An IVUS study reported a 6.7% incidence of intramural hematomas in 905 patients with 1,025 lesions undergoing PCI.1) Because one-half of intramural hematomas involve the distal reference artery and/or luminal narrowing, intramural hematomas are sometimes considered to represent coronary spasm.1) The possible mechanism underlying intramural hematomas involves blood entering the medial space via a dissection point along with longitudinal spread to a more normal vessel wall.1),10) One-third of intramural hematomas detected by IVUS show no abnormalities on coronary angiogram; however, intramural hematomas are associated with a high rate of non-Q-wave myocardial infarction and thus need repeat revascularization to prevent sudden death.1) Therefore, performing an IVUS examination plays a major role in the detection of intramural hematomas during PCI.1)
Fujii et al.7) reported the IVUS morphologic features of 67 CTOs just after guidewire passage or small balloon inflation. This study demonstrated that multiple small calcium deposits and intramural hematomas were the most common findings during CTO intervention. Presumably, a stiff guidewire likely penetrates into the medial space at the junction of a disease-free wall, translating into a higher chance of intramural hematoma formation.7) Similarly, in the presented case, a stiff guidewire entered into the medial space in the distal portion of the RCA and penetrated into the EEM at the RCA bifurcation site, resulting in intra- and extra-mural hematomas. Compared with an intramural hematoma, the incidence and mechanism of extramural hematomas are not well established. Mahr et al.2) reported a case of an extramural hematoma causing a reduced distal vessel diameter after coronary stenting, which was treated with another coronary stent. Similar to the mechanism of an intramural hematoma, dissection into the adventitia plays a beginning point of blood entry, which can be detected by IVUS.
From a clinical standpoint, a therapeutic strategy for intra- and/or extra-mural hematomas is a major issue during PCI. Several cases of intra- or extra-mural hematomas after PCI have been reported, which were successfully treated with coronary stenting.2),11-13) In our case, the blood entry site appeared as a dissection within the media and adventitia and resulted in combined intra- and extra-mural hematomas as detected by IVUS, although there was no evidence of extravasation of contrast media into the pericardium by coronary angiogram. Therefore, we implanted two DESs with an overlapping technique for entire lesion coverage. The final angiogram showed an acceptable result without any complications after successful coronary stenting, suggesting intra- and extra-mural hematomas were compressed and the entry site was sealed by the DESs. IVUS can identify intra- or extra-mural hematomas that cannot be detected by coronary angiogram after PCI in complex lesions. Furthermore, IVUS can also allow a strategy to treat complications after PCI.
References
- 1.Maehara A, Mintz GS, Bui AB, et al. Incidence, morphology, angiographic findings, and outcomes of intramural hematomas after percutaneous coronary interventions: an intravascular ultrasound study. Circulation. 2002;105:2037–2042. doi: 10.1161/01.cir.0000015503.04751.bd. [DOI] [PubMed] [Google Scholar]
- 2.Mahr P, Ge J, Haude M, Gorge G, Erbel R. Extramural vessel wall hematoma causing a reduced vessel diameter after coronary stenting: diagnosis by intravascular ultrasound and treatment by stent implantation. Cathet Cardiovasc Diagn. 1998;43:438–443. doi: 10.1002/(sici)1097-0304(199804)43:4<438::aid-ccd18>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Hirose M, Kobayashi Y, Kreps EM, et al. Luminal narrowing due to intramural hematoma shift from left anterior descending coronary artery to left circumflex artery. Catheter Cardiovasc Interv. 2004;62:461–465. doi: 10.1002/ccd.20138. [DOI] [PubMed] [Google Scholar]
- 4.Noh HJ, Choi JH, Song YB, et al. Intravascular ultrasound-guided troubleshooting in a large hematoma treated with fenestration using a cutting balloon. Korean Circ J. 2009;39:171–174. doi: 10.4070/kcj.2009.39.4.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oesterle SN, Limpijankit T, Yeung AC, et al. Ultrasound logic: the value of intracoronary imaging for the interventionist. Catheter Cardiovasc Interv. 1999;47:475–490. doi: 10.1002/(SICI)1522-726X(199908)47:4<475::AID-CCD19>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS): a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 7.Fujii K, Ochiai M, Mintz GS, et al. Procedural implications of intravascular ultrasound morphologic features of chronic total coronary occlusions. Am J Cardiol. 2006;97:1455–1462. doi: 10.1016/j.amjcard.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 8.Egglin TK, Dickey KW, Rosenblatt M, Pollak JS. Retrieval of intravascular foreign bodies: experience in 32 cases. AJR Am J Roentgenol. 1995;164:1259–1264. doi: 10.2214/ajr.164.5.7717243. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg SL, Colombo A, Maiello L, Borrione M, Finci L, Almagor Y. Intracoronary stent insertion after balloon angioplasty of chronic total occlusions. J Am Coll Cardiol. 1995;26:713–719. doi: 10.1016/0735-1097(95)00219-T. [DOI] [PubMed] [Google Scholar]
- 10.Murphy DA, Craver JM, King SB., 3rd Distal coronary artery dissection following percutaneous transluminal coronary angioplasty. Ann Thorac Surg. 1984;37:473–478. doi: 10.1016/s0003-4975(10)61134-4. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Recalde A, Moreno R, Jimenez-Valero S. Stenting of spontaneous intramural coronary haematoma: long-term consequences. Eur Heart J. 2008;29:1593. doi: 10.1093/eurheartj/ehm612. [DOI] [PubMed] [Google Scholar]
- 12.Souteyrand G, Motreff P. Acute vessel occlusion after coronary stenting: intravascular ultrasound diagnosis of extensive intramural haematoma. Eur Heart J. 2008;29:9. doi: 10.1093/eurheartj/ehm322. [DOI] [PubMed] [Google Scholar]
- 13.Werner GS, Figulla HR, Grosse W, Kreuzer H. Extensive intramural hematoma as the cause of failed coronary angioplasty: diagnosis by intravascular ultrasound and treatment by stent implantation. Cathet Cardiovasc Diagn. 1995;36:173–178. doi: 10.1002/ccd.1810360219. [DOI] [PubMed] [Google Scholar]