Abstract
Sodium–potassium ATPase (‘Na+ −K+ ATPase’) contributes to the maintenance of the resting membrane potential and the transmembrane gradients for Na+ and K+ in neurons. Activation of Na+ −K+ ATPase may be important in controlling increases in intracellular sodium during periods of increased neuronal activity. Down-regulation of Na+ −K+ ATPase activity is implicated in numerous CNS disorders, including epilepsy. Although Na+ −K+ ATPase is present in all neurons, little is known about its activity in different subclasses of neocortical cells. We assessed the physiological properties of Na+ −K+ ATPase in fast-spiking (FS) interneurons and pyramidal (PYR) cells to test the hypothesis that Na+ −K+ ATPase activity would be relatively greater in neurons that generated high frequency action potentials (the FS cells). Whole-cell patch clamp recordings were made from FS and PYR neurons in layer V of rat sensorimotor cortical slices maintained in vitro using standard techniques. Bath perfusion of Na+ −K+ ATPase antagonists (ouabain or dihydro-ouabain) induced either a membrane depolarization in current clamp, or inward current under voltage clamp in both cell types. PYR neurons were divided into two subpopulations based on the amplitude of the voltage or current shift in response to Na+ −K+ ATPase blockade. The two PYR cell groups did not differ significantly in electrophysiological properties including resting membrane potential, firing pattern, input resistance and capacitance. Membrane voltage responses of FS cells to Na+ −K+ ATPase blockade were intermediate between the two PYR cell groups (P < 0.05). The resting Na+ −K+ ATPase current density in FS interneurons, assessed by application of blockers, was 3- to 7-fold larger than in either group of PYR neurons. Na+ −K+ ATPase activity was increased either through direct Na+ loading via the patch pipette or by focal application of glutamate (20 mm puffs). Under these conditions FS interneurons exhibited the largest increase in Na+ −K+ ATPase activity. We conclude that resting Na+ −K+ ATPase activity and sensitivity to changes in internal Na+ concentration vary between and within classes of cortical neurons. These differences may have important consequences in pathophysiological disorders associated with down-regulation of Na+ −K+ ATPase and hyperexcitability within cortical networks.
Introduction
Na+ −K+ ATPase catalyses the transport of Na+ and K+ across the cell membrane and is important in establishing and maintaining the electrochemical gradient. The maintenance of this transmembrane gradient is vital to cell function at multiple levels, including Na+-coupled reuptake of glutamate (Balcar, 2002; O'Shea, 2002), glucose utilization (Honegger & Pardo, 1999; Magistretti, 2006), signal transduction (Liang et al. 2006) and modulation of cellular excitability and synaptic transmission (Ross & Soltesz, 2001; Reich et al. 2004; Kim et al. 2007). Changes in Na+ −K+ ATPase activity have been implicated in numerous CNS disorders (Lees, 1991; Kumar & Kurup, 2002), including those manifest by hyperexcitability such as epilepsy in humans (Rapport et al. 1975) and in several animal models of epileptogenesis (Donaldson et al. 1971; Anderson et al. 1994; Fernandes et al. 1996; Reime Kinjo et al. 2007). While the Na+ −K+ ATPase is ubiquitously expressed in all neurons our understanding of its activity in different types of neocortical cells remains limited.
Pyramidal (PYR) neurons represent the major source of excitatory output from neocortical layer V, a lamina that is the site of origin of interictal epileptiform discharge in both acute and chronic models of neocortical epileptogenesis (Connors, 1984; Prince & Tseng, 1993; Hoffman et al. 1994). The spike output of PYR cells is closely regulated by the action of inhibitory fast-spiking (FS) interneurons that synapse predominantly on PYR somata and proximal dendrites (Tamas et al. 1997). Regulation of FS interneuronal excitability is therefore important to normal and pathophysiological neocortical activity. In comparison to PYR cells, FS interneurons have a much higher firing frequency and can generate a sustained output in excess of 500 Hz with little spike frequency adaptation (McCormick et al. 1985; Connors & Gutnick, 1990 for review). This suggests that they possess an efficient mechanism for clearing increased [Na+] that would accumulate, particularly in their axons that have a high surface to volume ratio, and potentially suppress action potential firing. Activation of Na+ −K+ ATPase by increases in [Na+]i would serve to maintain the capacity to fire at high rates. There is little information available with respect to differences in Na+ −K+ ATPase activity in subgroups of neocortical neurons, even though such differences are important to the regulation of resting membrane potential, synaptic transmission, neuronal responses to injury and the development of hyperexcitability (Ross & Soltesz, 2000; Vaillend et al. 2002; Anderson et al. 2005). In the present experiments, we tested the hypothesis that FS interneurons have greater Na+ −K+ ATPase activity than PYR neurons in layer V, both at rest and during periods of high cellular activity.
Methods
Slice preparation
Protocols for all experiments were approved by the Stanford Institutional Animal Care and Use Committee. The authors have read, and the experiments comply with, the policies and regulations of The Journal of Physiology (Drummond, 2009). Male Sprague–Dawley rats (postnatal days (P)13–P24) or CD-1(ICR) mice (P15–P25) were deeply anaesthetized with 50 mg kg−1 sodium pentobarbital and decapitated. Brains were removed and coronal cortical slices (350 μm thick) of the somatosensory cortex were cut on a vibratome (VT 1000S; Leica, Nussloch, Germany) in a 4°C carboxygenated (95% O2–5% CO2) ‘cutting’ solution containing the following (in mm): 234 sucrose, 11 glucose, 24 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4 and 0.5 CaCl2. Slices were hemisected and incubated for 1 h at 32°C in carboxygenated artificial CSF (aCSF) containing (in mm): 126 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2 and 10 glucose, pH 7.4. Slices were then incubated at room temperature before being transferred to the recording chamber.
Electrophysiological recording
Slices submerged in aCSF were initially visualized under brightfield for identification of neocortical layer V (Paxinos & Watson, 1998). Whole-cell recordings were obtained from cortical pyramidal (PYR) neurons or fast-spiking (FS) interneurons using an upright microscope (Axioskop, Carl-Zeiss, Thornwood, NY, USA) fitted with infrared differential interference contrast optics. Regular spiking (RS) and intrinsically bursting (IB) PYR neurons were distinguished based on their current-clamp firing behaviour (Connors et al. 1982; Tseng & Prince, 1993; Guatteo et al. 1994). FS interneurons were identified visually by the lack of a large emerging apical dendrite and electrophysiologically by their firing behaviour in current clamp (McCormick et al. 1985). To facilitate identification of FS interneurons some recordings were made in transgenic mice in which the enhanced green fluorescent protein (EGFP) was specifically expressed in parvalbumin-positive neurons (Chattopadhyaya et al. 2004). These parvalbumin-containing cells were routinely identified electrophysiologically as FS interneurons. No difference was observed in data collected from rats or transgenic mice. All recordings were obtained at 32°C using borosilicate glass (WPI, Sarasota, FL, USA) microelectrodes (tip resistance, 2–3 MΩ) filled with intracellular solution containing the following (in mm): 70 potassium gluconate, 70 KCl, 2 NaCl, 10 Hepes, 10 EGTA, 2 MgCl2. The estimated ECl was approximately −16 mV, resulting in inward GABAA currents at a holding potential of −70 mV. Substitution of the internal solution for one containing a more physiological [Cl−]i (124 potassium gluconate, 16 KCl, 2 NaCl, 10 Hepes, 4 EGTA, ECl–52 mV) had no significant effect on the Na+ −K+ ATPase-sensitive current. Internal solution pH was adjusted to 7.3 using KOH as required. For intracellular labelling, biocytin 0.3–1% was included in the internal solution and sections processed as previously described (Salin et al. 1995). The electrode capacitance and bridge circuit were appropriately adjusted. The series resistance (Rs) of neurons chosen for analysis ranged between 6 and 30 MΩ (<20% of membrane input resistance) and was monitored for stability. Membrane potential was not corrected for a calculated 10 mV liquid junction potential. For measurement of the voltage sag induced by hyperpolarizing activated cationic current, the difference between the peak and steady-state membrane voltage recorded in response to a 1 s, −150 pA transmembrane current step was measured. The post-train afterhyperpolarization potential was measured from the peak hyperpolarized value to the recovered baseline following a 1 s, 150 pA depolarizing transmembrane current step. For frequency–current (f–I) slopes linear regressions were performed on plots of the average firing frequency against current (I) normalized to the threshold current (Ithreshold) that reliably produced a train of action potentials (100 pA for PYR neurons, 250 pA for FS interneurons. Similarly, we calculated an adaptation index as 100 × (1 −Flast/F2), where Flast corresponds to the firing rate of the last interspike interval and F2 the second interspike interval (modified from Tateno & Robinson, 2004). PYR neurons exhibited a high variability in the first interspike interval (in both PYR1 and PYR2 response groups) and as such the 2nd interval was chosen for analysis. A Multiclamp 700A patch-clamp amplifier (Axon Instruments, Union City, CA, USA) was used in either current- or voltage-clamp mode. Recordings were sampled at 20 kHz, filtered at 10 kHz, captured on an A–D interface (Digidata 1320A, Axon Instruments) and stored on a computer. Simultaneous continuous recordings were performed on a MiniDigi 1A, sampling at 1 kHz. For voltage-clamp recordings, the membrane potential was clamped at −70 mV. Data were analysed using pCLAMP (Axon Instruments), Origin (Microcal Software, Northampton, MA, USA), and Prism (GraphPad) software. Data are presented as means ± s.e.m. Statistical significance was tested with a one-way ANOVA with Tukey's multiple comparison test or a Student's paired t test. Differences were determined to be significant if P < 0.05. The Na+ −K+ ATPase current density for each cell was calculated as: (ΔVm/Rin)/Cm where ΔVm is the membrane depolarization induced by Na+ −K+ ATPase blockade, Rin the input resistance determined from the voltage response to an applied hyperpolarizing current step (1 s, 25–50 pA) and Cm the total capacitance calculated from the integrated area of the current response to a 40 ms, −5 mV voltage step. Membrane depolarization (Fig. 1B) or peak current (Fig. 2B) induced in FS or PYR neurons by a 30 s application of 100 μm dihydro-ouabain (DHO) were best fitted to single or double peak Gaussian distributions with the equation: y = y0+ (A/(w×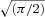 )) × exp(−2((x−xc)/w)2). Plots were performed in Origin 7.0 (OriginLabs, Northampton, MA, USA) and goodness of fit tested by the calculated coefficient of determination (R2) equal to: (total sum of squares – residual sum of squares)/total sum of squares.
)) × exp(−2((x−xc)/w)2). Plots were performed in Origin 7.0 (OriginLabs, Northampton, MA, USA) and goodness of fit tested by the calculated coefficient of determination (R2) equal to: (total sum of squares – residual sum of squares)/total sum of squares.
Figure 1. Dihydro-ouabain (DHO) induces a membrane depolarization in neocortical neurons.
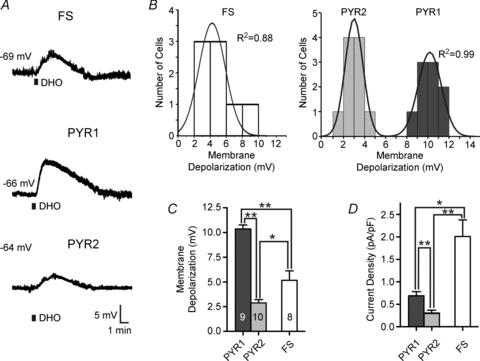
A, bath application of 100 μm DHO for 30 s induces a reversible membrane depolarization in a fast-spiking (FS) interneuron (top) and in two classes of pyramidal neuron (middle/bottom). Black bar represents period of DHO application. Resting membrane potential is listed to the left of each trace. B, population data of neuronal responses to DHO application. Left: data for FS interneurons are normally distributed (i.e. well fitted by a single peak Gaussian). Right: data for pyramidal neurons are best fitted by a 2-peak Gaussian. Goodness of fit calculated by the coefficient of determination (R2). Note the clear separation of responses into large amplitude (termed PYR1) and small amplitude (termed PYR2) responses. C, mean (±s.e.m.) membrane depolarization in response to DHO for all cell types. D, calculated current density (current/capacitance) for all cell types. *P > 0.05; **P > 0.01.
Figure 2. Heterogeneous inward current responses to Na+ −K+ ATPase blockade in different classes of neocortical neurons.
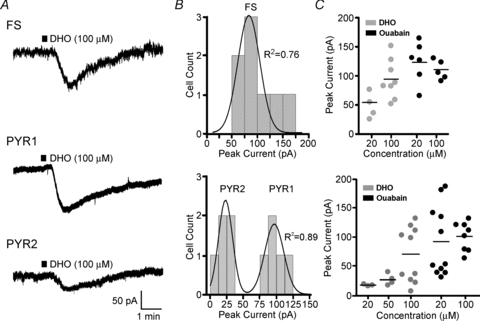
A, whole-cell voltage clamp responses to brief (30 s, black bar) application of 100 μm DHO, which induced a reversible inward current in all tested neurons. FS interneurons again showed an intermediate amplitude response between the two identified pyramidal types (larger amplitude (PYR1) and smaller amplitude (PYR2)). B, histograms of population responses to DHO in either the FS or PYR neurons. Again, the FS data were best fitted by a single peak Gaussian, and the PYR neurons by a 2-peak Gaussian. C, scatter plots of peak current responses to 30 s application of either the low affinity Na+ −K+ ATPase antagonist DHO (grey symbols; 20, 50 or 100 μm) or the high affinity Na+ −K+ ATPase antagonist ouabain (black symbols; 20, 100 μm) in FS (top) or PYR (bottom) neurons. Horizontal bars: mean values.
Experimental solutions
2-Amino-5-phosphonopentanoic acid (d-APV; 50 μm), 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 μm), 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288, 20 μm) and tetrodotoxin (TTX; 1.0 μm) were purchased from Ascent Scientific (Weston-super-Mare, UK), prepared from stock solutions and bath applied in various experiments. Cadmium chloride (200 μm), dihydro-ouabain (20–100 μm), picrotoxin (50 μm) and ouabain (1–100 μm) were purchased from Sigma-Aldrich (St Louis, MO, USA). NaCl was substituted for NaH2PO4 in experiments where cadmium was used. All vehicle concentrations (NaOH, DMSO, ethanol) were <0.5% of final and had no effect on recordings. For isolation of the Na+ −K+ ATPase activity, d-APV, DNQX, TTX and picrotoxin were routinely bath applied unless otherwise noted. Inclusion of TTX significantly reduced the occurrence of spreading depression and/or anoxic depolarization that may accompany blockade of the Na+ −K+ ATPase (Muller & Somjen, 2000; Anderson & Andrew, 2002); however, these events were observed in some cells that were eliminated from further analysis.
Na+-loading experiments
To increase [Na+]i, glutamate (20 mm) was locally delivered through a patch pipette (2–3 MΩ) by pressure ejection (31 kPa, 0.02–1.0 s pulse). For these experiments, DNQX was omitted from the bathing solution to allow AMPA activation, while d-APV was maintained to limit the potential inhibition of the Na+ −K+ ATPase by Ca2+ entering through activated NMDA receptors (Fukuda & Prince, 1992a). However, potential inhibition of the Na+ −K+ ATPase in FS interneurons through activation of Ca2+-permeable AMPA receptors (Angulo et al. 1999) could not be eliminated following glutamate application. Reproducibility of the glutamate responses was confirmed by monitoring responses elicited by two pre-puffs (0.1 s) prior to the test puff (1.0 s), all applied 30 s apart. These pre-puffs elicited short (<5 s), small amplitude (<200 pA) responses that fully recovered well before the delivery of the test puff. This stimulus sequence was repeated every 3 min for 3–5 trials and the results averaged. While the response to the 1st pre-pulse showed some variability, possibly due to a ‘cold barrel’ effect, the responses to the 2nd pre-pulse and test pulse were consistent across trials for puff durations ≤1 s. Responses to puff durations >1.0 s were inconsistent across trials and omitted from the analysis. For calculation of Na+ −K+ ATPase activity, the averaged direct glutamate response (DGR) obtained in the presence of DHO was digitally subtracted from the control glutamate response using pCLAMP software (Axon Instruments). The resulting trace is the current sensitive to blockade with DHO and is indicative of the glutamate-induced Na+ −K+ ATPase activity. Integration of this current will therefore yield the underlying Na+ −K+ ATPase charge. Addition of the Ca2+ chelator BAPTA to the patch electrode solution, bath perfusion of the Ca2+ channel antagonist cadmium (200 μm) and the hyperpolarization-activated mixed cationic channel (Ih) blocker ZD7288 (20 μm) had no effect on the Na+ −K+ ATPase response to the glutamate puff. Therefore, data from cells exposed to these agents were grouped and analysed with those from cells whose recordings were obtained with normal pipette and bath solutions. In separate experiments, [Na+]i was increased by partially substituting sodium gluconate for potassium gluconate in the patch electrode solution.
Results
Whole-cell recordings were obtained from 96 PYR and 71 FS neurons from layer V of sensorimotor cortex. Cells were both visually and electrophysiologically identified as previously described (Kawaguchi & Kubota, 1993; Cauli et al. 1997; Xiang et al. 1998; Bacci et al. 2003). Identification of FS interneurons was aided in the transgenic mice by the fluorescence of EGFP expressed in parvalbumin-positive neurons.
Resting Na+ −K+ ATPase activity varies between different types of neocortical neurons
Bath perfusion of dihydro-ouabain (DHO, 100 μm) for 30 s to either PYR or FS neurons under current clamp evoked a membrane depolarization in all cells tested. In FS interneurons, DHO induced a mean peak depolarization of 5.2 ± 0.8 mV (Fig. 1A). In contrast, DHO perfusion elicited more variable depolarizations in PYR neurons (Fig. 1A, middle and bottom). The response amplitude distributions from FS interneurons (n = 8) were well fitted with a single peak Gaussian (R2 = 0.89), while those of PYR neurons (n = 19) had a bimodal distribution (R2 = 0.99) (Fig. 1B) (see Methods for more details). PYR neurons thus fell into two significantly different groups based on the amplitude of their DHO-induced membrane depolarization. The mean peak amplitudes of responses in these two groups were 10.6 ± 0.4 mV (PYR1 neurons) and 2.7 ± 0.3 mV (PYR2 neurons) (P < 0.0001; Fig. 1C). We next examined the properties of these three cell groups (FS, PYR1 and PYR2) and their responses to Na+ −K+ ATPase blockade in more detail.
Although responses to DHO application in PYR1 cells tended to have a faster rise time (1.7 ± 0.1 min) it was not significantly different from either the FS (1.9 ± 0.3 min) or the PYR2 (1.8 ± 0.2 min) groups (P = 0.46 and P = 0.20, respectively). As the recorded membrane depolarization may be sensitive to differences in cell size and permeability, we examined the current density for each cell type calculated from the input resistance, DHO-induced membrane depolarization and whole-cell capacitance (see Methods). This measure revealed that the Na+ −K+ ATPase current density in FS interneurons (2.0 ± 0.4 pA pF−1) was approximately 3–7 times greater than that in the PYR1 (0.7 ± 0.1 pA pF−1) or PYR2 (0.3 ± 0.1 pA pF−1) groups (P < 0.01 and P < 0.001, respectively; Fig. 1D). The PYR neuron groups were themselves significantly different from each other (P < 0.01). Similar results were also obtained when somatic surface areas were estimated from biocytin-filled cells of each group (data not shown). Thus, FS interneurons and PYR neurons differ in their sensitivity to Na+ −K+ ATPase blockade, presumably due to differences in the resting state of their Na+ −K+ ATPase activity.
The difference in resting Na+ −K+ ATPase activity could be due to differences in the number of functional Na+ −K+ ATPase molecules and/or a difference in rate of Na+ −K+ ATPase activity. We included ATP/GTP in the internal pipette solution in an effort to increase and equalize the forward Na+ −K+ ATPase rate across the different cell types (Gadsby & Nakao, 1989; Ross & Soltesz, 2000). The inclusion of ATP/GTP increased the amplitude of the response to DHO (100 μm) application above control levels in PYR neurons (15.2 ± 3.8 mV, n = 10) but had no effect on FS interneurons (5.3 ± 0.5 mV, n = 5). The lack of effect on FS interneurons suggests that the forward Na+ −K+ ATPase rate is not limited by ATP/GTP levels in these neurons. Addition of ATP/GTP also hyperpolarized the resting membrane potential in PYR neurons (−67.5 ± 2.2 mV) and FS interneurons (−69.8 ± 1.3 mV). The inclusion of ATP/GTP in the patch pipette internal solution prevented grouping of the PYR neurons on the basis of their responses to blockade of the Na+ −K+ ATPase with control internal solution as previously described (Fig. 1B). Consequently the data for PYR neurons were combined as no direct paired comparison with control data was possible. However, responses to blockade with DHO in PYR neurons loaded with ATP/GTP did fall into low (8.1 ± 0.7 mV, n = 6) and high (17.1 ± 2.9 mV, n = 4) amplitude groups. Independent of the PYR grouping, the results of this experiment clearly indicate that increasing intracellular ATP/GTP failed to equalize the DHO-sensitive Na+ −K+ ATPase activity between PYR and FS neurons (P < 0.05). These results indicate that the difference in calculated Na+ −K+ ATPase-dependent current density between cell types is primarily due to a difference in the number of Na+ −K+ ATPase molecules in the cell membrane, rather than a difference in ATP/GTP limited rate.
To directly examine the current elicited by Na+ −K+ ATPase blockade we conducted experiments under voltage clamp. At a holding potential of −70 mV, bath application of 100 μm DHO for 30 s induced a transient inward current in all cell groups. Increasing the duration of DHO application from 30 s to 5 min did not increase the amplitude of the response, but significantly reduced recovery to resting levels (data not shown). In FS interneurons, the responses were normally distributed with a mean (±s.e.m.) peak inward current of 93.1 ± 12.1 pA (n = 8) (Fig. 2A and B). In PYR neurons, two groups could again be clearly identified. The first group of large amplitude responders (PYR1) had a mean peak inward current of 104.7 ± 5.5 pA (n = 5), while the second group (PYR2) had a smaller peak inward current of 26.1 ± 6.2 pA (n = 5) that was significantly different from the FS interneurons (P < 0.001) and the PYR1 group (P < 0.0001). Finally, responses to a series of drug concentrations were tested using DHO and a higher affinity Na+ −K+ ATPase antagonist, ouabain (Fig. 2C). In FS interneurons, 20 μm DHO induced an inward current (52.9 ± 16.4 pA, n = 4) that was significantly smaller than that elicited by 100 μm DHO (93.1 ± 12.1 pA as above, P < 0.05). Inward currents elicited by application of 20 or 100 μm ouabain (124.2 ± 19.8 pA, n = 6 and 108.8 ± 7.4 pA, n = 5, respectively) were not significantly different from those induced by 100 μm DHO (P = 0.44). In PYR neurons, application of 20 or 50 μm DHO induced inward currents of 18.5 ± 1.3 pA (n = 3) and 27.4 ± 6.9 pA (n = 4), respectively. Interestingly, the distinct grouping of PYR neuron responses was not present at either lower doses of DHO (20 and 50 μm) or at a higher dose of ouabain (100 μm). The two groups of PYR cell responses were again evident when 20 μm ouabain (PYR1 = 152.0 ± 14.7 pA, n = 5, PYR2 = 45.0 ± 3.8 pA, n = 6) was applied (P < 0.0001). This suggests that the observed difference in Na+ −K+ ATPase density between the two groups of PYR neurons is accompanied by a differential sensitivity to blockade of the Na+ −K+ ATPase by DHO or ouabain.
The intrinsic membrane properties of FS interneurons were significantly different from both PYR groups; however, there were no significant differences between the two PYR neuron groups (Table 1). Specifically, there was no correlation between the amplitude of the DHO-induced membrane depolarization and numerous intrinsic properties (Table 1). Using previously described criteria (Connors et al. 1982) we classified the firing behaviour of the PYR neurons and found that they were predominantly regular spiking (n = 16), although a few intrinsically bursting neurons were recorded in both PYR groups (n = 3) (Fig. 3A, and Table 1). There was no correlation between firing behaviour, frequency–current plots or adaptation index and the amplitude of responses to DHO application. While DHO application induced an expected leftward shift in the membrane voltage–current curve (Fig. 3B), there was no significant DHO-induced change in the input resistance of the three cell types (Fig. 3C). The laminar location and morphological identity of 18 PYR neurons was confirmed with intracellular biocytin labelling. There were no distinct differences in location or general cell morphology (Fig. 3D). Consequently, the amplitude of the PYR neuron response to blockade of resting Na+ −K+ ATPase activity was consistently used in the remaining experiments to classify the neurons as belonging to the PYR1 or PYR2 group.
Table 1.
Intrinsic membrane properties of different groups of recorded neurons
| PYR1 (n = 9) | PYR2 (n = 10) | FS (n = 8) | |
|---|---|---|---|
| DHO – membrane depolarization (mV) | 10.0 ± 0.4* | 2.7 ± 0.3 | 5.2 ± 0.8†* |
| Resting membrane potential (mV) | −64.1 ± 0.9 | −62.0 ± 1.3 | −67.4 ± 1.5* |
| Input resistance (MΩ) | |||
| Control | 119.7 ± 19.5 | 116.6 ± 15.8 | 80.9 ± 8.5†* |
| DHO | 127.6 ± 20.5 | 129.8 ± 16.4 | 79.3 ± 8.4†* |
| Capacitance (pF) | 125.5 ± 22.3 | 116.9 ± 13.5 | 38.2 ± 5.3†* |
| Ih (mV) | 4.30 ± 0.8 | 4.35 ± 0.8 | 1.17 ± 0.3†* |
| Post-train AHP (mV) | 4.32 ± 0.8 | 3.72 ± 0.7 | 0.95 ± 0.1†* |
| Firing properties | |||
| Type (RS/IB) | 8/1 | 8/2 | |
| Mean firing frequency (Hz) | 14.8 ± 2.6 | 14.1 ± 3.0 | 73.4 ± 35.3†* |
| f–I slope | 7.4 ± 0.2 | 7.0 ± 0.4 | 120.8 ± 14.3†* |
| Adaptation index | 18.5 ± 3.1 | 20.3 ± 4.8 | 9.1 ± 5.9 |
| Mean age (postnatal days) (range) | 19.8 ± 3.0 (17–27) | 17.8 ± 1.0 (16–27) | 21.0 ± 0.9 (18–27) |
Values ± x are means ± s.e.m. Ih, membrane potential sag, reflective of hyperpolarization-activated cation current; AHP, afterhyperpolarization potential; RS, regular spiking; IB, intrinsically bursting; f–I, frequency–current. Firing properties were calculated for the first depolarizing step (in 50 pA increments) that reliably produced action potentials (100 pA for PYR, 250 pA for FS).The f–I slope and adaptation index were calculated as described in the Methods.
P < 0.05 statistically different from PYR1.
P < 0.05 statistically different from PYR2.
Figure 3. DHO does not alter input resistance in any of the different neuronal types.
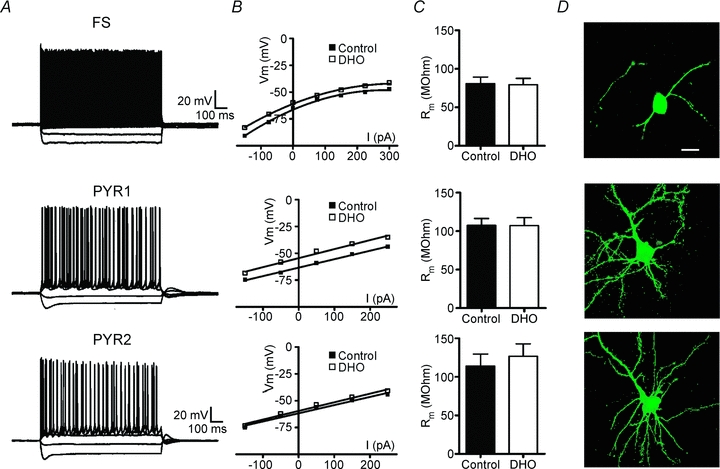
A, current clamp recordings in response to applied intracellular current steps (−150 to 300 pA, 1 s) in a FS, PYR1 or PYR2 neuron. Note the similarity in the recordings from both types of PYR neurons. B, V–I plots of cells recorded in A in control or during the peak membrane depolarization in response to DHO application (100 μm, 30 s). C, mean (±s.e.m.) membrane resistance (Rm) calculated from current steps applied in control or during DHO. Na+ −K+ ATPase blockade by DHO did not significantly alter Rm in any of the cell types. Additionally, no difference was observed between the PYR1 and PYR2 groups (P = 0.73 control, P = 0.33 with DHO). D, representative biocytin-filled neurons of each recorded cell type. Scale bar: 20 μm applies for all images.
Na+ −K+ ATPase activity induced by increased intracellular Na+ varies among classes of neocortical neurons
It is clear that both FS interneurons and PYR1 neurons have more active resting Na+ −K+ ATPase activity than PYR2 neurons. However, only a portion of the total Na+ −K+ ATPase molecules are phosphorylated and thus active at rest and sensitive to pharmacological blockade (Forbush & Hoffman, 1979; Antonelli et al. 1989). To test the Na+ −K+ ATPase capacity of the different cell groups we induced Na+ −K+ ATPase activity by intracellularly loading cells with Na+ using two methods. First, we focally applied 20 mm glutamate to slices while recording the resulting neuronal currents in FS and PYR neurons. In previous experiments in hippocampus, similar glutamate puffs were shown to be an indicator of Na+ −K+ ATPase activity (Thompson & Prince, 1986; Fukuda & Prince, 1992a,b;). In the present experiments under voltage clamp, the glutamate puff induced a fast, large inward current that quickly decayed, followed by a transient outward current in all cells. An example from an FS interneuron is displayed in Fig. 4A, Control. The glutamate puff was then repeated during blockade of the Na+ −K+ ATPase by bath application of 100 μm DHO. The resulting current is thus independent of Na+ −K+ ATPase activity and results primarily from the direct glutamate response (DGR) mediated by ionotropic glutamate receptors (Fig. 4A, DGR). These DGR currents were then averaged and digitally subtracted from the average control responses thereby revealing the isolated DHO-sensitive Na+ −K+ ATPase current (Fig. 4A, Na+ −K+ ATPase Activity) (see Methods for further details). A comparison between the neuronal types (Fig. 4B) revealed that the Na+ −K+ ATPase charge in FS interneurons (13.7 ± 2.2 nC, n = 12) was much greater than that in either PYR1 (2.8 ± 0.3 nC, n = 3) or PYR2 neurons (1.5 ± 0.6 nC, n = 5; P < 0.05). PYR neuron grouping was determined as above by the amplitude of the response to blockade of resting Na+ −K+ ATPase activity. Next we tested for a potential difference in sensitivity to the glutamate puffs between neuronal groups by varying the duration of the glutamate puff (0.02–1.0 s) applied to each type of neuron. At glutamate puff durations of 0.5 s and greater, FS interneurons showed more Na+ −K+ ATPase charge than either PYR cell type (P < 0.05; Fig. 4C). In contrast, no statistically significant difference between the PYR groups could be determined in the Na+ −K+ ATPase charge for any puff duration tested (Fig. 4C).
Figure 4. Glutamate puff-induced activation of the Na+ −K+ ATPase.
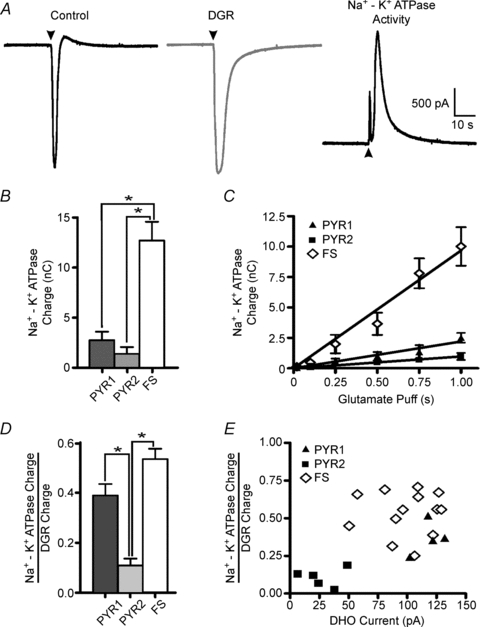
Glutamate (20 mm) was locally delivered through a patch-pipette by a brief pressure pulse (31 kPa, 1 s). A: left, representative trace from a FS interneuron under voltage clamp in response to a glutamate puff in control. Glutamate evoked a large amplitude, fast rising inward current followed by a rebound outward current. Middle, following blockade of the Na+ −K+ ATPase with 100 μm DHO the glutamate puff is repeated and the DGR recorded. Right, digital subtraction of the DGR response from the control response reveals the underlying DHO-sensitive Na+ −K+ ATPase current (Na+ −K+ ATPase activity). B, Na+ −K+ ATPase charge (mean ± s.e.m.) for FS, PYR1 and PYR2 neurons calculated as the area under the Na+ −K+ ATPase activity curve and then averaged across trials (see Methods for more details). PYR neurons were grouped based on the amplitude of the current induced by DHO as in Fig. 1. C, comparison of Na+ −K+ ATPase charge for the three groups across multiple glutamate puff durations. D, an estimate of the fraction of Na+ −K+ ATPase activity induced by the glutamate puff (Na+ −K+ ATPase charge) against the total induced non-Na+ −K+ ATPase activity (DGR charge) (mean ± s.e.m.). E, fractional glutamate puff-induced Na+ −K+ ATPase activity is plotted against the resting Na+ −K+ ATPase activity (DHO current). *P > 0.05, Vm = −70 mV.
Neocortical neurons differ in a wide range of properties (e.g. morphological, synaptic, receptor complement) that may differentially influence their sensitivity to activation by a glutamate puff. As stated, during blockade of the Na+ −K+ ATPase with DHO, the resulting charge induced by a glutmate puff (Fig. 4A, middle) would be indicative of the cell's direct response to glutamate (DGR), independent of Na+ −K+ ATPase activity. As a result, by normalizing the Na+ −K+ ATPase charge (a measure of Na+ −K+ ATPase-induced activity) to the DGR charge (a measure of non-Na+ −K+ ATPase-induced activity), we obtained an estimate of the induced Na+ −K+ ATPase activity independent of any variance in application or responsiveness to the glutamate puff across cell types. The results indicated that both FS and PYR1 neurons exhibited significantly greater normalized charge than PYR2 neurons (P < 0.05; Fig. 4D). This suggests that FS and PYR1 neurons are more sensitive to activation of Na+ −K+ ATPase induced by increases in [Na+]i. Finally, a comparison of this measure of induced Na+ −K+ ATPase activity (Na+ −K+ ATPase charge/DHO charge) in individual cells against their respective resting Na+ −K+ ATPase activity (DHO current) revealed a separation of the two PYR groups based on both resting and induced Na+ −K+ ATPase activity and a similarity in response between FS and PYR1 neurons (Fig. 4E). Therefore, resting Na+ −K+ ATPase activity is a strong indicator of induced Na+ −K+ ATPase activity for these cell types.
To directly test the potential for differential sensitivity to Na+-induced Na+ −K+ ATPase activity across cell types, we increased the concentration of Na+ in the patch pipette solution to 40 or 70 mm. These concentrations are known to activate both the α1 and α3 Na+ −K+ ATPase isoforms (Blanco & Mercer, 1998). We then compared the induced current resulting from perfusion with various concentrations of Na+ −K+ ATPase antagonists in the Na+-loaded neurons with that obtained using the control (2 mm Na+) intracellular solution. After achieving whole-cell configuration, the cells were dialysed for a minimum of 10 min with the higher Na+ internal solutions, and a stable baseline holding current achieved for a minimum of 3 min before a series of successive ouabain concentrations (1, 20 and 100 μm) were applied to each cell. Representative traces of responses to ouabain from PYR1- and PYR2-type neurons are shown in Fig. 5A. For these experiments, ouabain was chosen for its high affinity, and lack of washout. Therefore, stable baseline levels could be recorded for each concentration while minimizing the potential for partial drug washout. Two distinct groups of amplitude responses induced by 20 μm ouabain were evident in Na+-loaded PYR neurons, consistent with the previous results obtained from non-loaded PYR neurons (Fig. 2C). Consequently, PYR grouping in these experiments was based on the amplitude of the response to 20 μm ouabain. Application of 1 μm ouabain had little effect on any of the cell types (Fig. 5B). When exposed to 20 or 100 μm ouabain, PYR1 neurons loaded with 70 mm Na+ generated more current than comparably Na+-loaded PYR2 or FS neurons (Fig. 5B). Interestingly, the percentage increase in response to 100 μm ouabain was similar for both PYR1 (199.5% of control levels) and PYR2 neurons (172.4% of control) loaded with 70 mm Na+. This suggests that high internal Na+ concentrations (70 mm) equally activate the available Na+ −K+ ATPase molecules in both PYR groups, thereby supporting our initial finding that PYR1 neurons have a greater total number of Na+ −K+ ATPase molecules than PYR2. PYR1 neurons were also more sensitive to Na+ loading than PYR2 neurons, as internal perfusion with both 40 and 70 mm Na+ increased the Na+ −K+ ATPase current blocked by 100 μm ouabain above the control value (P < 0.05; Fig. 5B). In FS interneurons, increases in internal Na+ had no effect on the response to 1 or 20 μm ouabain. However, in FS cells loaded with 70 mm Na+, the Na+ −K+ ATPase current blocked by 100 μm ouabain was significantly increased (227.9 ± 17.6 pA) compared to that recorded in control 2 mm[Na+]i (109.5 ± 9.5 pA) or 40 mm[Na+]i (139.5 ± 4.6 pA) (P < 0.01; Fig. 5B).
Figure 5. Increasing internal Na+ concentration increases resting Na+ −K+ ATPase activity in all cell types.
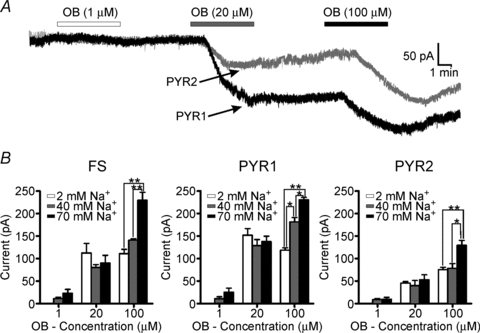
A, voltage clamp trace from a PYR1 (black) or PYR2 (grey) neuron loaded with 70 mm Na+ internally through the patch pipette. After sufficient time was allowed for dialysis of the Na+ (> 10 min) and stability achieved in the baseline recording, ouabain (OB) was applied at various concentrations (1, 20 and 100 μm). Application of 20 μm ouabain produced two distinct groups of responses in PYR neurons, consistent with our previous findings in non-loaded neurons, and was used for PYR neuron grouping (PYR1 and PYR2). B, mean (±s.e.m.) current recorded from FS (n = 18), PYR1 (n = 10) or PYR2 (n = 14) neurons in different internal Na+ concentrations. Cells were loaded with control (2 mm), 40 or 70 mm Na+. Loading with 70 mm Na+ increased the current induced by 100 μm, but not lower concentrations of ouabain, in all cell types. Only PYR1 neurons were more sensitive to 40 mm Na+ compared to control (2 mm). *P > 0.05, **P > 0.01. Vm = −70mV.
Discussion
Na+ −K+ ATPase activity in cortical neurons
We studied the activity of the Na+ −K+ ATPase in cortical layer V fast-spiking (FS) interneurons and pyramidal (PYR) neurons to test the hypothesis that Na+ −K+ ATPase function would vary between cell types and would be significantly more pronounced in fast-spiking interneurons. As expected, pharmacological blockade of the Na+ −K+ ATPase resulted in a membrane depolarization under current clamp or an increase of inward current under voltage-clamp conditions. PYR cells could be clearly separated into two groups based on the amplitude of responses to blockade of Na+ −K+ ATPase. PYR1 neurons comprised 48% of the PYR population and had significantly greater Na+ −K+ ATPase-dependent currents than PYR2 cells. In contrast, the response of FS interneurons was homogeneous and intermediate in amplitude between that of the two groups of PYR neurons. However, when cell size (membrane capacitance) was taken into account, FS interneurons possessed a 3- to 7-fold greater Na+ −K+ ATPase-dependent current density than either of the PYR groups. Despite their smaller cell size, the input resistance of FS interneurons is lower than that of PYR cells (Table 1) as previously reported (Angulo et al. 1999; Bacci et al. 2003). The higher density and level of resting Na+ −K+ ATPase activity (Figs 1 and 3) could play a role in the maintenance of a more hyperpolarized resting membrane potential and maintenance of high frequency firing in FS cells in such ‘leaky’ neurons.
Under normal resting conditions only a portion of the total membrane-bound Na+ −K+ ATPase is phosphorylated and available to contribute to the measured change in membrane voltage or current when the Na+ −K+ ATPase is pharmacologically blocked. By increasing internal Na+, either directly (pipette Na+ loading) or indirectly (glutamate puff), we were able to assess each neuron's responsiveness to Na+ and their capacity to activate the Na+ −K+ ATPase. The result was greater activation of Na+ −K+ ATPase-dependent currents in FS interneurons and PYR1 cells than in PYR2 neurons. In the glutamate puff experiments it was possible to compare the resting Na+ −K+ ATPase activity, measured as the change in holding current during the initial Na+ −K+ ATPase blockade, with the increased Na+ −K+ ATPase-dependent current, measured as the component of charge induced by the glutamate puff that was sensitive to Na+ −K+ ATPase block by DHO (Figs 4 and 5). In this way, the relationship between resting Na+ −K+ ATPase activity and total Na+ −K+ ATPase activity activated by a Na+ load could be determined. FS and PYR1 neurons have both higher resting Na+ −K+ ATPase activity and greater ability to increase Na+ −K+ ATPase activity, allowing them to accommodate a wider range of Na+ loads with increases in Na+ −K+ ATPase activity.
The subgroups of PYR neurons differ in Na+ −K+ ATPase activity but not intrinsic properties
It is clear from the experimental data that two distinct groups of PYR neurons with varying Na+ −K+ ATPase activity exist in layer V cortex; however, we have been unable to detect any correlations between resting Na+ −K+ ATPase activity and any measured electrophysiological property. Responses from both PYR groups were observed on the same day, from the same animal and with the same stock of Na+ −K+ ATPase antagonists. As the Na+ −K+ ATPase antagonists, especially ouabain, are difficult to wash out, we recorded from only one neuron per slice to be certain that residual Na+ −K+ ATPase blockade was not contributing to our results. The presence of two distinct groups with no gradation in the distribution (Fig. 1B) helped rule out potential artifacts such as depth of recording in slice, slice health and/or drug penetration. Our data strongly suggest that the differences in recorded Na+ −K+ ATPase activity relate to differences in cell expression of Na+ −K+ ATPase and not to artifacts of recording conditions, slice preparation or other intrinsic properties of the recorded PYR neurons. However, we cannot rule out more subtle differences in the electrophysiological properties or morphology of the PYR 1 and 2 subgroups not tested in this study.
The Na+ −K+ ATPase is a protein multimer consisting of alpha (α) and beta (β) subunits (Lingrel, 1992). The α subunit has two neuronal forms (α1 and α3) that determine the major enzymatic and transporter properties of the molecule and confer sensitivity to blockade by Na+ −K+ ATPase antagonists (i.e. ouabain and DHO). Specifically, the α3 subunit is less sensitive to changes in Na+ and K+ and is much more sensitive to activation by ATP and blockade by Na+ −K+ ATPase antagonists than the α1 isoform (Blanco & Mercer, 1998). In situ analysis of the neocortex has shown protein levels for both the α1 and α3 isoform, with the α3 isoform being heavily expressed in PYR neurons (Hieber et al. 1991). In testing the sensitivity of PYR neurons to ouabain and DHO, we observed a distinct concentration range over which the PYR neuron grouping was evident. Low doses of ouabain (with high α3 affinity) separated the groups as did higher doses of DHO (with lower α3 affinity). Interestingly, higher doses of ouabain (100 μm) failed to separate the PYR groups. This concentration of ouabain (100 μm) would be expected to inhibit both the α1 and α3 isoforms (Blanco & Mercer, 1998). While the maximum Na+ −K+ ATPase current induced by 100 μm ouabain was similar to that observed with 20 μm ouabain, the small amplitude current responses were no longer evident. In the Na+-loading experiments, the PYR neurons with small responses to 20 μm ouabain (PYR2) also showed the smaller responses to 100 μm ouabain. These results suggest that the lack of grouping on resting Na+ −K+ ATPase activity with low dose DHO (20 or 50 μm) may be due to PYR2 neurons being non-responsive to this level of Na+ −K+ ATPase blockade. At higher doses a ceiling effect may be imposed such that the responses of PYR1 neurons are muted due to the limited number of Na+ −K+ ATPase molecules active at rest and thus sensitive to blockade. The Na+ −K+ ATPase capacity of PYR1 was not appreciated with modest challenges to the pump, but only observed when activated by a strong intracellular Na+ load (70 mm) Taken together, these findings suggest that there is a difference in the isoform composition of the two PYR groups. This is also well supported by the observed differences in Na+ and ATP sensitivity in the PYR neuron groups (Blanco & Mercer, 1998; Dobretsov & Stimers, 2005 for review). Similar results across neuronal subtypes have been recently reported in hippocampal subiculum neurons, where interneurons were more sensitive to blockade by ouabain than pyramidal neurons (Richards et al. 2007). The difference was attributed to differential expression of α isoforms of the Na+ −K+ ATPase. Here we show that such a difference in α isoform expression may exist between and even within subtypes of neocortical neurons. This is in line with studies showing that the membrane density of Na+ −K+ ATPase may vary between cell types and even within the membrane distribution of a single cell (Shyjan et al. 1990; Brines et al. 1991; Hieber et al. 1991; McGrail et al. 1991; Brines & Robbins, 1993). However, immunohistochemical results on biocytin-filled neurons from our experiments or from naive control animals were inconclusive. There was no apparent difference in association of the Na+ −K+ ATPase α1 or α3 isoforms between FS and PYR neurons or within PYR neuron subtypes (data not shown). The inability to distinguish between FS and PYR neuron Na+ −K+ ATPase immunoreactivity may be due to poor antibody penetration and/or the insensitivity of the antibody to detect small differences in membrane density that are more easily resolved at the electrophysiological level.
The Na+ −K+ ATPase significantly contributes to the resting membrane potential. However, here we found no significant difference in resting membrane potential between the PYR1 and PYR2 groups – although there was a trend towards PYR1 being more hyperpolarized. Several factors may contribute to this finding. The PYR neurons may have similar net resting Na+ −K+ ATPase activity but differ in relative α isoform-specific activity and thus sensitivity to blockade by the more α3-specific Na+ −K+ ATPase antagonists. At present, to our knowledge, no α1-specific antagonists exist. Preliminary experiments with the new α3 isoform-specific antagonist, Agrin-95 (Hilgenberg et al. 2006) have yielded similar differences in FS and PYR neuron resting Na+ −K+ ATPase activity to those described above. Actions of other ATPases (e.g. K+ or Ca2+), transporters (e.g. Na+/K+/2Cl− or Na+/Ca2+) or protein kinases may also differentially contribute in the PYR neuron groups. In addition, potential differences in local microenvironment (ionic or synaptic) due to architecture or even differences in glial localization may selectively alter the demand on resting Na+ −K+ ATPase activity. The two populations of PYR cells may therefore express different densities and isoforms of the Na+ −K+ ATPase to meet the challenges of their local environment.
The Na+ −K+ ATPase is a dynamically regulated membrane protein whose expression is controlled by activity, endogenous inhibitors and several intracellular messengers (Ross & Soltesz, 2001; Therien & Blostein, 2000; Kang et al. 2003; Dobretsov & Stimers, 2005). Detailed testing of the intrinsic properties between the two groups of PYR neurons failed to reveal any significant differences that correlated with differences in their Na+ −K+ ATPase activity. One possibility is that differences in local activity help to promote higher Na+ −K+ ATPase levels in one group of PYR neurons than the other. For example, differences in Na+ −K+ ATPase activity between neurons may reflect differences in the type or origin of afferent synaptic input to subgroups of cells (Senatorov & Hu, 1997). Na+ −K+ ATPase activity may both regulate and be regulated by release of several neurotransmitters (Phillis & Wu, 1981; Hernandez & Condes-Lara, 1992). The separation of the response of the PYR neurons into two electrophysiologically distinct groups required a relatively high dose of Na+ −K+ ATPase antagonists. At these concentrations the Na+ −K+ ATPase antagonists can cause neurotransmitter release and induce spreading depression if applied in the absence of NMDA antagonists or TTX (Muller & Somjen, 2000; Anderson & Andrew, 2002). In fact, glutamate has been shown to preferentially activate α3 on cerebellar and cerebral neurons (Dobretsov & Stimers, 2005). Therefore, in this study synaptic transmission was routinely blocked by bath application of d-APV, DNQX, picrotoxin and TTX. Although this aided in isolating the Na+ −K+ ATPase activity without contamination by synaptic currents, it prevented a detailed study of the potential reciprocal regulation of synaptic transmission and Na+ −K+ ATPase activity, or differences in synaptic input to the three groups of neurons examined here.
Conclusions
It is evident that expression of Na+ −K+ ATPase varies across and within types of cortical neurons and that differences extend to the state of resting Na+ −K+ ATPase activity as well as total Na+ −K+ ATPase capacity. Differences in Na+ −K+ ATPase activity within an otherwise homogeneous cell population would have an important impact on cellular function both at rest and especially during periods of high cellular activity. By defining the nature of these differences, we can begin to understand how they may contribute to control neuronal activities in functional states where there is increased demand for Na+ −K+ ATPase activity. For example, FS and PYR1 neurons may be better equipped than PYR2 neurons to ‘cope’ with states of excessive activity, such as those that occur during epileptiform discharges. The potential adaptive or maladaptive effects of high or low Na+ −K+ ATPase density and capacity during periods of hyperexcitability, and alterations in pathophysiological processes, such as those resulting from cortical injury and epileptogenesis, will be important to explore in future experiments.
Acknowledgments
We thank Isabel Parada for histological assistance. This work was supported by NIH grants NS12151 and NS59379.
Glossary
Abbreviations
- aCSF
artificial cerebrospinal fluid
- DGR
direct glutamate response
- DHO
dihydro-ouabain
- FS
fast spiking
- IB
intrinsically bursting
- Ih
membrane potential sag, reflective of hyperpolarization-activated cation current
- Na+ −K+ ATPase
sodium–potassium ATPase
- PYR
pyramidal
- RS
regular spiking
Author contributions
T.R.A.: collection, analysis and interpretation of data, drafting and revising the manuscript, final approval of the manuscript. J.R.H. and D.A.P.: drafting and revising the manuscript, conception and design of the experimental protocol, final approval of the manuscript.
References
- Anderson TR, Andrew RD. Spreading depression: imaging and blockade in the rat neocortical brain slice. J Neurophysiol. 2002;88:2713–2725. doi: 10.1152/jn.00321.2002. [DOI] [PubMed] [Google Scholar]
- Anderson TR, Jarvis CR, Biedermann AJ, Molnar C, Andrew RD. Blocking the anoxic depolarization protects without functional compromise following simulated stroke in cortical brain slices. J Neurophysiol. 2005;93:963–979. doi: 10.1152/jn.00654.2004. [DOI] [PubMed] [Google Scholar]
- Anderson WR, Franck JE, Stahl WL, Maki AA. Na,K-ATPase is decreased in hippocampus of kainate-lesioned rats. Epilepsy Res. 1994;17:221–231. doi: 10.1016/0920-1211(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Rossier J, Audinat E. Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J Neurophysiol. 1999;82:1295–1302. doi: 10.1152/jn.1999.82.3.1295. [DOI] [PubMed] [Google Scholar]
- Antonelli MC, Baskin DG, Garland M, Stahl WL. Localization and characterization of binding sites with high affinity for [3H]ouabain in cerebral cortex of rabbit brain using quantitative autoradiography. J Neurochem. 1989;52:193–200. doi: 10.1111/j.1471-4159.1989.tb10916.x. [DOI] [PubMed] [Google Scholar]
- Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J Neurosci. 2003;23:9664–9674. doi: 10.1523/JNEUROSCI.23-29-09664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcar VJ. Molecular pharmacology of the Na+-dependent transport of acidic amino acids in the mammalian central nervous system. Biol Pharm Bull. 2002;25:291–301. doi: 10.1248/bpb.25.291. [DOI] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- Brines ML, Gulanski BI, Gilmore-Hebert M, Greene AL, Benz EJ, Jr, Robbins RJ. Cytoarchitectural relationships between [3H]ouabain binding and mRNA for isoforms of the sodium pump catalytic subunit in rat brain. Brain Res Mol Brain Res. 1991;10:139–150. doi: 10.1016/0169-328x(91)90104-6. [DOI] [PubMed] [Google Scholar]
- Brines ML, Robbins RJ. Cell-type specific expression of Na+,K+-ATPase catalytic subunits in cultured neurons and glia: evidence for polarized distribution in neurons. Brain Res. 1993;631:1–11. doi: 10.1016/0006-8993(93)91179-v. [DOI] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW. Initiation of synchronized neuronal bursting in neocortex. Nature. 1984;310:685–687. doi: 10.1038/310685a0. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ, Prince DA. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982;48:1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Dobretsov M, Stimers JR. Neuronal function and alpha3 isoform of the Na/K-ATPase. Front Biosci. 2005;10:2373–2396. doi: 10.2741/1704. [DOI] [PubMed] [Google Scholar]
- Donaldson J, St Pierre T, Minnich J, Barbeau A. Seizures in rats associated with divalent cation inhibition of Na+-K+-ATP’ase. Can J Biochem. 1971;49:1217–1224. doi: 10.1139/o71-175. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes MJ, Naffah-Mazzacoratti MG, Cavalheiro EA. Na+K+ ATPase activity in the rat hippocampus: a study in the pilocarpine model of epilepsy. Neurochem Int. 1996;28:497–500. doi: 10.1016/0197-0186(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Forbush B, III, Hoffman JF. Evidence that ouabain binds to the same large polypeptide chain of dimeric Na,K-ATPase that is phosphorylated from Pi. Biochemistry. 1979;18:2308–2315. doi: 10.1021/bi00578a027. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Prince DA. Excessive intracellular Ca2+ inhibits glutamate-induced Na+-K+ pump activation in rat hippocampal neurons. J Neurophysiol. 1992a;68:28–35. doi: 10.1152/jn.1992.68.1.28. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Prince DA. Postnatal development of electrogenic sodium pump activity in rat hippocampal pyramidal neurons. Brain Res Dev Brain Res. 1992b;65:101–114. doi: 10.1016/0165-3806(92)90013-m. [DOI] [PubMed] [Google Scholar]
- Gadsby DC, Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J Gen Physiol. 1989;94:511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guatteo E, Bacci A, Franceschetti S, Avanzini G, Wanke E. Neurons dissociated from neocortex fire with ‘burst’ and ‘regular’ trains of spikes. Neurosci Lett. 1994;175:117–120. doi: 10.1016/0304-3940(94)91093-6. [DOI] [PubMed] [Google Scholar]
- Hernandez J, Condes-Lara M. Brain Na+/K+-ATPase regulation by serotonin and norepinephrine in normal and kindled rats. Brain Res. 1992;593:239–244. doi: 10.1016/0006-8993(92)91313-4. [DOI] [PubMed] [Google Scholar]
- Hieber V, Siegel GJ, Fink DJ, Beaty MW, Mata M. Differential distribution of (Na, K)-ATPase alpha isoforms in the central nervous system. Cell Mol Neurobiol. 1991;11:253–262. doi: 10.1007/BF00769038. [DOI] [PubMed] [Google Scholar]
- Hilgenberg LG, Su H, Gu H, O’Dowd DK, Smith MA. α3Na+/K+-ATPase is a neuronal receptor for agrin. Cell. 2006;125:359–369. doi: 10.1016/j.cell.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Hoffman SN, Salin PA, Prince DA. Chronic neocortical epileptogenesis in vitro. J Neurophysiol. 1994;71:1762–1773. doi: 10.1152/jn.1994.71.5.1762. [DOI] [PubMed] [Google Scholar]
- Honegger P, Pardo B. Separate neuronal and glial Na+,K+-ATPase isoforms regulate glucose utilization in response to membrane depolarization and elevated extracellular potassium. J Cereb Blood Flow Metab. 1999;19:1051–1059. doi: 10.1097/00004647-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Kang TC, Hwang IK, Park SK, An SJ, Nam YS, Kim DH, Lee IS, Won MH. Elevation of Na+-K+ ATPase immunoreactivity in GABAergic neurons in gerbil CA1 region following transient forebrain ischemia. Brain Res. 2003;977:284–289. doi: 10.1016/s0006-8993(03)02681-7. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol. 1993;70:387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- Kim JH, Sizov I, Dobretsov M, von Gersdorff H. Presynaptic Ca2+ buffers control the strength of a fast post-tetanic hyperpolarization mediated by the alpha3 Na+/K+-ATPase. Nat Neurosci. 2007;10:196–205. doi: 10.1038/nn1839. [DOI] [PubMed] [Google Scholar]
- Kumar AR, Kurup PA. Inhibition of membrane Na+-K+ ATPase activity: a common pathway in central nervous system disorders. J Assoc Physicians India. 2002;50:400–406. [PubMed] [Google Scholar]
- Lees GJ. Inhibition of sodium-potassium-ATPase: a potentially ubiquitous mechanism contributing to central nervous system neuropathology. Brain Res Brain Res Rev. 1991;16:283–300. doi: 10.1016/0165-0173(91)90011-v. [DOI] [PubMed] [Google Scholar]
- Liang M, Cai T, Tian J, Qu W, Xie ZJ. Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J Biol Chem. 2006;281:19709–19719. doi: 10.1074/jbc.M512240200. [DOI] [PubMed] [Google Scholar]
- Lingrel JB. Na,K-ATPase: isoform structure, function, and expression. J Bioenerg Biomembr. 1992;24:263–270. doi: 10.1007/BF00768847. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McGrail KM, Phillips JM, Sweadner KJ. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na,K-ATPase. J Neurosci. 1991;11:381–391. doi: 10.1523/JNEUROSCI.11-02-00381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Muller M, Somjen GG. Na+ dependence and the role of glutamate receptors and Na+ channels in ion fluxes during hypoxia of rat hippocampal slices. J Neurophysiol. 2000;84:1869–1880. doi: 10.1152/jn.2000.84.4.1869. [DOI] [PubMed] [Google Scholar]
- O'Shea RD. Roles and regulation of glutamate transporters in the central nervous system. Clin Exp Pharmacol Physiol. 2002;29:1018–1023. doi: 10.1046/j.1440-1681.2002.03770.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson . The Rat Brain in Stereotactic Coordinates. 4th edn. San Diego: Academic Press; 1998. [Google Scholar]
- Phillis JW, Wu PH. Catecholamines and the sodium pump in excitable cells. Prog Neurobiol. 1981;17:141–184. doi: 10.1016/0301-0082(81)90012-5. [DOI] [PubMed] [Google Scholar]
- Prince DA, Tseng GF. Epileptogenesis in chronically injured cortex: in vitro studies. J Neurophysiol. 1993;69:1276–1291. doi: 10.1152/jn.1993.69.4.1276. [DOI] [PubMed] [Google Scholar]
- Rapport RL, Harris AB, Friel PN, Ojemann GA. Human epileptic brain Na,K ATPase activity and phenytoin concentrations. Arch Neurol. 1975;32:549–554. doi: 10.1001/archneur.1975.00490500069008. [DOI] [PubMed] [Google Scholar]
- Reich CG, Mason SE, Alger BE. Novel form of LTD induced by transient, partial inhibition of the Na,K-pump in rat hippocampal CA1 cells. J Neurophysiol. 2004;91:239–247. doi: 10.1152/jn.00722.2003. [DOI] [PubMed] [Google Scholar]
- Reime Kinjo E, Arida RM, Mara de Oliveira D, da Silva Fernandes MJ. The Na+/K+ATPase activity is increased in the hippocampus after multiple status epilepticus induced by pilocarpine in developing rats. Brain Res. 2007;1138:203–207. doi: 10.1016/j.brainres.2006.12.068. [DOI] [PubMed] [Google Scholar]
- Richards KS, Bommert K, Szabo G, Miles R. Differential expression of Na+/K+-ATPase α-subunits in mouse hippocampal interneurons and pyramidal cells. J Physiol. 2007;585:491–505. doi: 10.1113/jphysiol.2007.144733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ST, Soltesz I. Selective depolarization of interneurons in the early posttraumatic dentate gyrus: involvement of the Na+/K+-ATPase. J Neurophysiol. 2000;83:2916–2930. doi: 10.1152/jn.2000.83.5.2916. [DOI] [PubMed] [Google Scholar]
- Ross ST, Soltesz I. Long-term plasticity in interneurons of the dentate gyrus. Proc Natl Acad Sci USA. 2001;98:8874–8879. doi: 10.1073/pnas.141042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin P, Tseng GF, Hoffman S, Parada I, Prince DA. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci. 1995;15:8234–8245. doi: 10.1523/JNEUROSCI.15-12-08234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatorov VV, Hu B. Differential Na+-K+-ATPase activity in rat lemniscal and non-lemniscal auditory thalami. J Physiol. 1997;502:387–395. doi: 10.1111/j.1469-7793.1997.387bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyjan AW, Cena V, Klein DC, Levenson R. Differential expression and enzymatic properties of the Na+,K+-ATPase α3 isoenzyme in rat pineal glands. Proc Natl Acad Sci USA. 1990;87:1178–1182. doi: 10.1073/pnas.87.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Somogyi P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J Neurosci. 1997;17:6352–6364. doi: 10.1523/JNEUROSCI.17-16-06352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno T, Robinson HPC. Threshold firing frequency-current relationships of neurons in rat somatosensory cortex: Type 1 and Type 2 dynamics. J Neurophysiol. 2004;92:2283–2294. doi: 10.1152/jn.00109.2004. [DOI] [PubMed] [Google Scholar]
- Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol. 2000;279:C541–C566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Prince DA. Activation of electrogenic sodium pump in hippocampal CA1 neurons following glutamate-induced depolarization. J Neurophysiol. 1986;56:507–522. doi: 10.1152/jn.1986.56.2.507. [DOI] [PubMed] [Google Scholar]
- Tseng GF, Prince DA. Heterogeneity of rat corticospinal neurons. J Comp Neurol. 1993;335:92–108. doi: 10.1002/cne.903350107. [DOI] [PubMed] [Google Scholar]
- Vaillend C, Mason SE, Cuttle MF, Alger BE. Mechanisms of neuronal hyperexcitability caused by partial inhibition of Na+-K+-ATPases in the rat CA1 hippocampal region. J Neurophysiol. 2002;88:2963–2978. doi: 10.1152/jn.00244.2002. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. GABAA receptor-mediated currents in interneurons and pyramidal cells of rat visual cortex. J Physiol. 1998;506:715–730. doi: 10.1111/j.1469-7793.1998.715bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


