Abstract
The influence of age on the central and peripheral contributors to exercise-induced hyperaemia is unclear. Utilizing a reductionist approach, we compared the peripheral and central haemodynamic responses to passive limb movement (exercise without an increase in metabolism) in 11 old (71 ± 9 years of age s.d.) and 11 young (24 ± 2 years of age) healthy subjects. Cardiac output (CO), heart rate (HR), stroke volume (SV), mean arterial pressure (MAP), and femoral blood flow of the passively moved and control legs were evaluated second-by-second during 2 min of passive knee extension at a rate of 1 Hz. Compared to the young, the old group exhibited a significantly attenuated increase in HR (7 ± 4%vs. 13 ± 7%s.d.), CO (10 ± 6%vs. 18 ± 8%) and femoral blood flow in the passively moved (123 ± 55%vs. 194 ± 57%) and control legs (47 ± 43%vs. 77 ± 96%). In addition, the change in vascular conductance in the passively moving limb was also significantly attenuated in the old (2.4 ± 1.2 ml min−1 mmHg−1) compared to the young (4.3 ± 1.7 ml min−1 mmHg−1). In both groups all main central and peripheral changes that occurred at the onset of passive knee extension were transient, lasting only 45 s. In a paradigm where metabolism does not play a role, these data reveal that both central and peripheral haemodynamic mechanisms are likely to be responsible for the 30% reduction in exercise-induced hyperaemia with age.
Introduction
The preponderance of evidence has revealed that aged humans have a 20–30% reduction in skeletal muscle blood flow during rest (Dinenno et al. 1999, 2001) and exercise (Proctor et al. 1998, 2003a; Beere et al. 1999; Poole et al. 2003; Wray et al. 2009a,b;). This attenuation in blood flow most likely contributes to age related declines in exercise tolerance, maximal oxygen consumption and overall physical functional capacity. Numerous peripheral factors are believed to contribute to the hyperaemic response at the onset of exercise including: the skeletal-muscle pump (Laughlin, 1987; Sheriff et al. 1993), mechanical-induced vasodilatation (Tschakovsky et al. 2004; Clifford et al. 2006; Kirby et al. 2007), mechanical distortion of arterioles (Segal, 2000) and flow-mediated dilatation (Pohl et al. 1986; Kooijman et al. 2008). Hyperaemia is also influenced by central (cardiac) factors through cardio-acceleration in response to muscle mechanoreceptor and chaemoreceptor feedback (Adreani et al. 1997; Adreani & Kaufman, 1998; Herr et al. 1999). Each of these peripheral and central factors, alone or in combination, could conceptually contribute to the blunted exercise hyperaemia with advancing age.
Recently, several investigators have determined the effects of age on muscle blood flow while minimizing the potential for central factors (i.e. cardiac output and sympathetic vasoconstriction) to limit the hyperaemic response during exercise (Lawrenson et al. 2003; Donato et al. 2006; Carlson et al. 2008; Kirby et al. 2009). Specifically, Donato et al. (2006) and Lawrenson et al. (2003) reported that the attenuated leg blood flow in healthy older adults during steady-state knee extension exercise was associated with a reduction in vascular conductance. In addition, Carlson et al. (2008) determined that older individuals display an impaired intensity-dependent contraction-induced rapid vasodilatation following single muscle contractions, a model that, due to the quick nature of a single contraction, eliminates the influence of central factors from the hyperaemic response. According to a follow-up study by Kirby et al. (2009), the diminished rapid vasodilatory capacity with age cannot be restored with antioxidant infusion and thus is not likely to be endothelium dependent. In combination, these investigations provide evidence of a peripheral vascular limitation to exercise-induced hyperaemia in the ageing population.
Over the past several years our group (Wray et al. 2005; McDaniel et al. 2010) and others (Nobrega & Araujo, 1993; Nurhayati & Boutcher, 1998; Ter Woerds et al. 2006; Gonzalez-Alonso et al. 2008) have utilized another reductionistic approach, passive exercise, to partition not only central and peripheral factors but also the metabolic and mechanical stimuli that may influence exercise induced hyperaemia. Specifically, this passive model, devoid of increased metabolism, exposes other central and peripheral factors that contribute to movement-induced hyperaemia. Thus, this model may help clarify whether metabolism is the major source for the age related immediate and steady state reduction in exercise-induced hyperaemia, or if there are other central and/or peripheral factors that also contribute to the reductions in blood flow observed with age.
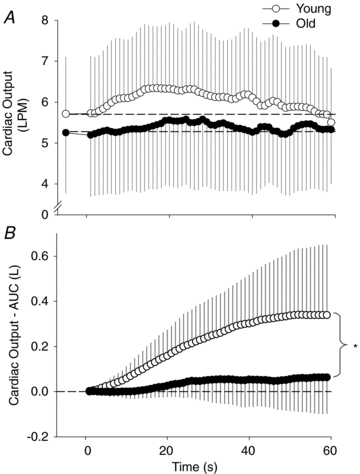
Therefore, the primary aim of this study was to compare the central and peripheral haemodynamic responses to passive limb movement between young and old humans to better elucidate the mechanisms that are responsible for the attenuated exercise-induced hyperaemic response with age. Despite the performance of no work by the subjects, and therefore no increase in metabolism, we hypothesized that blood flow in the passively moved leg would be attenuated in the older subjects. Based on the current understanding that CO at any oxygen consumption (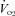 ) during submaximal exercise is similar between young and old, we hypothesized that the central haemodynamic (i.e. CO) response would be similar between the young and old group during passive exercise. Accordingly, we also hypothesized that peripheral factors such as mechanically induced and flow-mediated dilatation, known to be reduced with age, would be the major mechanisms responsible for the attenuated hyperaemic response in the old.
) during submaximal exercise is similar between young and old, we hypothesized that the central haemodynamic (i.e. CO) response would be similar between the young and old group during passive exercise. Accordingly, we also hypothesized that peripheral factors such as mechanically induced and flow-mediated dilatation, known to be reduced with age, would be the major mechanisms responsible for the attenuated hyperaemic response in the old.
Methods
Subjects and general procedures
Eleven healthy older men (71 ± 9 years of age s.d.) and 11 healthy young men (24 ± 2 years of age) participated in the current study. The protocol was approved by the Institutional Review Boards of the University of Utah and the Salt Lake City VA Medical Center and written informed consent was obtained from all subjects prior to their inclusion in the study. The study conformed with the Declaration of Helsinki. All studies were performed in a thermoneutral environment (22°C). Subjects reported to the laboratory in an overnight fasted state, and had not performed exercise within the past 24 h.
Passive exercise protocol
Prior to the protocol blood was collected for analysis of fasting glucose, a blood lipid panel (cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglycerides) and a complete blood count (haemoglobin, white blood cell, neutrophils, lymphocytes and monocytes). Following blood sampling, subjects lay supine for 20 min prior to the start of data collection. The initial protocol consisted of a 30 s resting baseline followed by a 2 min bout of passive knee extension. One minute prior to the start of the passive exercise a cuff, placed distal to the knee on the passively moved leg, was inflated to 250 mmHg eliminating blood flow to the lower leg. The cuff, which remained inflated throughout the entire 2 min protocol, eliminated fluctuations in blood flow to the lower leg as a consequence of movement related changes in gravitational and centrifugal forces. Initial pilot work revealed minimal effect of either cuffing or not cuffing the control leg (i.e. non-moved leg) in the same manner and consequently, for subject comfort, a cuff on the control leg was not applied in these studies. All passive exercise was achieved by the same member of our research team moving the subjects’ lower leg through the range of motion defined by 90 and 180 deg knee joint angles at 1 Hz. Real time feedback was provided by a position sensor to ensure a consistent range of motion and a metronome to maintain cadence. Prior to the start and throughout the protocol subjects were encouraged to remain passive, and resist any urge to assist with leg movement. In the rare instance that a subject assisted with or resisted the movement the protocol was terminated and repeated following 10 min of recovery. Throughout the protocol the control leg remained fully extended.
Measurements
Femoral blood flow
Simultaneous measurements of femoral arterial blood velocity and vessel diameter were performed in the passive (moving) and control (stationary) legs distal to the inguinal ligament and proximal to the bifurcation of the superficial and deep femoral artery with a Logic 7 and Logic e ultrasound systems (General Electric Medical Systems, Milwaukee, WI, USA) operated by two separate trained technicians. The Logic 7 and Logic e were equipped with linear array transducers operating at an imaging frequency of 14 and 12 MHz, respectively. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area. Blood velocity was obtained using the same transducers with a Doppler frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60 deg or less. The sample volume was maximized according to vessel size and was centred within the vessel based on real-time ultrasound visualization. Arterial diameter was measured and angle-corrected, and intensity weighted mean velocity (Vmean) values were then calculated using commercially available software (Logic 7 and Logic e). Using arterial diameter and Vmean, blood flow in the femoral artery was calculated as: Blood flow = Vmeanπ(vessel diameter/2)2× 60, where blood flow is in millilitres per minute.
Central variables
Electrocardiogram (ECG), stroke volume (SV), cardiac output (CO) and mean arterial pressure (MAP) were determined with a Finometer (Finapres Medical Systems BV, Amsterdam, the Netherlands). Stroke volume was calculated using the Modelflow method (Sugawara et al. 2003; Bogert & van Lieshout, 2005; de Wilde et al. 2009) which includes age, sex, height and weight in its algorithm (Beatscope version 1.1; Finapres Medical Systems). Cardiac output was then calculated as the product of HR and SV. Vascular conductance within the passively moved leg was calculated as leg blood flow/MAP.
Knee joint angle
During each protocol knee joint angle of the passive leg was continuously recorded using a Vishay Spectrol 360 degree Smart Position Sensor (Vashay Intertechnology Inc., Malvern, PA, USA) mounted on a BREG X2K knee brace (BREG, Inc., Vista, CA, USA) worn by the subjects.
Data acquisition
Throughout each entire protocol signals reflecting ECG, SV, CO, MAP and knee joint angle underwent A/D conversion and were simultaneously acquired (200 Hz) using commercially available data acquisition software (AcqKnowledge, Biopac Systems Inc., Goleta, CA, USA). In addition, the audio anterograde and retrograde signals from both Doppler ultrasound systems were acquired (10,000 Hz) to serve as a qualitative indicator of blood velocity changes and to ensure accurate temporal alignment of blood velocity measurements obtained from the Doppler systems and other variables collected (i.e. CO, HR, SV, MAP as well as the knee joint angle indicating the onset and offset of the passive exercise).
Thigh volume
Thigh volume was calculated, as previously described (Lawrenson et al. 2003), based on thigh circumference (three sites: distal, middle and proximal), thigh length and thigh skinfold measurements (Jones & Pearson, 1969).
Data analysis
The data acquisition software (AcqKnowledge, Biopac Systems) allowed second by second acquisition of HR, SV, CO, MAP, knee joint angle as well as the qualitative tracings that represented anterograde and retrograde blood velocities. From the velocity and femoral artery diameters, anterograde, retrograde and net blood flows were also calculated on a second-by-second basis for both the control and passively moved leg. Prior to analysis, all data were smoothed using a rolling 3 s average. Two-way repeated measures ANOVAs were used to determine if there was a significant main effect of time or interaction (group × time). If there was a significant main effect of time, simple contrasts (comparison of baseline to each 3 s average) were utilized to determine if there were significant changes from baseline for each variable. If the ANOVA and simple contrasts indicated a significant change from baseline, independent t tests were used to determine if the individual maximal absolute and relative changes differed between the young and old groups. Alpha was set at 0.05 for all comparisons. All data are presented as the mean ± standard deviation (s.d.).
Results
Subject characteristics
Results from the blood analyses revealed that only fasting glucose and lymphocyte count differed between the two groups. Only one young and one old subject had elevated triglycerides and cholesterol and two older subjects had elevated LDL; all other subjects were within the normal range for all variables. Body mass index (BMI) and thigh volume were not different between the two groups (Table 1).
Table 1.
Subject characteristics
| Young (n = 11) | Old (n = 11) | |
|---|---|---|
| Age (years) | 24 ± 2 | 71 ± 9 |
| Weight (kg) | 84.6 ± 9.0 | 83.7 ± 11.8 |
| Height (cm) | 182 ± 6 | 174 ± 9 |
| Lean thigh volume (l) | 7.5 ± 1.1 | 6.9 ± 1.1 |
| BMI | 24.9 ± 2.3 | 27.4 ± 2.2 |
| Femoral artery diameter (mm) | 98.4 ± 13.6 | 88.9 ± 6.4 |
| Glucose (mg dl−1) | 74.4 ± 9.8 | 95.7 ± 15.4* |
| Cholesterol (mg dl−1) | 145.7 ± 41.8 | 173 ± 41.51 |
| HDL (mg dl−1) | 41.7 ± 8.9 | 44.3 ± 9.8 |
| LDL (mg dl−1) | 96.0 ± 40.9 | 113.2 ± 37.02 |
| Triglycerides (mg dl−1) | 82.4 ± 38.21 | 118.4 ± 56.41 |
| Haemoglobin (g dl−1) | 15.2 ± 1.1 | 14.9 ± 1.2 |
| WBC (K ul−1) | 5.5 ± 0.6 | 5.7 ± 1.2 |
| Neutrophil (K ul−1) | 2.7 ± 0.6 | 3.6 ± 1.1 |
| Lymphocyte (K ul−1) | 2.0 ± .4 | 1.4 ± .4* |
| Monocyte (K ul−1) | 0.6 ± 0.1 | 0.5 ± 0.1 |
BMI, body mass index; HDL, high density lipoproteins; LDL, low density lipoprotein; WBC, white blood cells. Data are means ± s.d.
Significantly different from the young (α = 0.05). Superscripts indicate number of subjects whose values were outside the normal range.
Baseline and overall responses
There were no differences in baseline values between the young and old group for CO, SV, MAP, HR or blood flow in the passive leg. Resting blood flow in the control leg was significantly lower in the old group (Table 2). The repeated measures and contrast analysis revealed that CO, HR and blood flow in the passively moved leg increased significantly from baseline as a result of the passive movement in both young and old groups. The fluctuations in MAP and SV did not achieve statistical significance. In addition, blood flow in the control leg increased significantly from baseline in the young but not the old. All physiological changes that occurred following the onset of passive movement were transient in nature, returning to baseline within 1 min (Fig. 1). The only exceptions to this were the anterograde and retrograde blood flows in the passively moved leg which remained elevated throughout the entire protocol. Due to the slight variations in kinetics between individuals, the average maximal change is underestimated in Fig. 1, but accurately represented in Fig. 2.
Table 2.
Baseline values
| Young (n = 11) | Old (n = 11) | |
|---|---|---|
| CO (l min−1) | 5.7 ± 1.6 | 5.3 ± 1.8 |
| HR (BPM) | 55.8 ± 6.8 | 59.4 ± 7.4 |
| SV (ml) | 103.5 ± 26.2 | 96.4 ± 36.6 |
| MAP (mmHg) | 92.7 ± 7.7 | 96.6 ± 10.9 |
| Passive leg blood flow (ml min−1)# | 207 ± 40 | 164 ± 138 |
| Control leg blood flow (ml min−1)# | 232 ± 96 | 140 ± 68* |
CO, cardiac output; HR, heart rate; SV, stroke volume; MAP, mean arterial pressure.
Bilateral blood flow was only successfully measured on 9 of the subjects within each group.
Significant difference from young (P < 0.05).
Figure 1. Relative changes (mean ± s.d.) in central and peripheral variables at the onset of passive knee extension.
These figures are presented to illustrate the general trends observed with passive exercise for the young and old groups. The first 60 s are a 3 s rolling average, whereas the data in the last minute are presented as 12 s averages.
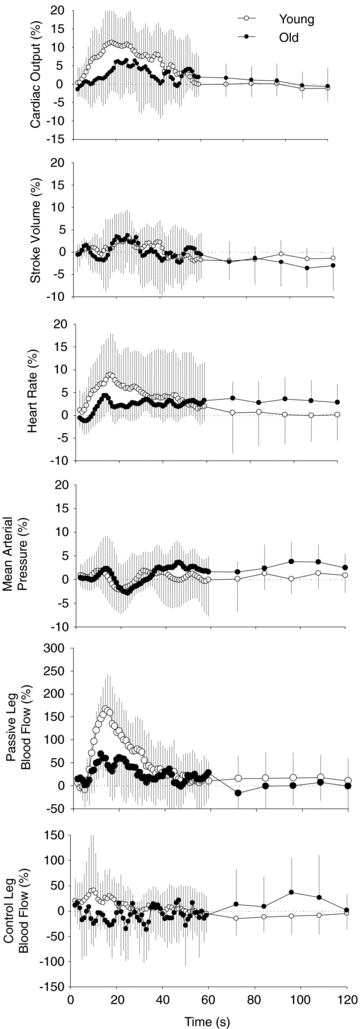
Figure 2. Mean (± s.d.) maximum change for CO, SV, MAP, HR and blood flow in the passive and control leg.
*Significant attenuation in the old group compared to the young group (P < 0.05). Vertical dashed line separates variables associated with the right and left axes.
Central responses
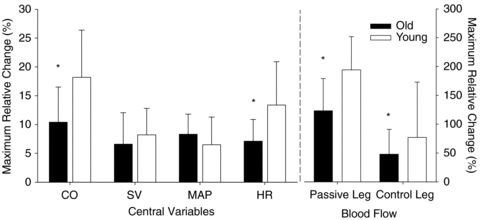
Following the onset of passive movement the old group displayed an attenuated absolute increase in CO and HR compared to the young (0.64 ± 0.57 l min−1versus 1.0 ± 0.4 and 4.2 ± 2.1 versus 7.3 ± 4.1 bpm, for the old and young, respectively). The maximal relative increases in CO and HR were also attenuated in the old compared to the young group (Fig. 2). In addition, the cumulative increase in CO, as measured by area under the curve across the first minute of passive knee extension, was reduced in the old group compared to the young (Fig. 3).
Figure 3. Cardiac output (mean ± s.d.) during passive knee extension.
A, cardiac output for the young and old groups during the first 60 s of passive knee extension. The point prior to 0 and the dashed horizontal lines represent baseline. B, summed second by second cardiac output across the first 60 s (area under the curve from A). *Significant attenuation in the old group compared to the young (P < 0.05).
Peripheral responses
The absolute change in blood flow (baseline to maximum) in the passively moved leg was attenuated in the old compared to the young (242 ± 133 versus 386 ± 146 ml min−1). The relative change in blood flow in the passively moved leg was also significantly reduced in the old compared to the young (Fig. 2). In addition, the absolute change in vascular conductance in the passive leg was attenuated in the old (2.4 ± 1.25 ml min−1 mmHg−1) compared to the young (4.3 ± 1.7 ml min−1 mmHg−1) as was the vascular conductance at maximal blood flow (4.3 ± 2.0 versus 6.3 ± 2.1 ml min−1 mmHg−1, for old and young, respectively). The transient increase in net blood flow in both groups resulted from an immediate increase in anterograde blood flow that was subsequently offset by a more gradual increase in retrograde blood flow (Fig. 4). In the control leg, the relative (Fig. 2) and absolute changes in blood flow were greater in the young compared to the old (152 ± 104 versus 52 ± 42 ml min−1 for the young and old, respectively). In fact, across the first minute of passive knee extension, the younger group demonstrated a cumulative increase in blood flow in the control leg whereas the older group demonstrated a cumulative reduction in blood flow (Fig. 5).
Figure 4. Net (A), anterograde (B) and retrograde (C) femoral blood flow from the passively moved limb.

The increase in net blood flow results from an immediate increase in anterograde blood flow. Subsequently, net blood flow returns to baseline due to the combination of a decrease in antegrade blood flow and a more gradual increase in retrograde blood flow that eventually offsets anterograde blood flow. Note that both anterograde and retrograde blood flow remain elevated throughout the duration of passive exercise. s.d. bars were omitted to maintain clarity.
Figure 5. Control leg blood flow (mean ± s.d.) during passive exercise.
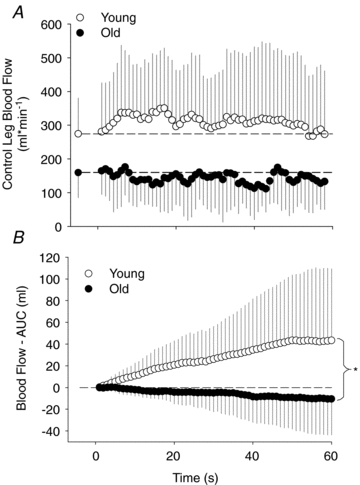
A, control femoral blood flow during the first 60 s of passive knee extension. Note, data reflecting the young group illustrate a net increase in blood flow across the first minute whereas data points from the old group remain near or below baseline. B, summed blood flow from the control leg across the first 60 s of passive knee extension (area under the curve from figure A). *Area under curve for the old is significantly lower than the young. Note the ‘steal effect’ from the control leg of the old group (P < 0.05).
Discussion
Utilizing high time resolution assessment (second-by-second) of cardiac output, stroke volume, heart rate, mean arterial pressure, and femoral blood flow, the central and peripheral factors responsible for hyperaemia at the onset of passive exercise were compared between old and young. This approach yielded several novel findings. In an exercise model that is devoid of increased metabolism, the old still exhibit a 30% reduction in blood flow following the onset of movement. This reduced blood flow is likely to have resulted from an attenuated HR driven increase in CO, while the reduced vascular conductance in the passively moved leg of the older subjects indicates that attenuated peripheral vasodilatation also contributes to this phenomenon. Together, these data indicate that both central and peripheral factors are responsible for the attenuated exercise-induced hyperaemia with age, and the mechanisms responsible for these age-associated differences are not dependent upon metabolic by-products produced during volitional exercise.
Effects of ageing on central parameters
In contrast to our hypothesis, following the onset of passive knee extension, the old group exhibited a reduced HR driven increase in CO compared to the old group. In fact, the absolute and relative increase in CO in the old group was nearly half that of the young group (Fig. 2). In addition, summing the second-by-second absolute increase in CO across the first minute (area under the curve) yielded a greater than twofold attenuation in CO for the old group compared to the young group (Fig. 3). It is interesting to note that MAP was not significantly elevated as a result of the increase in CO in either group. This conundrum may be explained by the simultaneous vasodilatation in the passive leg that is able to ‘absorb’ most of the increased blood flow associated with the increase in CO, minimizing the potential increase in MAP. In fact, our previous investigation supports this concept in that during an identical passive protocol, in which blood flow was occluded to the passive leg, there tended to be an immediate rise in MAP and a greater increase in blood flow to the control leg, compared to the trial without the femoral occlusion (McDaniel et al. 2010). These data support the concept that the vasodilatation in the passively moved leg is responsible for offsetting the increase in MAP.
There are several mechanisms that may contribute to the increase in HR observed at the onset of volitional exercise, including central command (i.e. feed-forward control) (Eldridge et al. 1985; Williamson et al. 2006), sympathetic stimulation (Warner & Cox, 1962; Robinson et al. 1966; Rowell, 1986), cardiac vagal withdrawal (Fagraeus & Linnarsson, 1976; McMahon & McWilliam, 1992; Vianna et al. 2008) and muscle afferents (i.e. feed-back control) (Coote et al. 1971; Stebbins et al. 1988; Adreani et al. 1997). The feedback signals from the muscle by group III and IV afferent neurons are stimulated by mechanical distortion and metabolic by-products, respectively. Due to the passive nature of the exercise utilized in this investigation, feed-forward stimulation from central command and feed-back stimulation from group IV afferents should be largely absent. It could be argued that occluding the lower limb would result in group IV feedback; however there is no evidence that longer (5 min) cuff occlusion during flow mediated dilatation protocols stimulates a cardiovascular response. In addition, the transient nature of the cardiovascular response does not agree with an occlusion-induced ever growing accumulation of metabolic byproducts which would be likely to yield a mounting stimulus and not a transient one. Furthermore, sympathetic stimulation has been shown not to be invoked until moderate intensity levels of active knee extension (Wray et al. 2004), and therefore it is unlikely to occur in this passive model especially since HR remained below 100 bpm (Robinson et al. 1966). Thus, in this paradigm the neural components most likely to be responsible for the immediate increase in HR are afferent feedback from group III mechanoreceptors and subsequent vagal withdrawal.
Although there is little information regarding the influence of age on mechanoreceptor sensitivity, there is evidence for reduced baroreceptor sensitivity and/or HR response to changes in carotid pressure with age (Ebert et al. 1992; Laitinen et al. 1998; Monahan, 2007; Fisher et al. 2009). Thus, it is possible that there is reduced global neuro-receptor (i.e. baroreceptor, chaemoreceptor, mechanoreceptor, etc.) sensitivity with age. With these factors in mind, the attenuated elevation in HR observed in the older group is likely to be a consequence of either reduced group III feedback, reduced vagal withdrawal, or reduced cardiac sensitivity to the changes in these stimuli. Further studies involving additional reductionist paradigms are needed to more fully elucidate this age-related dysfunction.
Effects of ageing on blood flow
Although not the focus of this investigation, baseline blood flow in both legs was numerically lower in the old, but these differences achieved statistical significance in the control leg only (Table 2: data only from subjects with successful bilateral blood flow measurements, n = 9). This somewhat equivocal finding reflects several studies in the literature which have concluded that age does not influence blood flow (Proctor et al. 2003b; Donato et al. 2006; Parker et al. 2007). In contrast, the data from the current investigation reveal that there is an attenuated increase in blood flow at the onset of passive exercise in the older subjects (Figs 1 and 2). In fact, the increased blood flow in the old subjects was over a 100 ml min−1 (∼30%) less than the increased blood flow in the young subjects. This is in agreement with previous reports that indicate there is a 20–30% reduction in skeletal muscle blood flow during exercise with age (Proctor et al. 1998, 2003a; Beere et al. 1999; Poole et al. 2003; Wray et al. 2009a,b;). As there were minimal changes in MAP in either group, the kinetics of vascular conductance was nearly identical to that of blood flow. The maximal change in conductance as well as conductance at maximal blood flow was reduced in the old compared to the young. Therefore, the differences in hyperaemia observed between the two groups were independent of changes in MAP.
The data reflecting an attenuated blood flow with age are in agreement with other investigations that also made an effort to reduce the influence of central haemodynamic factors, by employing small muscle mass exercise, and revealed an attenuated blood flow response in older subjects (Lawrenson et al. 2003; Hammer & Boegehold, 2005; Donato et al. 2006; Wray & Richardson, 2006; Carlson et al. 2008; Kirby et al. 2009; Jackson et al. 2010). For example, Donato et al. (2006) reported that older subjects exhibited a reduced femoral blood flow and conductance during knee extension exercise compared to younger controls. In addition, Carlson et al. (2008) and Kirby et al. (2009) both reported an attenuated blood flow response following a single contraction in old compared to young subjects. The current data extend this work to reveal that exercise-induced hyperaemia is attenuated with age even in a paradigm where an increase in metabolism is not invoked.
Although hyperaemia was attenuated in the old, both young and old groups demonstrated an initial rise in blood flow at the onset of passive exercise. This hyperaemia was due to the substantially greater initial increase in anterograde blood flow compared to retrograde blood flow (Fig. 4). However, this mismatch was transient and within 20–40 s following the onset of passive movement the anterograde blood flow declined but still remained greater than baseline. At that point the remaining elevation in anterograde blood flow above baseline was offset by the delayed gradual rise in retrograde blood flow, resulting in the return of net blood flow to baseline levels. Interestingly, this initial overshoot in blood flow is similar to the hyperperfusion observed at the onset of active exercise (Laughlin & Armstrong, 1983) prior to the achievement of steady state blood flow that is appropriately matched with metabolism (Armstrong et al. 1985; Glenn et al. 1987). Thus, a similar mechanism may be present in the passive exercise model, which results in the initial hyperaemic response followed by a steady state flow that matches metabolic demand, which during passive exercise is equal to the metabolic demand during baseline.
Blood flow in the control leg also exhibited different responses between the young and old groups. Specifically, in the young subjects there was a similar but attenuated hyperaemia in the control leg compared to the passive leg (Fig. 1). However, during the first minute of passive knee extension, the older subjects demonstrated a reduction in blood flow in the control leg concurrently with the hyperaemic response in the passive leg (Fig. 1). This is illustrated more clearly when the second-by-second increases in blood flow are summed across the first minute of passive exercise (Fig. 5). After 1 min there is a net increase in blood flow in the control leg of the young subjects and a net decrease in blood flow in the older subjects. Thus, in the older subjects there appears to be a blood flow ‘steal’ from the non-moving limb that results from local vasodilatation, and subsequent drop in pressure, in the passively moved leg that cannot be fully supported by their smaller increase in CO. In contrast, the CO increase in the young subjects appears to be sufficient to sustain increased blood flow in the passively moved leg as well as the non-moving control leg. In fact, in terms of absolute blood volume in the young group, the increase in CO was double that of the combined increase in blood flow to the passive and control leg. This difference can be explained by the likely parallel increase in blood flow to other vascular beds (e.g. arms, viscera, etc.) that were not accounted for in this investigation as a result of the increased cardiac output.
As previously mentioned, the main central and peripheral changes were transient in nature. With passive exercise there are neither descending motor command signals nor increased metabolism (Gonzalez-Alonso et al. 2008; Hellsten et al. 2008) to yield metaboreceptor afferent signals typically associated with active exercise. It is likely that this lack of motor command and metaboreceptor afferent signals, in addition to an adaptation of the mechanoreceptors (Baum et al. 1995) resulting in decreased type III afferent feedback, facilitates the fall in CO back toward baseline values. Thus at approximately 45 s after the onset of limb movement, CO returns to baseline values, shortly followed by the concomitant drop in control leg blood flow to baseline (Fig. 1).
In general, the data acquired from our young subjects are similar to our previous, more exploratory, investigation in this age group (McDaniel et al. 2010). Specifically, our previous results indicated that all physiological responses to passive exercise were transient in nature with only anterograde and retrograde blood flow remaining elevated for the duration of the protocol (Fig. 4). In addition, similar to the current investigation, both increased CO and vascular conductance were deemed to be major contributors to the hyperaemic response in the moving limb. Overall, the older group studied in this investigation displayed similar physiological trends, although smaller in magnitude, to their younger counterparts in this and the prior investigation (McDaniel et al. 2010).
Several limitations of the current study in terms of subject population and blood flow response should be addressed. Specifically, this study includes only males and thus the results may not translate to females. In addition, although not statistically significant, there was an 8% difference in thigh volume between the young and the old groups. This is in agreement with the well documented observation known as sarcopaenia. Of course, it is possible that a smaller muscle volume contributed to the attenuation in blood flow observed in the older group. However, as already indicated, in this sample of the ageing population, neither thigh volume nor resting blood flow in the passive leg were significantly different between the two groups. Therefore, we contend that the large difference in the hyperaemic response at the onset of movement was not the result of differences in muscle mass.
Conclusion
The findings of the present study indicate that human ageing is associated with a 30% attenuated hyperaemic response following the onset of passive movement. This reduction in blood flow is similar to that observed during active exercise, which, due to the current paradigm, cannot be explained by metabolic differences. The reduced blood flow response in older subjects appears to be both central and peripheral in origin, attributable to the concomitant attenuation of the HR driven increase in CO and vascular conductance.
Acknowledgments
Support was provided by National Institute of Health grant PO1 HL 109183.
Glossary
Abbreviations
- CO
cardiac output
- ECG
electrocardiogram
- HR
heart rate
- MAP
mean arterial pressure
- SV
stroke volume
Author contributions
All experiments were performed at the Salt Lake VA Medical Center-GRECC. J.M., D.W.W. and R.S.R conceived and designed the experiments. J.M., M.A.H, S.I., A.S.F. and J.D.T. collected, analyzed and interpreted the data. J.M. and R.S.R. drafted the manuscript.
References
- Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol. 1997;82:1811–1817. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol. 1998;84:1827–1833. doi: 10.1152/jappl.1998.84.6.1827. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Vandenakker CB, Laughlin MH. Muscle blood flow patterns during exercise in partially curarized rats. J Appl Physiol. 1985;58:698–701. doi: 10.1152/jappl.1985.58.3.698. [DOI] [PubMed] [Google Scholar]
- Baum K, Selle K, Leyk D, Essfeld D. Comparison of blood pressure and heart rate responses to isometric exercise and passive muscle stretch in humans. Eur J Appl Physiol Occup Physiol. 1995;70:240–245. doi: 10.1007/BF00238570. [DOI] [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol. 2005;90:437–446. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol. 2008;294:H1963–1970. doi: 10.1152/ajpheart.01084.2007. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol. 2006;572:561–567. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde RB, Geerts BF, Cui J, Van Den Berg PC, Jansen JR. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia. 2009;64:762–769. doi: 10.1111/j.1365-2044.2009.05934.x. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented α-adrenergic vasoconstriction. J Physiol. 2001;536:977–983. doi: 10.1111/j.1469-7793.2001.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290:H272–278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol Heart Circ Physiol. 1992;263:H798–803. doi: 10.1152/ajpheart.1992.263.3.H798. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol. 1985;59:313–337. doi: 10.1016/0034-5687(85)90136-7. [DOI] [PubMed] [Google Scholar]
- Fagraeus L, Linnarsson D. Autonomic origin of heart rate fluctuations at the onset of muscular exercise. J Appl Physiol. 1976;40:679–682. doi: 10.1152/jappl.1976.40.5.679. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Kim A, Young CN, Ogoh S, Raven PB, Secher NH, Fadel PJ. Influence of ageing on carotid baroreflex peak response latency in humans. J Physiol. 2009;587:5427–5439. doi: 10.1113/jphysiol.2009.177998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn GM, Laughlin MH, Armstrong RB. Muscle blood flow and fiber activity in partially curarized rats during exercise. J Appl Physiol. 1987;63:1450–1456. doi: 10.1152/jappl.1987.63.4.1450. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol. 2008;586:2405–2417. doi: 10.1113/jphysiol.2008.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer LW, Boegehold MA. Functional hyperaemia is reduced in skeletal muscle of aged rats. Microcirculation. 2005;12:517–526. doi: 10.1080/10739680591003396. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R975–982. doi: 10.1152/ajpregu.00677.2007. [DOI] [PubMed] [Google Scholar]
- Herr MD, Imadojemu V, Kunselman AR, Sinoway LI. Characteristics of the muscle mechanoreflex during quadriceps contractions in humans. J Appl Physiol. 1999;86:767–772. doi: 10.1152/jappl.1999.86.2.767. [DOI] [PubMed] [Google Scholar]
- Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol. 2010;588:2269–2282. doi: 10.1113/jphysiol.2010.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol. 1969;204:63P–66P. [PubMed] [Google Scholar]
- Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol. 2007;583:861–874. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol. 2009;587:1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol. 2008;586:1137–1145. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen T, Hartikainen J, Vanninen E, Niskanen L, Geelen G, Lansimies E. Age and gender dependency of baroreflex sensitivity in healthy subjects. J Appl Physiol. 1998;84:576–583. doi: 10.1152/jappl.1998.84.2.576. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperaemia. Am J Physiol Heart Circ Physiol. 1987;253:H993–1004. doi: 10.1152/ajpheart.1987.253.5.H993. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Rat muscle blood flows as a function of time during prolonged slow treadmill exercise. Am J Physiol Heart Circ Physiol. 1983;244:H814–824. doi: 10.1152/ajpheart.1983.244.6.H814. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperaemia: response to passive limb movement. J Appl Physiol. 2010;108:76–84. doi: 10.1152/japplphysiol.00895.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SE, McWilliam PN. Changes in R-R interval at the start of muscle contraction in the decerebrate cat. J Physiol. 1992;447:549–562. doi: 10.1113/jphysiol.1992.sp019017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R3–R12. doi: 10.1152/ajpregu.00031.2007. [DOI] [PubMed] [Google Scholar]
- Nobrega AC, Araujo CG. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc. 1993;25:37–41. [PubMed] [Google Scholar]
- Nurhayati Y, Boutcher SH. Cardiovascular response to passive cycle exercise. Med Sci Sports Exerc. 1998;30:234–238. doi: 10.1097/00005768-199802000-00010. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Herr MD, Proctor DN. Sex differences in leg vasodilation during graded knee extensor exercise in young adults. J Appl Physiol. 2007;103:1583–1591. doi: 10.1152/japplphysiol.00662.2007. [DOI] [PubMed] [Google Scholar]
- Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol. 2003;284:H1251–1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol. 2003a;95:1963–1970. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA, Leuenberger UA. Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J Appl Physiol. 2003b;94:1859–1869. doi: 10.1152/japplphysiol.00898.2002. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Robinson BF, Epstein SE, Beiser GD, Braunwald E. Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res. 1966;19:400–411. doi: 10.1161/01.res.19.2.400. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford University Press; 1986. [Google Scholar]
- Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand. 2000;168:511–518. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol Heart Circ Physiol. 1993;265:H1227–1234. doi: 10.1152/ajpheart.1993.265.4.H1227. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol. 1988;65:1539–1547. doi: 10.1152/jappl.1988.65.4.1539. [DOI] [PubMed] [Google Scholar]
- Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand. 2003;179:361–366. doi: 10.1046/j.0001-6772.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- Ter Woerds W, De Groot PC, van Kuppevelt DH, Hopman MT. Passive leg movements and passive cycling do not alter arterial leg blood flow in subjects with spinal cord injury. Phys Ther. 2006;86:636–645. [PubMed] [Google Scholar]
- Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperaemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol. 2004;96:639–644. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- Vianna LC, Ricardo DR, Araujo CG. Training-related changes in the R-R interval at the onset of passive movements in humans. Braz J Med Biol Res. 2008;41:825–832. doi: 10.1590/s0100-879x2008000900014. [DOI] [PubMed] [Google Scholar]
- Warner HR, Cox A. A mathematical model of heart rate control by sympathetic and vagus efferent information. J Appl Physiol. 1962;17:349–355. doi: 10.1152/jappl.1962.17.2.349. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Fadel PJ, Mitchell JH. New insights into central cardiovascular control during exercise in humans: a central command update. Exp Physiol. 2006;91:51–58. doi: 10.1113/expphysiol.2005.032037. [DOI] [PubMed] [Google Scholar]
- Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol. 2005;565:1053–1060. doi: 10.1113/jphysiol.2005.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Fadel PJ, Keller DM, Ogoh S, Sander M, Raven PB, Smith ML. Dynamic carotid baroreflex control of the peripheral circulation during exercise in humans. J Physiol. 2004;559:675–684. doi: 10.1113/jphysiol.2004.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil S, Carlier PG, Richardson RS. Multiparametric NMR-based assessment of skeletal muscle perfusion and metabolism during exercise in elderly persons: preliminary findings. J Gerontol. 2009a;64:968–974. doi: 10.1093/gerona/glp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil SS, Carlier PG, Richardson RS. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol. 2009b;297:H1870–1875. doi: 10.1152/ajpheart.00709.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Richardson RS. Aging, exercise, and limb vascular heterogeneity in humans. Med Sci Sports Exerc. 2006;38:1804–1810. doi: 10.1249/01.mss.0000230342.86870.94. [DOI] [PubMed] [Google Scholar]


