Abstract
Since protein kinase-dependent modulation of motoneuronal excitability contributes to adaptive changes in breathing, we hypothesized that cGMP-dependent pathways activating protein kinase G (PKG) modulate motoneuronal inspiratory drive currents and long-term plasticity. In a medullary slice preparation from neonatal rat (postnatal days 0–4) generating spontaneous respiratory-related rhythm, hypoglossal (XII) motoneuronal inspiratory drive currents and respiratory-related XII nerve activity were recorded. Focal application of a PKG activator, 8-bromoguanosine-3′,5′-cyclomonophosphate (8-Br-cGMP), to voltage-clamped XII motoneurones decreased inspiratory drive currents. In the presence of tetrodotoxin (TTX), 8-Br-cGMP decreased the exogenous postsynaptic inward currents induced by focal application of AMPA. Intracellular dialysis of XII motoneurones with an inhibitory peptide to PKG (PKGI) increased endogenous inspiratory-drive currents and exogenous AMPA-induced currents. Application of 8-Br-cGMP with PKGI had no further effect on spontaneous or evoked currents, confirming that the observed effects were induced by PKG. However, PKG differentially increased longer-term plasticity. Three 3 min applications (separated by 5 min) of the α1-adrenergic agonist phenylephrine (PE) in combination with 8-Br-cGMP yielded greater in vitro long-term facilitation than PE alone. These data indicate the presence of a cGMP/PKG-dependent signalling pathway in XII motoneurones that modulates inspiratory drive currents and plasticity of XII motoneurones, possibly contributing to their adaptation during physiological challenges, such as sleep and exercise.
Introduction
Adaptive changes in breathing are essential to maintain blood-gas homeostasis. A compromised ability to make such adaptations may underlie conditions such as obstructive sleep apnoea (OSA), where flaccidity of upper airway muscles, including the genioglossus muscle of the tongue, during non-rapid eye movement (REM) and REM sleep leads to collapse and obstruction of the airway precipitating nocturnal hypoxia (Horner & Bradley, 2008). OSA affects a substantial fraction of the adult population and has severe health consequences including neurodegeneration and increased incidence of cardiac failure and stroke (Shamsuzzaman et al. 2003). Failure of a form of adaptive motoneuronal plasticity known as long-term facilitation (LTF) may underlie OSA (Mahamed & Mitchell, 2008). LTF is characterized in vivo by increased respiratory motoneuronal output in response to episodic but not continuous bouts of hypoxia in adult (Baker & Mitchell, 2000; Fuller et al. 2000; Mitchell et al. 2001) and neonatal (McKay et al. 2004) rats, as well as in adult humans during sleep (Babcock & Badr, 1998; Shkoukani et al. 2002). In vitro LTF (ivLTF) in hypoglossal (XII) motoneurones, which innervate the genioglossus muscle, can be induced by episodic application of α-methyl-5-HT (Bocchiaro & Feldman, 2004) or phenylephrine (Neverova et al. 2007), the latter response being protein kinase C (PKC), but not protein kinase A (PKA) dependent.
XII motoneurones receive excitatory (Funk et al. 1993) and inhibitory (Saywell & Feldman, 2004) inputs from premotor neurones in respiratory rhythmic brainstem slices from neonatal rodents. Protein kinase activity contributes to neuronal plasticity at glutamatergic and GABAergic synapses in XII motoneurones (Bocchiaro et al. 2003; Saywell & Feldman, 2004; Neverova et al. 2007). Given the manifold roles of protein kinases in modulating the excitability of XII motoneurones and the abundance of PKG in XII motoneurones (de Vente et al. 2001), we investigated the role of PKG in XII motoneuronal plasticity. Elsewhere PKG is involved in synaptic plasticity. For example, cerebellar postsynaptic long-term depression (LTD; Levenes et al. 1998) or long-term potentiation (LTP; Lev-Ram et al. 2002) can be induced by stimulation of a cGMP-dependent pathway. cGMP-dependent pathways are active postsynaptically during induction of hippocampal LTD (Wu et al. 1998) and presynaptically during induction of hippocampal LTP (Arancio et al. 1996). Based on its role in other neurones and evidence in vivo for a role of PKG in the control of genioglossus activity (Aoki et al. 2006), we hypothesized that PKG could affect motoneuronal excitability and impact ivLTF (Bocchiaro & Feldman, 2004; Neverova et al. 2007).
Here we demonstrate that stimulating the cGMP-dependent pathway in XII motoneurones depresses inspiratory drive but has an opposite effect on ivLTF. Stimulation of the PKG pathway increases ivLTF relative to that induced by phenylephrine (PE) alone, yet it is not sufficient on its own to induce facilitation. These data further illuminate the very different and important roles played by protein kinases in modulating short-term excitability and long-term plasticity in XII motoneurones.
Some of these data have been published in abstract form.
Methods
Slice preparation and ethical approval
All animal procedures were performed according to National Institutes of Health guidelines and approved by the Office for the Protection of Research Subjects, University of California Research Committee. In addition experiments comply with the policies and regulations documented in Drummond (2009), which the authors have read. Experiments were performed on neonatal (postnatal days 0–4; Sprague–Dawley rats; n = 48; Charles River Laboratories International Inc., Wilmington, MA, USA) anaesthetized in initial experiments by hypothermia for a minimum of 3 min or in later experiments with isoflurane inhalation (5 ml for 15 min). Surgical anaesthesia was assessed by the absence of limb withdrawal to noxious pinch. Rats were then rapidly decerebrated.
A medullary slice was prepared that retains a sufficient proportion of the respiratory network to generate a respiratory-related rhythm (Smith et al. 1991). Briefly, the brainstem and upper cervical cord were isolated and bathed in artificial cerebrospinal fluid (ACSF) composed of (in mm): NaCl 128.0, KCl 3.0, CaCl2 1.5, MgCl2 1.0, NaHCO3 23.5, NaH2PO4 0.5, d-glucose 30.0, pH 7.4, gassed with 95% O2–5% CO2 pH 7.4 at room temperature. The dura mater, superficial blood vessels and the cerebellum were removed. The remaining brainstem was mounted on a chuck and serial transverse sections (200–300 μm) were cut with a Vibratome VT 100 (Vibratome, Bannockburn, IL, USA) until the identifiable landmarks of the compact formation of the nucleus ambiguus and the inferior olive could be seen. Then a transverse 700 μm slice including the preBötzinger complex (preBötC) and the XII motor nucleus and rootlets was cut. The slice was transferred to a recording chamber and superfused (≥5 ml min−1) with ACSF containing elevated K+ (9 mm) to sustain a stable respiratory-related output. The slice was maintained at a constant temperature of 28°C.
XII nerve recording
A suction electrode was applied to the cut ends of the XII nerve rootlets and discharges from the XII nerve recorded, amplified 1000–5000× and filtered at 1 kHz using a conventional amplifier. Population discharges of the XII nerve rootlets were then rectified and integrated using a Paynter filter (τ = 100 ms). Signals were digitized and stored using Digidata analog-to-digital converters and pCLAMP software (Molecular Devices, Sunnyvale, CA, USA). The rhythmic burst discharges of the XII nerve defined the inspiratory period.
Voltage-clamp recording
XII motoneurones (classified according to the criteria of Funk et al. 1993) were visualized using IR-DIC microscopy. Whole-cell voltage-clamp recordings (holding potential (Vh) = −70 mV) were made from XII motoneurones using electrodes pulled from borosilicate glass on an electrode puller (Model P-97, Sutter Instrument Co., Novato, CA, USA), and filled with patch solution composed of (in mm); 120 potassium gluconate, 11 EGTA, 5 NaCl, 1 CaCl2, 10 Hepes, 2 ATP (Mg2+ salt), pH 7.3 adjusted with KOH (resistance 4–8 MΩ). To help confirm the neurones as motoneurones, Lucifer yellow (Molecular Probes, Eugene, OR, USA) was included in the patch solution to intracellularly label the neurones. Neurones were subsequently examined under an epifluorescence microscope (Axioskop, Carl Zeiss MicroImaging, Thornwood, NY, USA) to confirm their location, examine their morphology and identify axons projecting in the XII nerve tract.
The patch-clamp electrode was advanced toward neurones under positive pressure. Once the electrode tip approached a neurone, positive pressure was released and a gigaohm seal formed by application of negative pressure. Neurones were then ruptured by an additional brief application of negative pressure. Access resistance was monitored and was always <30 MΩ. Cells with large or unstable access resistances were rejected. Intracellular signals were acquired using an Axopatch 1D amplifier filtered using a −3 dB Bessel filter and digitized at 10 kHz via a Digidata 1200 interface with a software filter (bandpass: 2 Hz to 5 kHz) in pCLAMP software (Molecular Devices). Junction potentials between bath solution and electrode were corrected and whole-cell capacitance was compensated.
Data analysis
Averages of integrated XII nerve activity and respiratory-related membrane currents were constructed using the rising phase of the integrated XII nerve activity to trigger acquisition of a 5 s epoch of membrane current and integrated XII nerve discharge. Averages of 10 consecutive respiratory cycles were constructed. Recordings were analysed off-line using Clampex software (Molecular Devices), DataView (W. Hitler, http://www.st-andrews.ac.uk/~wjh/dataview/) and exported to Origin (OriginLab Corp., Northampton, MA, USA). For ivLTF measurements, peak amplitudes of all integrated XII nerve bursts occurring in 5 min windows centred at 15, 30, 45, or 60 min post-ivLTF protocol were averaged and normalized relative to the 30 min pre-protocol control period.
Results are given as means ± s.d.t tests were used to determine statistical significance between paired groups of observations. P ≤ 0.05 was termed significant. For experiments where repeated measures were involved, repeated measures ANOVA (one-way or two-way mixed) was conducted first to determine a significant influence of the factor in question (P ≤ 0.05). Then protected repeated measures t tests were conducted to compare paired groups of observations (Cohen & Lea, 2004). A natural logarithmic transformation was used on normalized data used for statistical tests.
Power analysis was conducted by measuring the cumulative non-central F-distribution for a given effect size that fell below the critical value of the central F-distribution that represented a 5% chance of falsely rejecting a valid null hypothesis. Degrees of freedom and variance estimates for the power analysis were taken from the parent ANOVA. SAS (SAS Institute, Cary, NC, USA) was used to calculate non-central and central F-distributions.
Drugs and drug application
Drugs were dissolved in ACSF for bath application: 8-bromoguanosine-3′,5′-cyclomonophosphate sodium salt (8-Br-cGMP; 100 μm; Sigma-Aldrich, St Louis, MO, USA or Tocris Bioscience, Ellisville, MO, USA), tetrodotoxin (TTX; 1 μm; Sigma), phenylephrine hydrochloride (PE; 10 μm; Sigma). For intracellular dialysis, the membrane-impermeable inhibitory peptide of PKG (PKGI; 100 μm; Sigma) was placed in the patch solution. For application into the whole XII motor nucleus, 8-Br-cGMP (100 μm) was injected from pressure-ejection pipettes (5 p.s.i., 5–6 μm tip diameter, ejection duration 10 s) over XII motoneurones. Similarly, (±)-α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid hydrate (AMPA) (10 μm; Sigma) was applied via pressure-ejection pipettes (15–20 p.s.i., tip 1–2 μm diameter, injection duration 100 ms, interval of 10 s) positioned within 5 μm of the motoneuronal soma.
Results
8-Br-cGMP depresses inspiratory drive currents
To determine if activation of the cGMP-dependent pathway affected endogenous inspiratory drive currents, we recorded from XII motoneurones in whole-cell patch-clamp mode. We ejected 8-Br-cGMP (100 μm) focally over the motoneurone. There was a significant effect of the treatment (P < 0.01; n = 6, one-way repeated measures ANOVA). In particular, 8-Br-cGMP decreased inspiratory drive currents almost immediately to 66 ± 11% of control (P < 0.01; n = 6; repeated measures protected t test comparing treatment with 8-Br-cGMP to control). Currents returned to their control value 94 ± 9% (n = 6; not significant (n.s.); repeated measures protected t test comparing post-treatment to control) within 5 min after terminating ejection (Fig. 1). The return of the currents to baseline within 5 min indicated that the effects were not long-lasting, and post-8-Br-cGMP currents were not monitored for subsequent intracellular experiments.
Figure 1. Focal application of 8-Br-cGMP depresses inspiratory drive currents.
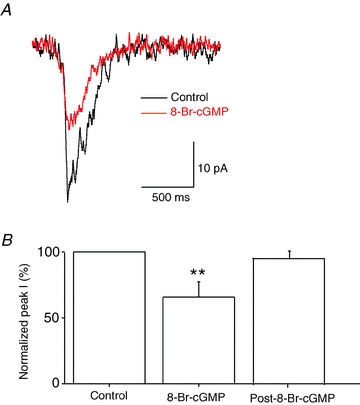
A, endogenous glutamatergic inspiratory drive currents in a XII motoneurone before (black line) and ∼1 min after (red line) focal application of 100 μm 8-Br-cGMP. Traces are averages of 10 individual currents. B, peak endogenous current amplitude decreased following the focal application of 8-Br-cGMP (n = 6; P < 0.01 (**); repeated measures t test) returning to control within 5 min.
8-Br-cGMP depresses exogenous AMPA-induced currents
To determine whether 8-Br-cGMP acted postsynaptically to depress inspiratory drive currents, XII motoneurones were synaptically isolated by bath application of TTX (1 μm). Excitatory inspiratory drive to XII motoneurones is mediated almost exclusively via AMPA receptors in neonatal rodent in vitro preparations (Funk et al. 1993). Pressure ejection of AMPA (10 μm) to excite postsynaptic AMPA receptors induced an inward current. Currents induced by successive (100 ms) AMPA ejections at 10 s intervals (to reduce the possibility of receptor desensitization) were constant for at least 60 min (see example control trace Fig. 3B). Bath application of 8-Br-cGMP (100 μm) decreased the amplitude of AMPA currents within minutes to 77 ± 10% of control (n = 7; P < 0.01; paired t test; Fig. 2).
Figure 3. Potentiation of endogenous excitatory drive by inhibition of PKG activity.

A, endogenous glutamatergic inspiratory drive current right after (black line) and 30 min after (red line) establishing whole-cell patch on a XII motoneurone with electrode filled with 100 μm PKGI. B, time course of effect of dialysis with PKGI on endogenous motoneuronal currents. Current increased to its maximal value and stabilized within 15 min after break-in. Example control trace (green) demonstrates the stability typical of endogenous motoneuronal currents in untreated cells following break-in. C, increase in endogenous peak current amplitude 30 min after establishing whole-cell patch conditions (n = 5; P < 0.01 (**); paired t test).
Figure 2. Postsynaptic exogenous AMPA-induced currents are depressed by 8-Br-cGMP.

A, continuous recording showing effect of 100 μm 8-Br-cGMP bath application on exogenous AMPA-induced currents. Whole-cell currents were generated by focal application of 10 μm AMPA after bath application of 1 μm TTX. B, AMPA-induced current before (black line) and several minutes after (red line) initiation of bath application of 8-Br-cGMP. AMPA ejection at arrow. C, peak AMPA-induced current amplitude following focal application of 8-Br-cGMP (n = 7; P < 0.01 (**); paired t test).
Potentiation of endogenous excitatory drive by inhibition of PKG activity
The above results suggest that stimulation of PKG depresses inspiratory drive currents. To determine whether PKG is endogenously active in XII motoneurones, XII motoneurones were intracellularly dialysed via the patch pipette with an inhibitory peptide for PKG (PKGI) and Lucifer yellow (to determine post hoc if the motoneurone had been successfully dialysed). After patch formation, the amplitude of the endogenous inspiratory currents progressively increased to 144 ± 17% relative to values preceding break-in (n = 5; P < 0.01; paired t test); this value peaked within 10–30 min (Fig. 3), at which time the amplitude remained stable for over an hour.
PKG-dependent mechanisms directly depress AMPA receptor currents
To exclude the possibility that 8-Br-cGMP directly affected AMPA receptors (Lei et al. 2000), motoneurones were dialysed with PKGI after bath application of TTX, and AMPA was focally ejected. Once a steady state was reached and the currents fully potentiated, 8-Br-cGMP was bath applied (100 μm). If 8-Br-cGMP exerted any direct (or indirect not via PKG) effects on AMPA receptors we would have expected to see a further change in the AMPA currents; no such effect was observed (Fig. 4) as the currents remained stable at 99 ± 5% of control (n = 4; n.s.).
Figure 4. PKG-dependent mechanisms directly depress AMPA receptor currents.

A, single AMPA-induced currents in a motoneurone dialysed with PKGI for at least 30 min before (black line) and several minutes after (red line) 100 μm 8-Br-cGMP bath application. B, no change in the peak AMPA-induced current amplitude following bath application of 8-Br-cGMP. The current amplitude was 99 ± 5% (n = 4; n.s.).
Stimulation of PKG-dependent mechanisms facilitates ivLTF
Episodic application of PE induces long-lasting increases in the amplitude of XII nerve activity and AMPA-induced motoneuronal currents. These increases are dependent on PKC but not PKA (Neverova et al. 2007). As stimulation of PKG pathways decreased motoneuronal excitability, we hypothesized that stimulation with 8-Br-cGMP during induction would decrease ivLTF magnitude.
To test this hypothesis, we superfused slices with three 3 min episodes of PE (10 μm) or PE and 8-Br-cGMP (100 μm) at 5 min intervals, to determine if ivLTF was affected. During the application of 8-Br-cGMP with PE, the level of tonicity appeared to be greater than for PE alone (Fig. 5A). For long-term effects, a two-way repeated measures ANOVA with time as the repeated measure (4 time points: 15, 30, 45 and 60 min post-treatment) and treatment (PE vs. PE and 8-Br-cGMP) as the second independent variable, showed the effects of treatment (F(1,14) = 4.678, P < 0.05, n = 8 slices for each treatment) and time (F(3,42) = 22.81, P < 0.001, n = 8 slices for each treatment) were significant, while the interaction between time and treatment (F(3,42) = 0.126, P > 0.05, n = 8 slices for each treatment) was not significant, revealing that 8-Br-cGMP significantly affected facilitation of XII nerve activity.
Figure 5. Activation of PKG facilitates induction of ivLTF.

A, integrated XII (∫XII) nerve activity in response to episodic PE application (10 μm; upper trace), 8-Br-cGMP (100 μm; middle trace) or PE and 8-Br-cGMP together (10 μm and 100 μm, respectively, lower trace). B, co-application of 8-Br-cGMP with PE significantly increased ivLTF relative to PE alone (P < 0.05 (*); two-way repeated measures ANOVA). Filled circles, PE alone; filled squares, co-application of PE and 8-Br-cGMP. Horizontal bars show group mean responses. C, episodic application of 8-Br-cGMP alone did not significantly increase ∫XII nerve activity (105 ± 12% at 60 min; n = 9 slices; n.s.; one-way repeated measures ANOVA). D, power analysis for the probability of failing to detect a long-term effect of episodically applied 8-Br-cGMP on XII nerve activity plotted as a function of the size of the effect.
The difference in facilitation at 60 min post-treatment between slices treated with PE (120 ± 15%) or PE with 8-Br-cGMP (136 ± 25%) could have been due to an independent effect of 8-Br-cGMP that linearly added to the effect of PE alone or an interaction between the two drugs that led to facilitation that was greater than a linear sum of their independent effects. We applied 8-Br-cGMP alone to slices in the same episodic protocol as before, three 3 min episodes with 5 min intervals, with no observable acute effect (Fig. 5A). To consider 8-Br-cGMP's long-term effects independently of those of PE, we would have expected to see an effect size equal to the difference between episodic application of PE or PE with 8-Br-cGMP, i.e. ∼16% on average. However, a one-way repeated measures ANOVA showed that there was a much smaller, non-significant effect resulting from episodic application of 8-Br-cGMP (105 ± 12%; n.s.; n = 9 slices; repeated measures ANOVA; Fig. 5C). We therefore analysed the power of our study, i.e. the probability of detecting an ∼16% difference given the number of slices (n = 9) and the error variance. The power was >95%, meaning that there was a <5% chance that this study missed an effect size large enough to account for the difference in levels of facilitation seen between PE and PE with 8-Br-cGMP (Fig. 5D). Therefore, the more likely explanation was that an interaction between the effects of these two drugs accounted for the difference in facilitation. Together these data suggest that 8-Br-cGMP serves to enhance facilitation of XII nerve activity brought on by episodic application of PE rather than acting independently.
Discussion
This study demonstrates diverse roles for the cGMP/PKG signalling pathway in controlling motoneuronal excitability and long-term plasticity. In the case of motoneuronal excitability, focal application of 8-Br-cGMP, a PKG activator, significantly decreased excitatory inspiratory drive currents. There was a significant postsynaptic component to these effects that was shown by patching motoneurones that were synaptically isolated with TTX and then focally applying AMPA to mimic endogenous currents (Bocchiaro et al. 2003; Bocchiaro & Feldman, 2004; Neverova et al. 2007). Activation of PKG induced a significant depression of AMPA receptor-mediated currents in these neurones. Non-specific actions of PKG that might occur under current-clamp conditions can be excluded, e.g. effects upon ion channels affecting changes in membrane potential, because recordings were made under voltage-clamp conditions. Furthermore, the role played by PKG in motoneuronal excitability is constitutive since intracellular dialysis of PKGI, a membrane-impermeable peptide that inhibits PKG activity, potentiated inspiratory drive currents. Considered together, these data indicate that PKG is constitutively active in rhythmically firing XII motoneurones, dampening their excitability.
Intracellular cGMP can depress AMPA receptor currents and inhibit excitatory postsynaptic currents in hippocampal neurones through a phosphorylation-independent mechanism (Lei et al. 2000). Thus, cGMP modulation of excitatory transmission may involve a direct coupling to AMPA receptors. However, our data show that this action, if present in XII motoneurones, does not contribute significantly to regulation of motoneuronal excitability. Specifically, bath application of 8-Br-cGMP had no effect on the amplitude of AMPA currents in motoneurones dialysed with intracellular PKGI (Fig. 4). We should have seen a reduction in these currents if cGMP played a phosphorylation-independent role in depressing AMPA currents in our experiments, but we did not. Thus, we conclude that the main action of 8-Br-cGMP is via PKG activation, which, in turn, reduces AMPA receptor-mediated currents.
XII motoneurones, like neurones in many parts of the brain, exhibit both protein kinase-dependent synaptic plasticity and excitability (Bocchiaro et al. 2003; Saywell & Feldman, 2004; Neverova et al. 2007). For example, PKC activity is necessary for PE-induced ivLTF but does not constitutively modulate AMPA receptor-mediated currents (Neverova et al. 2007). In contrast, while PKA constitutively modulates excitatory and inhibitory currents (Bocchiaro et al. 2003; Saywell & Feldman, 2004), it is not necessary for ivLTF (Neverova et al. 2007).
PKG is unique compared to PKA and PKC, since its activity regulates both motoneuronal excitability and long-term plasticity. While depressing excitatory currents, it augments long-term motoneuronal facilitation when activated during induction of PE-induced ivLTF. Specifically, simultaneous episodic application of 8-Br-cGMP and PE enhanced ivLTF 60 min after induction relative to the use of PE alone.
A possible explanation for increased facilitation is the observation that there is an effect of PKG presynaptic to the motoneurone that is in addition to any postsynaptic effects within the motoneurone. For example, PKG pathway stimulation may lower the excitability of inhibitory neurones that synapse onto XII motoneurones (Saywell & Feldman, 2003), thereby lowering endogenous inhibition resulting in increased motoneurone activity. We do not favour this explanation because episodic 8-Br-cGMP application alone did not induce ivLTF.
We favour the explanation that PKG activation within XII motoneurones converges on and modulates intracellular pathways leading to ivLTF, possibly similar to the interactions observed between PKG and PKC during induction of ischaemic preconditioning (Costa et al. 2008). Ischaemic preconditioning, first discovered in myocardium (Murry et al. 1986), is a phenomenon whereby low doses of noxious insults like ischaemia or hypoxia protect cells from future, more severe insults and appears to be a general phenomenon, occurring in the brain, lungs, liver, intestine, and kidney as well as the heart (Shpargel et al. 2008).
Ischaemic preconditioning, like ivLTF, was first induced using short, episodic events (5 min episodes of ischaemia separated by 5 min intervals, Murry et al. 1986). Also, like ivLTF, ischaemic preconditioning in the heart and the brain is PKC dependent (Ytrehus et al. 1994; Ping et al. 1997). During myocardial ischaemic preconditioning, one mechanism of PKC activation is PKG dependent. Specifically, activation of myocardial cell membrane bradykinin and opioid receptors triggers a phosphatidylinositol 3-kinase/Akt/extracellular signal-regulated kinase/nitric oxide synthase pathway that creates nitric oxide, which in turn activates guanylyl cyclase producing cGMP and activating PKG. PKG then causes the opening of mitochondrial ATP-sensitive K+ channels, inducing reactive oxygen species (ROS) formation that activates PKC via redox signalling (Costa et al. 2008).
Similar to ischaemic preconditioning, pharmacological scavenging of ROS blocks phrenic and XII respiratory long-term facilitation in the anaesthetized, paralysed, and ventilated adult rats (MacFarlane & Mitchell, 2008). Acute intermittent hypoxia (AIH)-induced LTF may be related to ivLTF and shares many of the same signalling components (Feldman et al. 2005). We speculate that 8-Br-cGMP causes the production of additional ROS that augments PKC activity via PKG activation and opening of mitochondrial ATP-sensitive K+ channels. This in turn increases ivLTF relative to that induced by PE alone.
The increased tonicity of XII nerve activity when 8-Br-cGMP was present in addition to PE during the induction of ivLTF may be some indication of this interaction; however, studies correlating levels of tonicity during induction and the amount of facilitation long-term do not exist for ivLTF. For AIH LTF in vivo, most recent meta-analysis (Baker-Herman & Mitchell, 2008) indicates that the amplitude of phrenic bursts during the hypoxic ventilatory response is a significant predictor of LTF. The authors postulate two potential reasons for this correlation: (1) a stronger hypoxic response leads to greater release of serotonin in the vicinity of phrenic motoneurones, or (2) a limited dynamic range of phrenic motor output, which limits an increase in phrenic burst amplitude during hypoxia, may similarly limit phrenic increases during LTF. The authors allow, however, that there may be no causal relationship between phrenic burst amplitude during the hypoxic ventilatory response and phrenic amplitude at long-term time points. Whether an increase in amplitude of phrenic nerve bursts seen during induction of AIH LTF in vivo is related to enhanced tonicity in XII nerve activity in vitro is unknown. Similarly, whether the increased tonicity we observed during application of PE plus 8-Br-cGMP vs. PE alone was a part of inducing the observed increase in ivLTF or a separate short-term phenomenon remains unstudied.
In total, our study provides additional clarity to a complex picture of the roles played by protein kinases in the control of motoneuronal excitability and long-term plasticity. We propose that at the time of activation PKA and PKG exert constitutive antagonistic effects upon inspiratory drive currents, modulating motoneuronal excitability on a state-dependent, cycle-by-cycle time frame. Additional PKG activation with 8-Br-cGMP, occurring during ivLTF induction, augments long-term facilitation by increasing PKC activity via ROS production and redox signalling mechanisms.
Interestingly, both ischaemic preconditioning and LTF take advantage of what appears to be a common set of intracellular signalling mechanisms to promote survival against hypoxic/anoxic/ischaemic events, which makes both phenomena of therapeutic as well aetiological interest. For example, LTF is a proposed compensatory mechanism for obstructive sleep apnoea (Mahamed & Mitchell, 2008). Thus, exploiting the cGMP/PKG pathway is potentially of interest therapeutically in augmenting LTF responses in OSA patients. Alternatively, pathophysiological changes in the cGMP/PKG pathway could underlie decreased XII motoneurone excitability seen in OSA, again, offering a point for therapeutic intervention.
Acknowledgments
This work was supported by NIH Grant NS24742, Roman Reed Spinal Cord Research Fund of California. N.V.N. was a Roman Reed predoctoral fellow. W.E.B. is a Ruth L. Kirschstein NRSA pre-doctoral fellow (NS067933) and formerly a pre-doctoral fellow of the UCLA-NIH Neural Microcircuits Training Grant. We would like to thank Gordon Mitchell for his comments on the manuscript.
Glossary
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AIH
acute intermittent hypoxia
- 8-Br-cGMP
8-bromoguanosine-3′,5′-cyclomonophosphate
- cGMP
cyclic guanosine monophosphate
- ivLTF
in vitro long-term facilitation
- LTD
long-term depression
- LTP
long-term potentiation
- n.s.
not significant
- OSA
obstructive sleep apnoea
- PE
phenylephrine
- PKA
protein kinase A
- PKC
protein kinase C
- PKG
protein kinase G
- PKGI
inhibitory peptide of PKG
- preBötC
preBötzinger complex
- ROS
reactive oxygen species
- XII
hypoglossal
Author contributions
S.A.S., N.V.N. and J.L.F. conceived of the intracellular experiments assessing PKG's short-term effects on XII motoneurone excitability. S.A.S. and N.V.N. conducted the experiments and analysed the data. S.A.S., W.E.B. and J.L.F. conceived of the long-term facilitation experiments. The experiments were conducted by W.E.B., who also analysed the data. S.A.S. and W.E.B. were responsible for authoring final drafts of the manuscript with careful review provided by J.L.F. N.V.N. contributed to the preparation of early manuscripts.
References
- Aoki CRA, Liu H, Downey GP, Mitchell J, Horner RL. Cyclic nucleotides modulate genioglossus and hypoglossal responses to excitatory inputs in rats. Am J Respir Crit Care Med. 2006;173:555–565. doi: 10.1164/rccm.200509-1469OC. [DOI] [PubMed] [Google Scholar]
- Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD. Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell. 1996;87:1025–1035. doi: 10.1016/s0092-8674(00)81797-3. [DOI] [PubMed] [Google Scholar]
- Babcock M, Badr M. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–716. [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529:215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Resp Physiol Neurobiol. 2008;162:8–17. doi: 10.1016/j.resp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchiaro CM, Saywell SA, Feldman JL. Dynamic modulation of inspiratory drive currents by protein kinase A and protein phosphatases in functionally active motoneurons. J Neurosci. 2003;23:1099–1103. doi: 10.1523/JNEUROSCI.23-04-01099.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BH, Lea RB. Essentials of Statistics for the Social and Behavioral Sciences. Hoboken: John Wiley & Sons, Inc.; 2004. [Google Scholar]
- Costa ADT, Pierre SV, Cohen MV, Downey JM, Garlid KD. cGMP signaling in pre- and post-conditioning: the role of mitochondria. Cardiovasc Res. 2008;77:344–352. doi: 10.1093/cvr/cvm050. [DOI] [PubMed] [Google Scholar]
- de Vente J, Asan E, Gambaryan S, Markerink-van Ittersum M, Axer H, Gallatz K, Lohmann SM, Palkovits M. Localization of cGMP-dependent protein kinase type II in rat brain. Neuroscience. 2001;108:27–49. doi: 10.1016/s0306-4522(01)00401-8. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Neverova NV, Saywell SA. Modulation of hypoglossal motoneuron excitability by intracellular signal transduction cascades. Respir Physiol Neurobiol. 2005;147:131–143. doi: 10.1016/j.resp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Horner RL, Bradley TD. Update in sleep and control of ventilation 2007. Am J Respir Crit Care Med. 2008;177:947–951. doi: 10.1164/rccm.200801-051UP. [DOI] [PubMed] [Google Scholar]
- Lei S, Jackson MF, Jia Z, Roder J, Bai D, Orser BA, MacDonald JF. Cyclic GMP-dependent feedback inhibition of AMPA receptors is independent of PKG. Nat Neurosci. 2000;3:559–565. doi: 10.1038/75729. [DOI] [PubMed] [Google Scholar]
- Levenes C, Daniel H, Crepel F. Long-term depression of synaptic transmission in the cerebellum: cellular and molecular mechanisms revisited. Prog Neurobiol. 1998;55:79–91. doi: 10.1016/s0301-0082(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. PNAS. 2002;99:8389–8393. doi: 10.1073/pnas.122206399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience. 2008;152:189–197. doi: 10.1016/j.neuroscience.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol. 2004;557:13–18. doi: 10.1113/jphysiol.2004.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Simulated apnoeas induce serotonin-dependent respiratory long-term facilitation in rats. J Physiol. 2008;586:2171–2181. doi: 10.1113/jphysiol.2007.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EBJ. Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Murry C, Jennings R, Reimer K. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Neverova NV, Saywell SA, Feldman JL. 2002 Neuroscience Meeting Planner, Program No. 856.5. Washington, D.C.: Society for Neuroscience; 2002. The role of cGMP-mediated signaling pathway in modulation of hypoglossal (XII) motoneuron excitability. Online. [Google Scholar]
- Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of α1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci. 2007;27:4435–4442. doi: 10.1523/JNEUROSCI.2803-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping P, Zhang J, Qiu Y, Tang X-L, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms ɛ and η in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- Saywell SA, Feldman JL. 2003 Neuroscience Meeting Planner, Program No. 499.16. Washington, DC: Society for Neuroscience; 2003. Protein kinases differentially modulate inhibition in respiratory phased hypoglossal (XII) motoneurons. Online. [Google Scholar]
- Saywell SA, Feldman JL. Dynamic interactions of excitatory and inhibitory inputs in hypoglossal motoneurones: respiratory phasing and modulation by PKA. J Physiol. 2004;554:879–889. doi: 10.1113/jphysiol.2003.054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea: Implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- Shkoukani M, Babcock MA, Badr MS. Effect of episodic hypoxia on upper airway mechanics in humans during NREM sleep. J Appl Physiol. 2002;92:2565–2570. doi: 10.1152/japplphysiol.00938.2001. [DOI] [PubMed] [Google Scholar]
- Shpargel KB, Jalabi W, Jin Y, Dadabayev A, Penn MS, Trapp BD. Preconditioning paradigms and pathways in the brain. Cleve Clin J Med. 2008;75:S77–S82. doi: 10.3949/ccjm.75.suppl_2.s77. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang Y, Rowan MJ, Anwyl R. Evidence for involvement of the cGMP-protein kinase G signaling system in the induction of long-term depression, but on long-term potentiation, in the dentate gyrus in vitro. J Neurosci. 1998;18:3589–3596. doi: 10.1523/JNEUROSCI.18-10-03589.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ytrehus K, Liu Y, Tsuchida A, Miura T, Liu GS, Yang XM, Herbert D, Cohen MV, Downey JM. Rat and rabbit heart infarction: effects of anesthesia, perfusate, risk zone, and method of infarct sizing. Am J Physiol Heart Circ Physiol. 1994;267:H2383–H2390. doi: 10.1152/ajpheart.1994.267.6.H2383. [DOI] [PubMed] [Google Scholar]


