Abstract
Passing current through mastoid electrodes (conventionally termed galvanic vestibular stimulation; GVS) evokes a balance response containing a short- and a medium-latency response. The origins of these two responses are debated. Here we test the hypotheses that they originate from net signals evoked by stimulation of otolith and semi-circular canal afferents, respectively. Based on anatomy and function, we predicted the directions of the stimulus-evoked net head rotation vector from the canals and the linear acceleration net vector from the otoliths. We tested these predictions in healthy adults by obtaining responses with the head in strategic postures to alter the relevance of the signals to the balance system. Cross-covariance between a stochastic waveform of stimulating current and motor output was used to assess the balance responses. Consistent with the canal hypothesis, with the head pitched down the medium-latency EMG response was abolished while the short-latency EMG response was maintained. The results, however, did not support the otolith hypothesis. The direction of the linear acceleration signal from the otoliths was predicted to change substantially when using monaural stimuli compared to binaural stimuli. In contrast, short-latency response direction measured from ground-reaction forces was not altered. It was always directed along the inter-aural axis irrespective of whether the stimulus was applied binaurally or monaurally, whether the head was turned in yaw through 90 deg, whether the head was pitched down through 90 deg, or combinations of these manipulations. We conclude that a net canal signal evoked by GVS contributes to the medium-latency response whilst a net otolith signal does not make a significant contribution to either the short- or medium-latency responses.
Introduction
A small direct current passed between the mastoids of a standing subject (conventionally termed galvanic vestibular stimulation; GVS) induces a reflexive whole-body response that has been attributed to action of the vestibular system (Coats & Stoltz, 1969; Nashner & Wolfson, 1974; Lund & Broberg, 1983; Britton et al. 1993) on the balance system (Day et al. 1997). When measured electromyographically, the response comprises two components: a short-latency (∼60 ms) and a medium-latency (∼110 ms) response of opposite sign (Britton et al. 1993; Fitzpatrick et al. 1994). Although both components are generally considered to be of vestibular origin, the reason for differences in their latency and sign remains a matter of debate. An early suggestion was that the two responses represent processing of the vestibular signal in two distinct brain regions that utilise different descending pathways, e.g. vestibulospinal and reticulospinal tracts (Britton et al. 1993). This may explain latency differences but it does not provide a compelling explanation for sign differences. More recently, Cathers and colleagues (2005) put forward another hypothesis. They suggested that the two responses arise from two different parts of the vestibular system, with the medium-latency component being driven by rotational signals from semicircular canals and the short-latency component being driven by linear acceleration signals from the otoliths. If this were true it would have important implications. It would mean that for the first time the otolith and semicircular canal contributions to human balance control could be independently studied simply through measurement of the two responses. Here we test this hypothesis.
It is straightforward to show theoretically that GVS should evoke a net head rotation signal from semicircular canal afferents (Schneider et al. 2002; Fitzpatrick & Day, 2004) and there are a number of lines of evidence which support the theory (Day & Cole, 2002; Fitzpatrick et al. 2002, 2006; Schneider et al. 2002, 2009; Day & Fitzpatrick, 2005a). However, the presence and potency of any net linear acceleration signal arising from mass stimulation of the whole population of otolith afferents is less certain. If the short-latency component of the GVS-evoked response represents the response to this net otolith signal then its direction should agree with that predicted from theoretical considerations of otolith structure and function. Based on morphology of the utricular and sacular maculae and on the mirror symmetry of the left and right labyrinths we generate predictions about the direction of the balance response that can be expected under different stimulation conditions and different head postures. We then go on to compare these predictions with measures of the short-latency response directions obtained empirically.
Methods
Adults aged 18–38 with no known history of neurological or vestibular problems took part in these experiments (8 in experiment 1; 20 in experiment 2). The study was approved by the University College London Research Ethics Committee and conformed to the Declaration of Helsinki. All participants gave written, informed consent.
Theory and predictions
Three principles must be considered to infer the origin of reflexes evoked by mastoid stimulation.
First, electrical stimulation modulates the activity of both otolith and canal afferents (Lowenstein, 1955; Goldberg et al. 1982, 1984; Kim & Curthoys, 2004) so that net signals of both linear and rotational head motion can potentially be evoked. We assume that net linear and rotational signals from each side are dictated by vector summation of the responses of all afferents based on directional sensitivities of the hair cells they innervate (Schneider et al. 2002; Fitzpatrick & Day, 2004). Since the population contains hair cells with different directional sensitivities, net zero vectors through cancellation across the population are possibilities, although not predicted from current knowledge of hair cell orientations (see below).
Second, the direction of the net response to binaural stimulation arises from the vector summation of the responses evoked on each side (Day et al. 2010). Bi-directionality of afferent responses to opposite polarity currents (Goldberg et al. 1984) and left–right anatomical symmetry dictates that, under binaural–bipolar stimulation, any net linear vector must be inter-aurally directed and the axis of any net rotation vector must lie in the mid-sagittal plane of the head, regardless of the net directions on each side of the head (Fig. 1A and C). Monaural responses to same-polarity stimulation of each side of the head should be symmetrical about the mid-sagittal plane (compare right ear in Fig. 1A with left ear in Fig. 1A).
Figure 1. Impact of left–right mirror symmetry on head motion signals evoked by electrical mastoid stimulation.
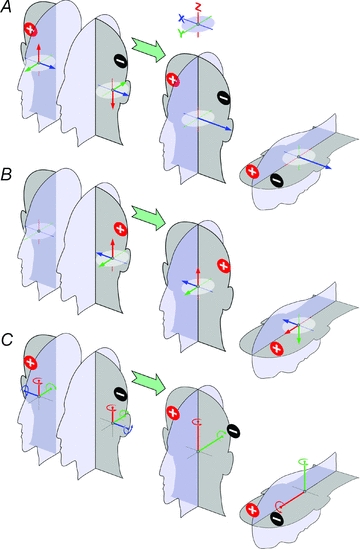
The coloured vectors in these figures, indicating equal XYZ component 3D signals of motion evoked by electrical vestibular stimulation, are arbitrary and for illustrative purpose. A, binaural bipolar stimulation must lead to a net inter-aural linear signal: opposite polarity currents have opposite effects on the firing of vestibular afferents (Goldberg et al. 1984) and so evoke oppositely directed vectors (compare left ears in A and B). Because left–right vestibular organs are symmetrical about the mid-sagittal plane, vectors are mirrored about the mid-sagittal plane for the same stimulus to opposite sides of the head (compare right ear in A with left ear in B). The result of this is the net binaural bipolar vector can only be inter-aurally directed or zero. Pitch orientation of the head does not affect orientation of this net vector in the earth horizontal plane (right-most drawing). B, with monaural stimulation, vector summation preserves any vertical and anterior component. With the head upright, the head-vertical (red) component is an earth vertical linear motion signal, which has little relevance for balance. When the head is pitched forward, this component becomes a balance relevant earth-horizontal motion signal whilst the head sagittal vector (green) becomes a vertical motion signal. C, by the same principles as in A, the resultant angular vector to binaural bipolar stimulation must lie in the mid-sagittal plane of the head.
Third, balance responses reflect the significance of the evoked signals of head motion to the balance system. Thus, we assume the balance system responds to signals of earth-horizontal acceleration and pitch/roll motion about earth horizontal axes. We assume earth vertical linear accelerations and yaw rotation about earth vertical axes are not relevant for balance control. By changing the position of the head, the relevance of the evoked vectors can be altered (Figs 1 and 2A).
Figure 2. Experimental set-up and measurement of short-latency direction.
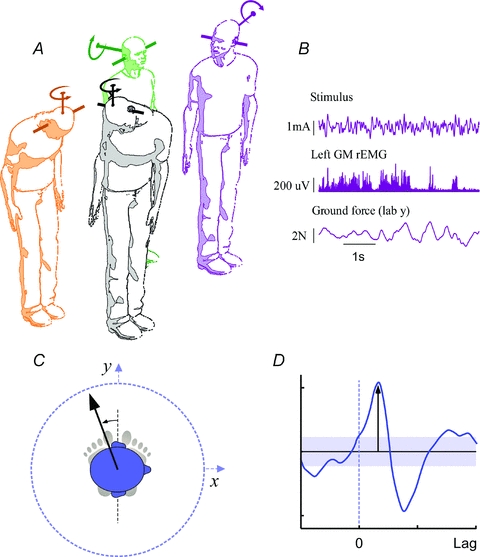
A, postures adopted in this study. Clockwise from left: head forward-down, head forward-upright, head right-upright, head right-down. The orientation of predicted linear (Fig. 1A) and rotational head motion (Fitzpatrick & Day, 2004) vectors evoked by binaural–bipolar GVS are shown. Pitching the head down changes the orientation of the rotation vector from one that signals roll to one that signals yaw, but the functional relevance of the inter-aural linear vector is unchanged (Cathers et al. 2005). B, example of raw data from head right upright binaural experiment (stimulus waveform, left gastrocnemius rectified EMG, and anteroposterior force). C, measurement of short-latency direction. The linear arrow shows the positive direction of the axis along which the largest positive value for the first peak in the SVS–force cross covariance (D) was found (method detailed in Mian & Day, 2009). The direction is represented with respect to the inter-aural line. The shaded segment in D represents the 95% confidence interval for significance of correlation.
Knowledge of the anatomy and orientation of the semicircular canals has allowed the precise orientation of the binaural GVS rotational vector within the head's mid-sagittal plane to be modelled at −18.8 deg and then validated experimentally with a high degree of certainty (Day & Fitzpatrick, 2005a). The abolition of the medium-latency balance response when this vector is vertical provides compelling evidence that the medium-latency component is driven by the canals (Cathers et al. 2005). Observations in the current study replicate this, using a variant stimulus mode.
The existence of a GVS binaural bipolar net linear vector depends only on the presence of a medio-lateral component of the net vector from each labyrinth since antero-posterior and vertical components cancel owing to mirror symmetry (Fig. 1A). There is morphological basis for net medio-lateral components. Because hair cells are on average oriented oppositely on either side of the macular striola, the unequal surface area of the pars lateralis (53%) and pars medialis (47%) of the horizontal human macula utriculi (Tribukait & Rosenhall, 2001) could lead to a GVS-evoked population vector from each labyrinth with net medio-lateral components (Fitzpatrick & Day, 2004). When the head is pitched down, an inter-aural linear vector would remain relevant to the balance system (Figs 1A and 2A). Thus, an inter-aural short-latency response evoked by binaural bipolar stimulation that persists with the head pitched down provides evidence against canal mediation and is consistent with an otolith origin. However it does not exclude a non-vestibular source.
The response to monaural stimulation is crucial for further testing the hypothesis that a net otolith signal produces the short-latency reflex. There is a morphological basis for expecting a non inter-aural net unilateral vector, especially when the head is pitched down. The pars superior (56%) and the pars inferior (44%) occupy unequal areas of the human macula sacularis (Tribukait et al. 2005). Using the same argument as for the utricle, it follows that a net infero-superior component can be expected from inequality in saccular areas either side of the striola. With the head pitched down this would be transformed into an antero-posterior component of linear acceleration (see Fig. 1B).
Based on existing information (Corvera et al. 1958; Lindeman, 1969; Rosenhall, 1972; Takagi & Sando, 1988; Merchant et al. 2000; Tribukait & Rosenhall, 2001; Naganuma et al. 2001, 2003; Tribukait et al. 2005) we have made an estimate of the direction of the monaural otolith vector to supplement the qualitative reasoning offered above. The estimate, detailed in Supplemental Material, is that monaural anodal GVS would evoke a linear acceleration signal directed medial, 23 deg anterior to the inter-aural line and 52 deg upward from Reid's plane, and opposite for cathodal GVS. When Reid's plane is pitched 18.8 deg nose up from horizontal (targeted head upright position in current experiments), the earth horizontal component of the estimated net anodal GVS linear acceleration vector is directed 1 deg anterior from medial and when Reid's plane is pitched 71.2 deg nose down (target head down position) it is directed 53 deg anterior from medial. Due to the relative complexity of hair cell arrangements and limitations to existing morphological data (see supplemental material for discussion), the precise orientation of the net vector from the otoliths cannot be estimated with the same level of confidence as the net rotation vector from the canals. However, the important aspect of this predicted monaural otolith vector is not its precise orientation, but rather that it supports the notion advanced in the previous paragraph that the monaural otolith vector is likely to contain a non-inter-aural component. Thus, if the short-latency response were due to a net GVS otolith vector, with monaural stimulation it would be expected to deviate from the inter-aural line with the head upright and/or pitched down (symmetrically for same-polarity stimulation of opposite sides –Fig. 1).
Experimental approach
The short and medium-latency components of the GVS-evoked response are, by definition, electromyographic events, but it is not possible to determine their directions from EMG records without recording from the wide range of muscles that contribute to the response (Ali et al. 2003) and weighting them appropriately. A direct approach is to measure the net mechanical output arising from each EMG event. The ground-reaction shear force, which accelerates the body's centre of mass horizontally, offers a means of doing this. GVS evokes a shear force response with two components similar to the EMG response (Marsden et al. 2002; Day & Guerraz, 2007). In theory, therefore, it should be possible to infer the direction of the short-latency EMG response by measuring the direction of the early shear-force component. In practise, however, the early force response, measured in individuals, is often small and variable making it difficult to reliably extract it from background noise in reasonable duration experiments. Cross-covariance between a random waveform of stimulating current (stochastic vestibular stimulation; SVS) and motor output can evoke equivalent responses to GVS (Dakin et al. 2007), and with force used to measure motor output, response direction can be estimated (Mian & Day, 2009). In our experience, the advantage of the stochastic method is that clean responses can be obtained with higher fidelity relative to the data collection time and objective methods can be applied to differentiate responses from background noise.
Protocol and set-up
The first experiment (8 participants) determined responses to binaural stimulation in four postures: (i) head forward and upright, (ii) head forward and down, (iii) head right and upright, and (iv) head right and down (Fig. 2A). Head forward trials were performed before head right trials and within these, head-upright and head-down trials alternated. Stimulus amplitude was 0.52 mA root mean square (r.m.s.) and 2 mA peak.
The second experiment (20 participants) determined responses to monaural stimulation on each side in two postures: (i) head forward and upright, and (ii) head forward and down. Eight participants were tested only with the head upright and a 0.6 mA r.m.s. stimulus. This had been increased slightly over the binaural levels because responses to monaural stimuli are smaller (Day et al. 2010). To obtain more robust force responses, the stimulus was further increased to 0.9 mA r.m.s. for 12 additional participants who were tested in both head postures. Stimulus side alternated every six trials and head pitch every trial.
For each condition tested, 180 s of data was collected, split into six 30 s trials. For each trial, participants stood without shoes and with the medial borders of the feet touching whilst vestibular stimulation was delivered.
The target yaw rotation of the inter-aural line was 90 deg in the head-right conditions. Target head pitch had Reid's plane inclined 18.8 deg nose up in the head-upright conditions and 71.2 deg nose down in the head-down conditions. Reid's plane intersects the external auditory meati and lower orbital margins and these postures were chosen so that the estimated rotation vector of the signal evoked from the semicircular canals is horizontal and vertical, respectively (Fig. 2A). Subjects attained the requested head posture relative to feet by directing a head-fixed laser at a nominated target by rotation and flexion of the neck and trunk. Once in position, the eyes were closed and the stimulus commenced. Seated rest was available between trials.
The stochastic stimulus was delivered from an isolated current source between 10 cm2 carbon rubber electrodes coated with conducting gel. For binaural stimulation they were attached over the mastoid processes and for monaural stimulation they were over one mastoid process and the lateral end of the ipsilateral clavicle. The stimulus was a zero mean, bandwidth-limited waveform created by band-pass filtering computer generated white noise. The bandwidth of 1–20 Hz used in this study evokes large, easily measured short latency force responses (Dakin et al. 2010).
Measurement and analysis
Ground reaction forces from a force plate (Kistler 9286AA, Winterthur, Switzerland), surface EMG (Experiment 1) from Ag–AgCl electrodes over the medial gastrocnemius of each leg, and the variable-current stimulus through an isolation amplifier (MT8, MIE Medical Research, UK), were all recorded at 1 kHz (Fig. 2B). The position of head (tragus and the lower orbital margin on each side) and body (c7 spinous process) landmarks were tracked at 50 Hz (CODA, Charnwood Dynamics, Rothley, UK). All data were recorded synchronously using the CODA acquisition system.
Head landmarks were used to determine horizontal plane orientation of the inter-aural line and the pitch orientation of Reid's plane. To determine body sway, we calculated the angle between the 3D line connecting c7 and the ground-projected mean position of c7, and a vertical passing through this ground point. The total cumulative angular excursion during a trial was divided by trial duration to represent body sway (deg s−1).
As previously, un-normalised cross-covariance between stimulus and rectified EMG (Dakin et al. 2007) and between stimulus and shear force (Mian & Day, 2009) were calculated for individual subjects and for group pooled data through the cumulant density function (Halliday et al. 1995). Analysis parameters were: 4.096 s non-overlapping segments (the initial 1.328 s of each trial were discarded as excess data), 42 per subject per condition, giving a 0.244 Hz frequency resolution. Statistical 95% confidence intervals were calculated separately for single-subject and pooled cumulant density functions (Halliday et al. 1995). For the single-subject analysis undertaken in this study (short-latency force responses; see below) we had decided a priori to include only responses in excess of the 95% confidence level. With the chosen stimulus parameters, all short-latency force responses exceeded this level.
Significant cross-covariance indicates association between stimulus and motor output at a particular lag. For SVS–EMG relationships, positive and negative values indicate an association between SVS and EMG activation and between SVS and EMG inhibition, respectively. For SVS–force relationships, positive and negative values indicate association between SVS and force signals of the same and opposite polarities, respectively. For example, a positive SVS–force (Lab X axis) relationship is due to association between a positive current (anode right/cathode left in our binaural convention) and a rightward force component as well as association between negative current (anode left/cathode right) and leftward force component. For brevity, when referring to the motor output associated with SVS we shall generally only refer to the output associated with an anode right/cathode left current (binaural) and anode mastoid/cathode clavicle current (monaural), the interpretation for the opposite currents being anti-polar (EMG) and anti-podal (force).
To estimate the direction of the short-latency response, the stimulus–force cross-covariance was calculated for 360 one-degree increments of the horizontal force axis (Mian & Day, 2009). The axis that yielded the largest positive magnitude for the first peak in the force response was deemed its direction, and is represented relative to the inter-aural axis (Fig. 2C and D). Following the previous paragraph, the direction represents the responses to binaural anode-right/cathode-left or monaural anode-mastoid/cathode-clavicle stimuli.
To assess congruence between measured response directions and reference directions (inter-aural line/morphology-based predictions), confidence intervals for mean direction for each experimental condition were calculated using circular statistics (Upton, 1986), and lack of overlap between these intervals and the reference directions was taken as evidence to reject the statistical null hypothesis of congruence (Zar, 2010).
Results
Subjects did not become grossly unstable as a result of the stimulus: mean r.m.s. body sway during stimulation ranged from 0.33 ± 0.10 deg s−1 with the head forward-upright to 0.38 ± 0.12 deg s−1 with the head right-down. These averages were no more than 23% greater than we typically measure in subjects without stimulation, but otherwise under identical conditions (unpublished data).
Target head orientation angles were well attained. Measured pitch values for Reid's plane were 16 ± 5 deg (mean and standard deviations of subjects’ average angles; target 18.8 deg) and −71 ± 6 deg (target −71.2 deg) for the head upright and head down conditions, respectively. Measured values for the horizontal orientation of the inter-aural line were 1 ± 4 deg (target 0 deg) and –87 ± 6 deg (target −90 deg) for head forward and head right conditions respectively.
Muscle reflexes
Binaural stochastic stimulation evoked short-latency and medium-latency responses in gastrocnemius EMG with the same characteristics that have been reported previously with GVS. That is, with the head upright and facing forwards, responses from the left and right muscles had opposite shape waveforms (Fig. 3A left; cf. Lee Son et al. 2008; Day et al. 2010); with the head upright and facing right, the responses from left and right muscles had the same shape but were larger in the left leg (Fig. 3A right; cf. Britton et al. 1993); with the head pitched down, the medium-latency peak was almost abolished while the short-latency peak remained intact (Fig. 3A red traces; cf. Cathers et al. 2005).
Figure 3. EMG and force responses.
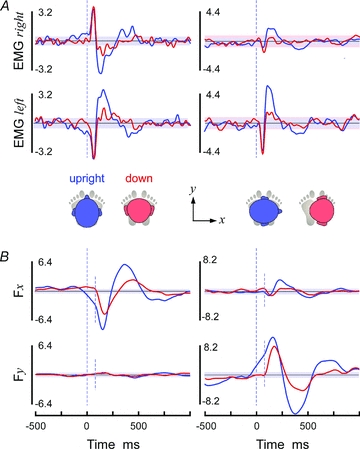
Cross covariance calculated using pooled data from 8 subjects (experiment 1) is shown. Shaded bands represent the 95% confidence intervals. A, gastrocnemius EMG responses for head upright (blue) and pitched down (red). The left graphs are with the head forward and the right with the head turned 90 deg to the right. Data are from right (upper graphs) and left (lower graphs) legs. Units are mA ⋅μV ×10−1. B, shear ground reaction forces relative to the axis of the feet (positive Fx = rightward, positive Fy = aneterior). Units are mA ⋅ N ×10−2. Small, dotted vertical lines explained in main text.
Force responses
The ground reaction shear force provides the direction of the reflexes evoked in all postural muscles. It represents the direction of the total balance response to the stimulus and not just that of a particular muscle. Like the EMG response, with the head upright the characteristic short- and medium-latency responses of opposite and appropriate sign are observed with binaural stimulation (Fig. 3B). With the head facing forward, the peak responses were approximately medio-lateral (Fig. 3B left) and with the head facing right they were approximately antero-posterior relative to the feet (Fig. 3B right). The polarity of the peaks indicated that the short-latency peak was directed toward the cathode and the medium-latency peak towards the anode.
The shape of the SVS–force relationship was influenced by head pitch. With the head down, the short-latency peak rose sharply and monophasically. However with the head upright, the short-latency peak appeared to start on top of a separate underlying wave, with a clear inflexion at its commencement (small dotted lines in Fig. 3B). Absence of this phenomenon with the head pitched down suggests the underlying wave was unrelated to the short-latency muscle response and accounted for the larger magnitude of the first force peak when the head was upright compared to down.
Direction of short-latency peak to binaural and monaural stimulation
Figure 4 shows directions of the short-latency force measured from individual subjects. As predicted by theoretical considerations of symmetry, binaural stimulation must create a net otolith response directed along the inter-aural axis regardless of the direction of the unilateral response, and this was observed for every head alignment (Fig. 4A and B).
Figure 4. Directions of the short-latency responses.
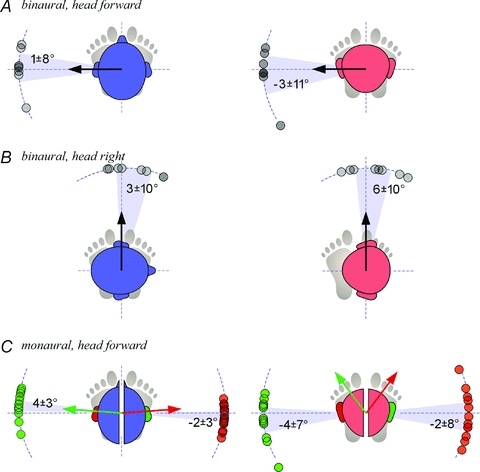
Data points for individual subjects are shown overlapped. Blue heads (left column) are upright and red heads (right column) are pitched down. The shaded sectors and numerical annotations represent the 95% confidence intervals of the mean directions relative to the inter-aural lines (dotted axes extending to perimeter). For each condition, measured data points were clustered within a small arc without circular discontinuities; therefore it was feasible to report directions relative to the pole of the inter-aural line about which the sample was clustered. A, binaural–bipolar stimulation with the head forward (n = 8; experiment 1). The black arrow represents the prediction that responses will be directed along the inter-aural line. B, binaural–bipolar stimulation with the head turned 90 deg to the right (n = 8; experiment 1). C, monaural stimulation on each side (n = 20; experiment 2). The coloured arrows represent morphology-based predicted response vectors for stimulation of each side (right = green, left = red). To relate the arrows with the responses, consider the following. Whilst stochastic stimulation involves random alternating current, the measured responses are equivalent, for binaural stimulation, to anode-right/cathode-left galvanic currents, and for monaural stimulation, to anode-mastoid galvanic currents (see Methods). We assume the balance response would be in the direction of the linear acceleration evoked by the stimulus, opposite to the equivalent tilt (Day & Fitzpatrick, 2005b). With binaural stimulation, the direction of the inter-aural acceleration is predicted to be to the cathode side (supplemental material). With monaural stimulation, the arrows denote direction of the earth-horizontal projection (upright: 4 deg anterior of medial axis; down 53 deg anterior of medial axis) of estimated head acceleration evoked by anodal stimulation (Supplemental Material), accounting for average measured Reid's plane orientation relative to earth-horizontal (upright: Reid's plane16 deg anterior up; down: 71 deg anterior down).
We made the prediction that monaural stimulation will evoke net linear acceleration vectors that deviate from the inter-aural line. According to this prediction, it is not possible for balance relevant (earth horizontal) components of the monaural vector to be inter-aural in both the head upright and head down postures. Furthermore, to ascribe any deviations from the inter-aural line to an otolith signal, the deviations must be mirrored about the mid-sagittal plane for same polarity stimulation on different sides of the head (Fig. 1). However, with monaural stimulation, short-latency response directions remained essentially directed along the inter-aural axis without any evidence of a mirrored deviation for left vs. right stimulation (Fig. 4C). The only statistically significant deviation from the inter-aural line was a small magnitude deviation (95% confidence interval of mean: 4 ± 3 deg; P = 0.02) for head upright, monaural right stimulation that was not mirrored for monaural left stimulation.
Figure 4C also depicts the directions predicted from the estimated net otolith signal based on anatomy and morphology of the utricle and saccule (Supplemental Material) and measured head orientation. In head upright monaural conditions, the estimate was close to the measured response directions (and overlapped the 95% confidence interval of monaural right responses). In the case of the head down monaural conditions, the difference between estimated and measured directions were vast (P < 0.0001).
Discussion
In this series of experiments we measured properties of the short-latency and medium-latency response to mastoid stimulation using the correlation between stochastic electrical current and motor output. We observed a disappearance of the medium-latency EMG response to binaural stimulation when the head was pitched down, indicating it has a canal and not an otolith origin, replicating Cathers et al. (2005). However, our data do not support Cathers et al.'s hypothesis that the short-latency response has an otolith origin. The results show that the direction of the short-latency response to mastoid stimulation was immutable. It was always directed along the inter-aural axis irrespective of whether the stimulus was applied binaurally or monaurally, whether the head was turned in yaw through 90 deg, whether the head was pitched down through 90 deg, or combinations of these manipulations. Based on the anatomy and morphology of the human utricular and saccular maculae, an inter-aural response direction was expected for otolith-driven behaviour arising from binaural stimulation, but not from monaural stimulation. The data, therefore, are inconsistent with the otolith hypothesis.
A potential difficulty of analysis was that with the head upright the short-latency force response was superimposed on a slower waveform. The source of this wave is unknown but it was not present when the head was pitched down. Its presence is likely to have produced an artifactual lift in magnitude of the short-latency force response with head upright relative to down. A concern is that it may also have distorted the direction of the response. However, with the head down, and the response restricted just to the short-latency component, this complication in the force data was absent. Furthermore, it is in this head position that we strongly predict that the monaural response should deviate substantially from the inter-aural line. In this posture, directions of the short-latency responses remained inter-aural thus providing significant evidence of inconsistency with the otolith model.
There is a relatively limited amount of human data to make predictions of properties of the net otolith vector. With the data available we made predictions based on the assumptions that afferents of hair cells from different regions of the otoliths are uniformly stimulated, that the saccule and utricle are equally capable of evoking balance responses, and that the maculae areas with respect to the striola represent a fair estimate of afferent numbers. Of course, it is possible that despite our predictions for a non inter-aural orientation bias in the otolith vector, the vector actually happens to have no sagittal components (i.e. an X-component only in Fig. 1B). However, in addition to our predictions there is prior empirical evidence suggesting that selective stimulation of otolith afferents by 500 Hz bone-conducted vibration (Curthoys et al. 2006) is capable of evoking a non inter-aural net linear vector. Thus, the balance response vector to 500 Hz vibration over one mastoid was found to have a significant antero-posterior component (Welgampola & Day, 2006), although the otolith afferent responses to vibration and GVS are not necessarily the same. We also assumed no current spread from stimulus electrodes to the contralateral labyrinth. This has not been proven, but the only way it would violate our prediction of a non-inter-aural response would be if monaural stimulation activated both labyrinths in a similar way to binaural bipolar stimulation. Since response magnitudes to monaural stimulation are substantially smaller than to binaural stimulation (e.g. Day et al. 2010) and polarity-dependent balance responses are not evoked when unilateral vestibular nerve lesioned patients are stimulated on their lesioned side (Watson & Colebatch, 1997), current spread to the contralateral side is unlikely to be more than modest.
Our focus here was short and medium-latency balance responses to GVS. What can be said of otolith involvement in other aspects of responses evoked by GVS? It may be expected that the response to a constant linear acceleration signal evoked from the otoliths would be tonic (i.e. a maintained tilt). In line with this it is known that GVS produces a relatively static tilted posture after a few seconds of stimulation (Day et al. 1997). Indeed, the GVS-evoked response has been hypothesised to be due to an otolith-mediated altered estimate of verticality (Inglis et al. 1995; Hlavacka et al. 1996). However, since a deafferented subject was observed to continue rotating throughout prolonged stimulation (Day & Cole, 2002), the maintained tilt is more likely to be due to proprioceptive re-afference from the sway acting to oppose the canal-evoked rotation. The waveform of the deafferented subject's response resembled the sum of position and velocity components, which could be interpreted as otolith and canal responses, respectively (Day & Cole, 2002), but equally it could simply be the dynamic response to a pure canal signal. When the canal-evoked balance response is abolished, Cathers et al. (2005) showed that GVS does not produce a maintained tilt response. Further evidence against otolith involvement comes from an absence of altered static head or body-tilt perception to GVS (Bisdorff et al. 1996; Watson et al. 1998), and that GVS-evoked postural tilt was unrelated to visual or non-visual estimates of verticality (Wardman et al. 2003). Eye movements evoked by GVS do have features consistent with both otolith and canal reflexes (Zink et al. 1998; Watson et al. 1998; Kleine et al. 1999; Séverac Cauquil et al. 2003), and tilt of the subjective visual vertical (Watson et al. 1998; Wardman et al. 2003) may be interpreted as an otolith effect. However, pure canal signals have been shown (i) to account for the presence and inter-individual variation of both the tonic and phasic ocular torsion evoked by GVS (Schneider et al. 2002) and (ii) to evoke tilts of the subjective visual vertical (Pavlou et al. 2003).
What is the origin of the short-latency response?
If we reject the idea that the short-latency response is a net otolith response, where does it come from? The hypothesis put forward by Britton et al. (1993) was that the short and medium-latency responses arise from the same vestibular signal being processed in two areas of the brain. But the observation that head pitch selectively abolishes only the medium-latency response argues against this hypothesis. Apart from the selective effect of head pitch, other lines of evidence also seem to favour the idea that the two responses arise from different receptors. The two responses have different stimulus thresholds (Fitzpatrick et al. 1994), different effects of ageing (Welgampola & Colebatch, 2002), different effects of current rise time (Rosengren & Colebatch, 2002), and different bandwidths of coherence between SVS and EMG (Dakin et al. 2007). The short-latency response direction's close link with head yaw direction is necessary but not sufficient for a vestibular origin, although it does suggest that it has a balance function. A balance function is also evident from the observation that the response in the legs disappears when the subject is seated or stabilising an equivalent mechanical inverted pendulum (Britton et al. 1993; Fitzpatrick et al. 1994).
A non-vestibular source that might be considered for the short-latency response is through stimulation of cutaneous afferents. However, it was reported that skin anaesthesia at the electrode site had no impact on the short-latency response (Baldissera et al. 1990). GVS-induced facilitation of T-reflexes was recently attributed to a cutaneous effect (Ghanim et al. 2009) but this was a non-polarity-dependent effect, distinguishing it from properties of GVS-evoked balance responses. Watson & Colebatch (1997) described a non-polarity and non-head position dependent reflex in some unilateral vestibular neurectomy patients at the approximate latency of the normal short-latency response, which they speculated could have cutaneous origin. Such a reflex could have biased the apparent direction of the short-latency response measured in our study, but the evidence would suggest that its effect would be very minor under normal conditions.
Another possibility comes from a noteworthy study by Spiegel (1942). He showed that head rotation towards the anode following galvanic stimulation of decerebrate cats depends on the integrity of the 8th cranial nerve and of the vestibular nuclei. After their ablation, a head rotation in the opposite direction towards the cathode (apparently suppressed or over-ridden with intact vestibular pathways) was released. A series of selective lesions localised this effect to afferent stimulation of the 5th and 9th–10th cranial nerves. Unfortunately polarity dependence and craniocentricity of the reversed reflex was not explicitly stated and latencies were not considered. Whilst it would be a big leap to associate a ‘hidden’ cephalogyric reflex in decerebrate cats with the short-latency balance response in humans, the observation does raise the possibility of an independent reflex of non-vestibular origin, not usually expressing overt signs of movement, and opposite to the anodally directed medium-latency vestibular reflex.
Conclusions
In this study we measured the direction of the short-latency response to mastoid stimulation using the correlation between stochastic electrical current and ground-reaction shear force. Our observations show that the short-latency response is directed along the inter-aural line under binaural and monaural stimulation conditions, both with the head up and pitched down through 90 deg. Contrary to our hypothesis, these observations indicate that the short-latency reflex does not represent the response to a net linear vector from otolith afferents.
Acknowledgments
The authors received research funding from the UK Medical Research Council, The Wellcome Trust, the National Health and Medical Research Council of Australia, the Natural Sciences and Engineering Research Council of Canada and the Michael Smith Foundation for Health Research. We thank Mr Daniel Voyce for expert technical support.
Glossary
Abbreviations
- GVS
galvanic vestibular stimulation
- SVS
stochastic vestibular stimulation
Author contributions
All authors contributed to conception and design of the study, interpretation of data, and drafting or critically revising the manuscript. O.S.M and C.J.D. collected and analysed the experimental data. All authors approved the final version of the manuscript. Experiments were conducted at UCL Institute of Neurology.
References
- Ali AS, Rowen KA, Iles JF. Vestibular actions on back and lower limb muscles during postural tasks in man. J Physiol. 2003;546:615–624. doi: 10.1113/jphysiol.2002.030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Tassone G. Effects of transmastoid electrical stimulation on the triceps brachii EMG in man. Neuroreport. 1990;1:191–193. doi: 10.1097/00001756-199011000-00003. [DOI] [PubMed] [Google Scholar]
- Bisdorff AR, Wolsley CJ, Anastasopoulos D, Bronstein AM, Gresty MA. The perception of body verticality (subjective postural vertical) in peripheral and central vestibular disorders. Brain. 1996;119:1523–1534. doi: 10.1093/brain/119.5.1523. [DOI] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Cathers I, Day BL, Fitzpatrick RC. Otolith and canal reflexes in human standing. J Physiol. 2005;563:229–234. doi: 10.1113/jphysiol.2004.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats AC, Stoltz MS. The recorded body-sway response to galvanic stimulation of the labyrinth: a preliminary study. Laryngoscope. 1969;79:85–103. doi: 10.1288/00005537-196901000-00004. [DOI] [PubMed] [Google Scholar]
- Corvera J, Hallpike CS, Schuster EH. A new method for the anatomical reconstruction of the human macular planes. Acta Otolaryngol. 1958;49:4–16. doi: 10.3109/00016485809134722. [DOI] [PubMed] [Google Scholar]
- Curthoys I, Kim J, McPhedran S, Camp A. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. 2006;175:256–267. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Luu BL, Van Den Doel K, Inglis JT, Blouin J. Frequency-specific modulation of vestibular-evoked sway responses in humans. J Neurophysiol. 2010;103:1048–1056. doi: 10.1152/jn.00881.2009. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Son GML, Inglis JT, Blouin J. Frequency response of human vestibular reflexes characterized by stochastic stimuli. J Physiol. 2007;583:1117–1127. doi: 10.1113/jphysiol.2007.133264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Cole J. Vestibular-evoked postural responses in the absence of somatosensory information. Brain. 2002;125:2081–2088. doi: 10.1093/brain/awf212. [DOI] [PubMed] [Google Scholar]
- Day BL, Fitzpatrick RC. Virtual head rotation reveals a process of route reconstruction from human vestibular signals. J Physiol. 2005a;567:591–597. doi: 10.1113/jphysiol.2005.092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Fitzpatrick RC. The vestibular system. Curr Biol. 2005b;15:R583–586. doi: 10.1016/j.cub.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Day BL, Guerraz M. Feedforward versus feedback modulation of human vestibular-evoked balance responses by visual self-motion information. J Physiol. 2007;582:153–161. doi: 10.1113/jphysiol.2007.132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Marsden JF, Ramsay E, Mian OS, Fitzpatrick RC. Non-linear vector summation of left and right vestibular signals for human balance. J Physiol. 2010;588:671–682. doi: 10.1113/jphysiol.2009.181768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Séverac Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol. 1997;500:661–672. doi: 10.1113/jphysiol.1997.sp022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC, Butler JE, Day BL. Resolving head rotation for human bipedalism. Curr Biol. 2006;16:1509–1514. doi: 10.1016/j.cub.2006.05.063. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004;96:2301–2316. doi: 10.1152/japplphysiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Marsden J, Lord SR, Day BL. Galvanic vestibular stimulation evokes sensations of body rotation. Neuroreport. 2002;13:2379–2383. doi: 10.1097/00001756-200212200-00001. [DOI] [PubMed] [Google Scholar]
- Ghanim Z, Lamy JC, Lackmy A, Achache V, Roche N, Penicaud A, Meunier S, Katz R. Effects of galvanic mastoid stimulation in seated human subjects. J Appl Physiol. 2009;106:893–903. doi: 10.1152/japplphysiol.90594.2008. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C, Smith CE. Responses of vestibular-nerve afferents in the squirrel monkey to externally applied galvanic currents. Brain Res. 1982;252:156–160. doi: 10.1016/0006-8993(82)90990-8. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data—Theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hlavacka F, Mergner T, Krizkova M. Control of the body vertical by vestibular and proprioceptive inputs. Brain Res Bull. 1996;40:431–434. doi: 10.1016/0361-9230(96)00138-4. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Shupert CL, Hlavacka F, Horak FB. Effect of galvanic vestibular stimulation on human postural responses during support surface translations. J Neurophysiol. 1995;73:896–901. doi: 10.1152/jn.1995.73.2.896. [DOI] [PubMed] [Google Scholar]
- Kim J, Curthoys IS. Responses of primary vestibular neurons to galvanic vestibular stimulation (GVS) in the anaesthetised guinea pig. Brain Res Bull. 2004;64:265–271. doi: 10.1016/j.brainresbull.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Kleine JF, Guldin WO, Clarke AH. Variable otolith contribution to the galvanically induced vestibulo-ocular reflex. Neuroreport. 1999;10:1143–1148. doi: 10.1097/00001756-199904060-00044. [DOI] [PubMed] [Google Scholar]
- Lee Son GM, Blouin J, Inglis JT. Short duration galvanic vestibular stimulation evokes prolonged balance responses. J Appl Physiol. 2008;105:1210–1217. doi: 10.1152/japplphysiol.01398.2006. [DOI] [PubMed] [Google Scholar]
- Lindeman HH. Studies on the morphology of the sensory regions of the vestibular apparatus with 45 figures. Ergeb Anat Entwicklungsgesch. 1969;42:1–113. [PubMed] [Google Scholar]
- Lowenstein O. The effect of galvanic polarization on the impulse discharge from sense endings in the isolated labyrinth of the thornback ray (Raja clavata) J Physiol. 1955;127:104–117. doi: 10.1113/jphysiol.1955.sp005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand. 1983;117:307–309. doi: 10.1111/j.1748-1716.1983.tb07212.x. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Castellote J, Day BL. Bipedal distribution of human vestibular-evoked postural responses during asymmetrical standing. J Physiol. 2002;542:323–331. doi: 10.1113/jphysiol.2002.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SN, Velázquez-Villaseñor L, Tsuji K, Glynn RJ, Wall C, Rauch SD. Temporal bone studies of the human peripheral vestibular system. Normative vestibular hair cell data. Ann Otol Rhinol Laryngol Suppl. 2000;181:3–13. doi: 10.1177/00034894001090s502. [DOI] [PubMed] [Google Scholar]
- Mian OS, Day BL. Determining the direction of vestibular-evoked balance responses using stochastic vestibular stimulation. J Physiol. 2009;587:2869–2873. doi: 10.1113/jphysiol.2009.171256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma H, Tokumasu K, Okamoto M, Hashimoto S, Yamashina S. Three-dimensional analysis of morphological aspects of the human saccular macula. Ann Otol Rhinol Laryngol. 2001;110:1017–1024. doi: 10.1177/000348940111001105. [DOI] [PubMed] [Google Scholar]
- Naganuma H, Tokumasu K, Okamoto M, Hashimoto S, Yamashina S. Three-dimensional analysis of morphological aspects of the human utricular macula. Ann Otol Rhinol Laryngol. 2003;112:419–424. doi: 10.1177/000348940311200506. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Wolfson P. Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of the human labyrinth. Brain Res. 1974;67:255–268. doi: 10.1016/0006-8993(74)90276-5. [DOI] [PubMed] [Google Scholar]
- Pavlou M, Wijnberg N, Faldon ME, Bronstein AM. Effect of semicircular canal stimulation on the perception of the visual vertical. J Neurophysiol. 2003;90:622–630. doi: 10.1152/jn.00960.2002. [DOI] [PubMed] [Google Scholar]
- Rosengren SM, Colebatch JG. Differential effect of current rise time on short and medium latency vestibulospinal reflexes. Clin Neurophysiol. 2002;113:1265–1272. doi: 10.1016/s1388-2457(02)00121-9. [DOI] [PubMed] [Google Scholar]
- Rosenhall U. Vestibular macular mapping in man. Ann Otol Rhinol Laryngol. 1972;81:339–351. doi: 10.1177/000348947208100305. [DOI] [PubMed] [Google Scholar]
- Schneider E, Bartl K, Glasauer S. Galvanic vestibular stimulation combines with Earth-horizontal rotation in roll to induce the illusion of translation. Ann N Y Acad Sci. 2009;1164:116–118. doi: 10.1111/j.1749-6632.2008.03732.x. [DOI] [PubMed] [Google Scholar]
- Schneider E, Glasauer S, Dieterich M. Comparison of human ocular torsion patterns during natural and galvanic vestibular stimulation. J Neurophysiol. 2002;87:2064–2073. doi: 10.1152/jn.00558.2001. [DOI] [PubMed] [Google Scholar]
- Séverac Cauquil A, Faldon M, Popov K, Day BL, Bronstein AM. Short-latency eye movements evoked by near-threshold galvanic vestibular stimulation. Exp Brain Res. 2003;148:414–418. doi: 10.1007/s00221-002-1326-z. [DOI] [PubMed] [Google Scholar]
- Spiegel EA. Cephalogyric reactions of non-labyrinthine origin. Am J Physiol. 1942;135:628–632. [Google Scholar]
- Takagi A, Sando I. Computer-aided three-dimensional reconstruction and measurement of the vestibular end-organs. Otolaryngol Head Neck Surg. 1988;98:195–202. doi: 10.1177/019459988809800303. [DOI] [PubMed] [Google Scholar]
- Tribukait A, Rosenhall U. Directional sensitivity of the human macula utriculi based on morphological characteristics. Audiol Neurootol. 2001;6:98–107. doi: 10.1159/000046815. [DOI] [PubMed] [Google Scholar]
- Tribukait A, Rosenhall U, Osterdahl B. Morphological characteristics of the human macula sacculi. Audiol Neurootol. 2005;10:90–96. doi: 10.1159/000083364. [DOI] [PubMed] [Google Scholar]
- Upton GJG. Approximate confidence intervals for the mean direction of a von mises distribution. Biometrika. 1986;73:525–527. [Google Scholar]
- Wardman DL, Taylor JL, Fitzpatrick RC. Effects of galvanic vestibular stimulation on human posture and perception while standing. J Physiol. 2003;551:1033–1042. doi: 10.1113/jphysiol.2003.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SR, Colebatch JG. EMG responses in the soleus muscles evoked by unipolar galvanic vestibular stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:476–483. doi: 10.1016/s0924-980x(97)00044-1. [DOI] [PubMed] [Google Scholar]
- Watson SRD, Brizuela AE, Curthoys IS, Colebatch JG, MacDougall HG, Halmagyi GM. Maintained ocular torsion produced by bilateral and unilateral galvanic (DC) vestibular stimulation in humans. Exp Brain Res. 1998;122:453–458. doi: 10.1007/s002210050533. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Selective effects of ageing on vestibular-dependent lower limb responses following galvanic stimulation. Clin Neurophysiol. 2002;113:528–534. doi: 10.1016/s1388-2457(02)00020-2. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Day BL. Craniocentric body-sway responses to 500 Hz bone-conducted tones in man. J Physiol. 2006;577:81–95. doi: 10.1113/jphysiol.2006.115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 5th ed. New Jersey, USA: Pearson Education; 2010. [Google Scholar]
- Zink R, Bucher SF, Weiss A, Brandt T, Dieterich M. Effects of galvanic vestibular stimulation on otolithic and semicircular canal eye movements and perceived vertical. Electroencephalogr Clin Neurophysiol. 1998;107:200–205. doi: 10.1016/s0013-4694(98)00056-x. [DOI] [PubMed] [Google Scholar]


