Abstract
A 52-year-old woman with rheumatoid arthritis who had been treated with prednisone and hydroxychloroquine for >12 years presented with chest discomfort and a seizure. She was diagnosed with restrictive cardiomyopathy combined with sick sinus syndrome. A myocardial muscle biopsy was performed to identify the underlying cardiomyopathy, which showed marked muscle fiber hypertrophy, fiber dropout, slightly increased interstitial fibrous connective tissue, and extensive cytoplasmic vacuolization of the myocytes under light microscopy. Electron microscopy of the myocytes demonstrated dense, myeloid, and curvilinear bodies. The diagnosis of hydroxychloroquine-induced cardiomyopathy was made based on the clinical, hemodynamic, and pathologic findings. This is the first case report describing chloroquine-induced cardiomyopathy involving the heart conduction system.
Keywords: Hydroxychloroquine; Cardiomyopathy, restrictive; Sick sinus syndrome
Introduction
Chloroquine-induced cardiomyopathy (CICMP) is very rare and difficult to diagnose because of the non-specific clinical manifestations. A 52-year-old woman presented with chest discomfort and a seizure. She had been treated with hydroxychloroquine >12 years for the treatment of rheumatoid arthritis. The patient was diagnosed with CICMP and sick sinus syndrome. This is the first case report of CICMP with sick sinus syndrome, which we present with a review of the relevant literature.
Case
A 52-year-old woman was hospitalized for evaluation of a 2-day-history of chest discomfort. She had rheumatoid arthritis for 13 years and had been treated with hydroxychloroquine for >12 years (total dose, 1,898 grams). In the emergency department, she had a generalized tonic-clonic seizure caused by sinus arrest (Fig. 1A). The initial vital signs were as follows: blood pressure, 130/80 mmHg; heart rate, 38 beats/minute; body temperature, 36.4℃; and respiratory rate, 20/minute. The physical examination revealed a mild degree of jugular venous distention, a regular, slow cardiac beat without murmurs, clear lung sounds, and no pitting edema in the extremities. The 12-lead ECG showed a junctional rhythm with sinus arrest (Fig. 1B). The laboratory findings included a hemoglobin level of 13.1 g/dL and a normal white blood cell count with a normal differential cell count. The blood urea nitrogen was 27 mmol/L, the serum creatinine was 2.02 mg/dL and the C-reactive protein was 0.94 mg/L. The antinuclear and anti-double stranded deoxyribonucleic acid (DNA) antibodies were negative, while the rheumatoid factor was positive. The creatine kinase was 294 UI/L with a normal myocardial band fraction. The aspartate aminotransferase level was 57 U/L, the alanine aminotransferase level was 48 U/L, and the alkaline phosphatase level was 49 UI/L. Chest radiography demonstrated marked cardiomegaly without pulmonary congestion (Fig. 2). Transthoracic echocardiography (TTE) revealed a markedly thickened left ventricular (LV) septum and posterior wall thickness, measuring 17.7 mm and 18.8 mm, respectively, with mild LV systolic dysfunction (ejection fraction, 44%). A slightly thickened RV free wall with mild RV systolic dysfunction was also noted. Both atria were enlarged and a mild degree of mitral regurgitation was present. The mitral inflow pattern was consistent with restrictive physiology. The early mitral inflow (E) velocity was 90.28 cm/s, the late mitral inflow (A) velocity was 36.77 cm/s, and the deceleration time was 216.64 milliseconds. The E/E' was 33.4, suggesting a high left ventricular end diastolic pressure (Fig. 3). Magnetic resonance imaging (MRI) showed diffuse wall thickening of the LV (septal wall thickness at the end-diastolic phase, 19 mm) and the RV free wall, suggesting hypertrophic cardiomyopathy. There was a small pericardial effusion. On delayed enhancement, multifocal patchy myocardial enhancement was noted in the lateral and septal walls (Fig. 4). The patient underwent myocardial biopsy to determine the underlying cause of the cardiomyopathy. Light microscopy showed vacuolated myocytes (Fig. 5A and B). Electron microscopy revealed abundant intra-myocyte lysosomes with numerous large, dense myelin figures occupying large portions of the myocyte sarcoplasm. Lysosomal inclusions with curvilinear substructures were also noted (Fig. 5C and D). There was no evidence of amyloid deposition, myocarditis, or an acute vasculitic process. Chloroquine toxicity was diagnosed based on the pathologic findings and the hydroxychloroquine was promptly discontinued. A permanent pacemaker was inserted for management of sick sinus syndrome. Four months later, the follow-up TTE demonstrated no significant interval change in LV systolic function or the LV wall thickness compared with the previous examination. However, LV diastolic function improved from restrictive physiology to pseudonormal relaxation. The patient's rheumatoid arthritis was subsequently controlled with low-dose prednisolone and analgesics.
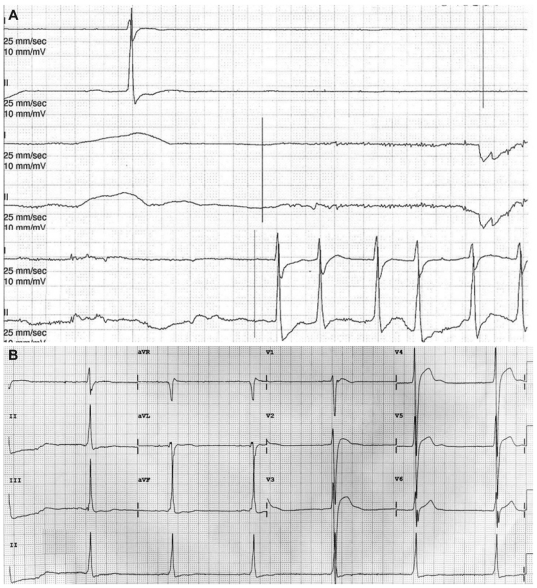
Fig. 1.
Initial electrocardiogram findings. A: the rhythm strip shows sinus pause. B: the 12-lead electrocardiogram shows a junctional rhythm with sick sinus syndrome.
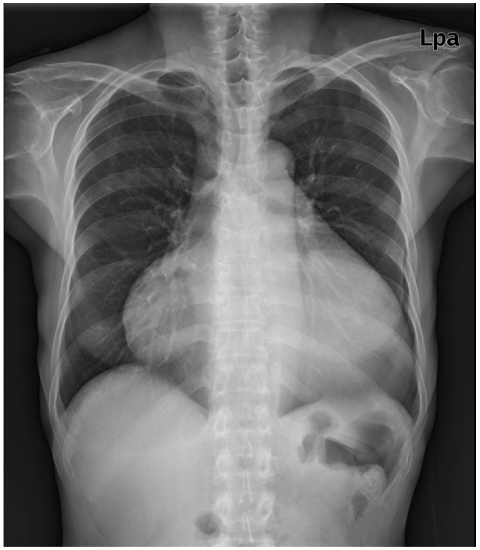
Fig. 2.
Chest radiography demonstrated marked cardiomegaly without pulmonary congestion.
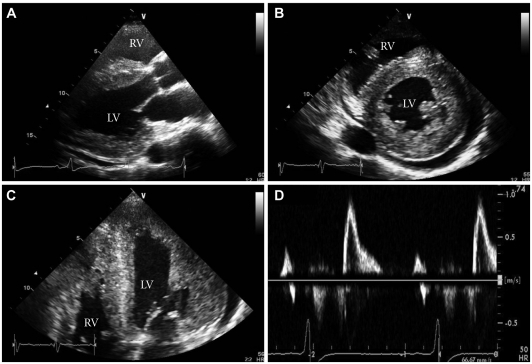
Fig. 3.
Two-dimensional echocardiography reveals severe concentric LV hypertrophy, mild LV systolic dysfunction, a small pericardial effusion, and increased RV free wall thickness with mild RV systolic dysfunction. A: parasternal long axis view. B: parasternal short axis view at the mid-ventricular level. C: magnified apical 4-chamber view. D: mitral inflow view (E velocity, 90.28 cm/s; A velocity, 36.77 cm/s; deceleration time, 216.64 ms; E/E': 33.4). LV: left ventricular, RV: right ventricular.
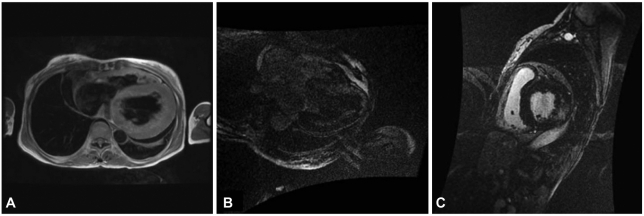
Fig. 4.
Cardiac MRI findings. A: the black-blood coronal axis view on cardiac MRI demonstrates marked concentric left ventricle hypertrophy, suggesting hypertrophic cardiomyopathy. Right ventricle wall thickening is also noted and there is a small pericardial effusion. On delayed enhancement, signal hyperenhancement after gadolinium administration is observed within the septal wall on the coronal axis view (B) and lateral wall on the short axis view (C).
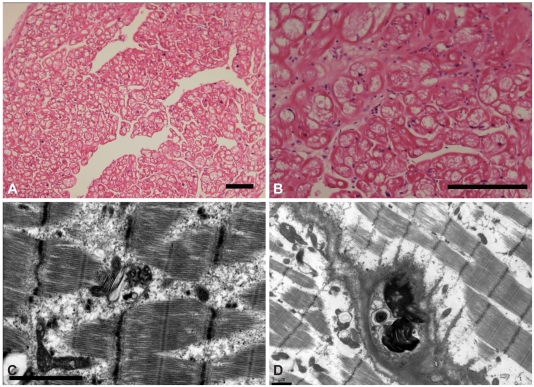
Fig. 5.
Myocardial biopsy findings. A and B: light microscopy of the myocardial biopsy specimen showing diffusely enlarged myocytes with extensive cytoplasmic vacuolization (hematoxylineosin staining; A: original magnification ×100, B: ×200 scale bar, 200 µm). C: curvilinear bodies. This appearance is typical and characteristic of chloroquine and hydroxychloroquine toxicity. D: the transmission electron microscopy of the skeletal muscle biopsy specimen displays central replacement and displacement of the sarcomeres by marked accumulation of secondary lysosomes, including myeloid bodies (scale bar 1 µm).
Discussion
Hydroxychloroquine is a well-known anti-malarial agent that is now used for the treatment of rheumatoid arthritis and other connective tissue disorders. However, prolonged and larger doses of chloroquine are usually required for non-malarial indications.1-3) These drugs are known to cause a variety of toxic effects, such as retinopathy,4) myopathy,5) neuromyopathy6) and cardiotoxicity. Conduction system abnormalities related to chloroquine include heart block and arrhythmias. Chloroquine cardiomyopathy is characterized by biatrial enlargement, biventricular thickening, and systolic and diastolic dysfunction.7),8) Chloroquine induces the formation of sarcoplasmic myelinoid and curvilinear bodies, which results in myocyte dysfunction. Sarcoplasmic accumulation of myelinoid and curvilinear bodies expands the myocytes, and as a result, the ventricular wall is abnormally thickened.1),2) Experimental studies on rabbits and biopsy specimens of human endomyocardium have established the toxicity of chloroquine in cardiac muscle. Chloroquine is also known to produce vacuolar myopathy in skeletal muscle,9),14) and this results in a specific ultrastructural pattern of pathologic findings, such as the accumulation of myeloid bodies, glycogen, and curvilinear bodies within the skeletal muscles.15),16) The proposed underlying mechanism is chloroquine-induced phospholipid storage in muscle cells. Chloroquine accumulates inside the cells by the action of lysosomes, and intracellular chloroquine increases the intralysosomal pH, which results in dysfunction of the lysosomal enzymes. Chloroquine induces dysfunction of the lysosomal enzymes by direct binding, leading to the accumulation of glycogen and phospholipids. Chloroquine also causes acute interference with mitochondrial oxidative metabolism. Accumulated glycogen and phospholipids lead to the formation of myelinoid (lamellar) and curvilinear bodies that cause dysfunction of myocytes.17),18)
Diagnosing the underlying cause of idiopathic restrictive cardiomyopathy is difficult, although TTE, cardiac MRI, and cardiac muscle biopsy can collectively yield essential findings.19) A large number of patients with restrictive cardiomyopathy have myocardial ischemia associated with chest pain.20)
In this case, vacuolated cardiomyocytes and curvilinear body formation were observed on light and electron microscopy, respectively, which are pathognomic findings of the intralysosomal accumulation of lipids. Cervera et al.1) recommended that a cardiac evaluation with an ECG and an ophthalmologic examination should be performed before the long-term administration of chloroquine. Chloroquine is usually not indicated if the patient presents with some cardiac conduction disorder in order to prevent cardiomyopathy or complete heart block. It is also recommended that an ECG exam should be performed 6 months after chloroquine treatment, then annual follow-up is needed when the total dose of chloroquine reaches ≥1,000 grams. There have been a few cases of CICMP, which have shown a favorable outcome with improvement in left ventricular systolic function after discontinuation of treatment,6) but most studies have reported persistent signs of cardiac dysfunction and histologic lesions up to 9 years after stopping treatment.
In this case, the patient had a 12-year history of RA, for which she had been treated with medications, including hydroxychloroquine. She did not complain of symptoms of heart failure and her symptoms were mainly due to sick sinus syndrome. Her echocardiograpic evaluation manifested the classic findings of restrictive cardiomyopathy by infiltrative disease, including biventricular hypertrophy, restrictive mitral and pulmonary vein inflow, and an increased LV end-diastolic filling pressure. Even though the follow-up echocardiographic parameters of systolic function did not show significant improvement 4 months after discontinuation of hydroxychloroquine, her symptoms had improved substantially. Further, the parameters of diastolic function improved, which suggested the possible recovery of myocardial function. The patient will be followed on a regular basis.
Herein we have reported a case of CICMP with a conduction disorder, in which the patient manifested typical findings of infiltrative restrictive cardiomyopathy by TTE and MRI. Her symptoms were successfully treated with the implantation of a permanent pacemaker. The myocardial biopsy findings and the patient's medical history confirmed the diagnosis. The cardiac function of patients who need long-term administration of chloroquine should be carefully monitored.
References
- 1.Cervera A, Espinosa G, Font J, Ingelmo M. Cardiac toxicity secondary to long term treatment with chloroquine. Ann Rheum Dis. 2001;60:301. doi: 10.1136/ard.60.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iglesias Cubero G, Rodrigue Reguero JJ, Rojo Ortega JM. Restrictive cardiomyopathy caused by chloroquine. Br Heart J. 1993;69:451–452. doi: 10.1136/hrt.69.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratliff NB, Estes ML, Myles JL, Shirey EK, McMahon JT. Diagnosis of chloroquine cardiomyopathy by endomyocardial biopsy. N Engl J Med. 1987;316:191–193. doi: 10.1056/NEJM198701223160405. [DOI] [PubMed] [Google Scholar]
- 4.Weiner A, Sandberg MA, Gaudio AR, Kini MM, Berson EL. Hydroxychloroquine retinopathy. Am J Ophthalmol. 1991;112:528–534. doi: 10.1016/s0002-9394(14)76853-9. [DOI] [PubMed] [Google Scholar]
- 5.Hughes JT, Esiri M, Oxbury JM, Whitty CW. Chloroquine myopathy. Q J Med. 1971;40:85–93. [PubMed] [Google Scholar]
- 6.Estes ML, Ewing-Wilson D, Chou SM, et al. Chloroquine neuromyotoxicit: clinical and pathologic perspective. Am J Med. 1987;82:447–455. doi: 10.1016/0002-9343(87)90444-x. [DOI] [PubMed] [Google Scholar]
- 7.Roos JM, Aubry MC, Edwards WD. Chloroquine cardiotoxicity: clinicopathologic features in three patients and comparison with three patients with Fabry disease. Cardiovasc Pathol. 2002;11:277–283. doi: 10.1016/s1054-8807(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 8.Keating RJ, Bhatia S, Amin S, Williams A, Sinak LJ, Edwards WD. Hydroxychloroquine-induced cardiotoxicity in a 39-year-old woman with systemic lupus erythematosus and systolic dysfunction. J Am Soc Echocardiogr. 2005;18:981. doi: 10.1016/j.echo.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Rewcastle NB, Humphrey JG. Vacuolar myopathy: clinical, histochemical, and microscopic study. Arch Neurol. 1965;12:570–582. doi: 10.1001/archneur.1965.00460300018003. [DOI] [PubMed] [Google Scholar]
- 10.Whisnant JP, Espinosa RE, Kierland RR, Lambert EH. Chloroquine neuromyopathy. Proc Staff Meet Mayo Clin. 1963;38:501–513. [PubMed] [Google Scholar]
- 11.Eadie MJ, Ferrier TM. Chloroquine myopathy. J Neurol Neurosurg Psychiatry. 1966;29:331–337. doi: 10.1136/jnnp.29.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mastaglia FL, Papadimitriou JM, Dawkins RL, Beveridge B. Vacuolar myopathy associated with chloroquine, lupus erythematosus and thymoma: report of a case with unusual mitochondrial changes and lipid accumulation in muscle. J Neurol Sci. 1977;34:315–328. doi: 10.1016/0022-510x(77)90149-6. [DOI] [PubMed] [Google Scholar]
- 13.Neville HE, Maunder-Sewry CA, McDougall J, Sewell JR, Dubowitz V. Chloroquine-induced cytosomes with curvilinear profiles in muscle. Muscle Nerve. 1979;2:376–381. doi: 10.1002/mus.880020509. [DOI] [PubMed] [Google Scholar]
- 14.Gerard JM, Stoupel N, Collier A, Flament-Durand J. Morphologic study of a neuromyopathy caused by prolonged chloroquine treatment. Eur Neurol. 1973;9:363–379. doi: 10.1159/000114244. [DOI] [PubMed] [Google Scholar]
- 15.Fedorko ME, Hirsch JG, Cohn ZA. Autophagic vacuoles produced in vitro: II. studies on the mechanism of formation of autophagic vacuoles produced by chloroquine. J Cell Biol. 1968;38:392–402. doi: 10.1083/jcb.38.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macdonald RD, Engel AG. Experimental chloroquine myopathy. J Neuropathol Exp Neurol. 1970;29:479–499. doi: 10.1097/00005072-197007000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Motan J, Topinka I, Dura J, Kvapilova H. Chloroquine cardiodystrophy. Vnitr Lek. 1978;24:1122–1128. [PubMed] [Google Scholar]
- 18.Baguet JP, Tremel F, Fabre M. Chloroquine cardiomyopathy with conduction disorders. Heart. 1999;81:221–223. doi: 10.1136/hrt.81.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung KY, Nam KB, Ko KK, et al. A case of idiopathic restrictive cardiomyopathy. Korean Circ J. 1990;20:260–264. [Google Scholar]
- 20.Bae EJ, Cheon EJ, Yun YS. Clinical profile and outcome of idiopathic restrictive cardiomyopathy in children. Korean Circ J. 2001;31:427–433. [Google Scholar]