Abstract
Background
The role of unicompartmental arthroplasty in managing osteoarthritis of the knee remains controversial. The Oxford medial unicompartmental arthroplasty employs a fully congruent mobile bearing intended to reduce wear and increase the lifespan of the implant. Long-term second decade results are required to establish if the design aim can be met.
Questions/purposes
We report the (1) 20-year survivorship for the Oxford mobile bearing medial unicompartmental knee arthroplasty; (2) reasons for the revisions; and (3) time to revision.
Methods
We reviewed a series of 543 patients who underwent 682 medial Oxford meniscal bearing unicompartmental knee arthroplasties performed between 1983 and January 2005. The mean age at implantation was 69.7 years (range, 48–94 years). The median followup was 5.9 years (range, 0.5 to 22 years). One hundred and forty-one patients (172 knees) died. None were lost to followup. The primary outcome was 20-year survival, a key variable in assessing the longevity of arthroplasty.
Results
The 16-year all cause revision cumulative survival rate was 91.0% (CI 6.4, 71 at risk) and survival was maintained to 20 years (91.0%, CI 36.2, 14 at risk). There had been 29 revision procedures: 10 for lateral arthrosis, nine for component loosening, five for infection, two bearing dislocations, and three for unexplained pain. In addition, five patients had undergone bearing exchange, four for dislocation and one for bearing fracture. The mean time to revision was 3.3 years (range, 0.3–8.9 years).
Conclusions
Mobile bearing unicompartmental knee arthroplasty is durable during the second decade after implantation.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
As part of the continued debate regarding unicompartmental knee arthroplasty, reports of the long-term clinical results are important to distinguish the complications and durability of different implants [1, 4–6, 14]. The Oxford meniscal knee arthroplasty (Biomet, Swindon, UK) was first used for unicompartmental replacement in 1982. In this design, an unconstrained mobile polyethylene bearing sits between a spherical femoral component and a flat tibial component (Fig. 1). It is the only unicompartmental prosthesis in which the bearing surfaces articulate congruously in all positions of the joint [10].
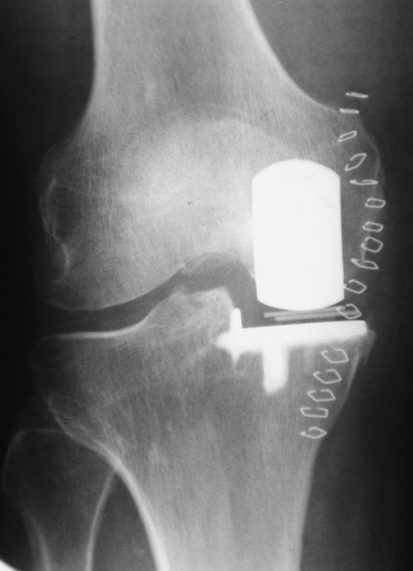
Fig. 1.
A radiograph of a Phase 3 Oxford medial unicompartmental knee arthroplasty taken the day after implantation is shown.
The designers reported a 98% survival at 10 years and encouraging clinical outcome scores for a small series of prostheses reaching beyond 10 years [9]. A larger series carried out in Skovde, Sweden, has previously reported 10- and 15-year survival of 95% and 94%, respectively, with successful clinical results at 10 years [13]. As with any implant, it is important to establish longer-term complications and durability.
We report (1) a second decade lifetable survival analysis for the Oxford mobile bearing medial unicompartmental knee arthroplasty; (2) reasons for the revisions; and (3) time to revision.
Patients and Methods
The study group consisted of 543 patients (300 females [55%] and 243 males [45%]) who had undergone a total of 682 Oxford medial unicompartmental knee arthroplasties between 1983 and the end of January 2005 in one institution. Skaraborgs Sjukhus Kärnsjukhuset is a group of three hospitals centered in the town of Skövde in Sweden. The mean age of the patients at operation was 69.7 years (range, 48–94 years). There were 404 unilateral procedures, 38 synchronous bilateral procedures, and 101 staged bilateral procedures. One hundred and forty-one patients had died (172 knees), leaving 402 patients with 510 arthroplasties who were still alive at the time of this review in June 2005. The mean age at implantation was 69.7 years (range, 48–94 years). The minimum followup was 0.5 years, and median 5.9 years (range 0.5 to 22 years). The lifetable shows the number of patients entering each year of the survival analysis (Table 1).
Table 1.
Lifetable analysis of entire study group
| Year | Number of UKA | Withdrawn with no revision (success or death) | Failures (all cause revision) | Number at risk | Failure rate (%) | Survival in each time period (%) | Cummulative survival (%) | 95% Confidence interval |
|---|---|---|---|---|---|---|---|---|
| 1 | 682 | 58 | 8 | 653.0 | 1.2 | 98.8 | 98.8 | 0.8 |
| 2 | 616 | 76 | 7 | 578.0 | 1.2 | 98.8 | 97.6 | 1.2 |
| 3 | 533 | 59 | 2 | 503.5 | 0.4 | 99.6 | 97.2 | 1.4 |
| 4 | 472 | 33 | 3 | 455.5 | 0.7 | 99.3 | 96.6 | 1.6 |
| 5 | 436 | 43 | 3 | 414.5 | 0.7 | 99.3 | 95.9 | 1.9 |
| 6 | 390 | 45 | 5 | 367.5 | 1.4 | 98.6 | 94.5 | 2.3 |
| 7 | 340 | 29 | 1 | 325.5 | 0.3 | 99.7 | 94.3 | 2.5 |
| 8 | 310 | 27 | 1 | 296.5 | 0.3 | 99.7 | 93.9 | 2.6 |
| 9 | 282 | 26 | 1 | 269.0 | 0.4 | 99.6 | 93.6 | 2.8 |
| 10 | 255 | 38 | 0 | 236.0 | 0.0 | 100.0 | 93.6 | 3.0 |
| 11 | 217 | 35 | 0 | 199.5 | 0.0 | 100.0 | 93.6 | 3.3 |
| 12 | 182 | 17 | 1 | 173.5 | 0.6 | 99.4 | 93.1 | 3.6 |
| 13 | 164 | 26 | 0 | 151.0 | 0.0 | 100.0 | 93.1 | 3.9 |
| 14 | 138 | 28 | 1 | 124.0 | 0.8 | 99.2 | 92.3 | 4.5 |
| 15 | 109 | 24 | 0 | 97.0 | 0.0 | 100.0 | 92.3 | 5.1 |
| 16 | 85 | 28 | 1 | 71.0 | 1.4 | 98.6 | 91.0 | 6.4 |
| 17 | 56 | 17 | 0 | 47.5 | 0.0 | 100.0 | 91.0 | 7.8 |
| 18 | 39 | 13 | 0 | 32.5 | 0.0 | 100.0 | 91.0 | 14.2 |
| 19 | 26 | 10 | 0 | 21.0 | 0.0 | 100.0 | 91.0 | 23.6 |
| 20 | 16 | 4 | 0 | 14.0 | 0.0 | 100.0 | 91.0 | 36.2 |
| 21 | 12 | 3 | 0 | 10.5 | 0.0 | 100.0 | 91.0 | 50.2 |
| 22 | 9 | 5 | 0 | 6.5 | 0.0 | 100.0 | 91.0 | 74.5 |
Six hundred sixty-one knees (522 patients) were treated for primary anteromedial osteoarthritis (OA) and one knee (one patient) for secondary OA after an intraarticular fracture. Thirteen knees (13 patients) were treated for osteonecrosis. These 675 knees (536 patients) fulfilled all currently used indications for the operation: disabling knee pain, a noninflammatory arthropathy or osteonecrosis, full-thickness cartilage in the lateral compartment, a functionally intact ACL, fixed flexion deformity no greater than 15, passively correctable varus deformity, and no previous high tibial osteotomy. Within the total series of procedures, seven knees (seven patients) did not meet the current indications for the procedure. Four patients (four patients) had arthroplasty after a failed high tibial osteotomy; in two patients (two knees), the ACL was absent; one patient (one knee) had inflammatory arthritis.
Within the series, 125 were Phase 1 prostheses (1983 to 1988), 271 Phase 2 (1989 to 1999, with introduction of new instruments), and 286 Phase 3 (1999 to present, with introduction of a small incision technique). All surgery was performed by one of three surgeons (US, GG, BT). Phase 1 and 2 procedures were performed through a midline incision with eversion of the patella, while a short incision with patellar subluxation was used for Phase 3. A flat tibial base plate and a spherical femoral component are inserted, with balancing of the flexion and extension gaps completed with incremental removal of bone from the distal femur. In Phase 1 this was performed with a saw blade, but Phase 2 saw the introduction of a mill, which has been retained for use in Phase 3. The procedure is completed by insertion of a fully congruent, unconstrained mobile bearing. In all cases the components were cemented.
Patients were routinely mobilized fully weight-bearing the day after surgery under the supervision of a physiotherapy team. Patients used crutches as required during their initial mobilization to aid balance and to improve initial confidence. Ongoing outpatient physiotherapy was employed to ensure patients regained an adequate range of flexion.
Within this hospital patients are routinely followed up at 1, 6 and 10 years with clinical assessment and HSS scores. For the purposes of this study a new survival analysis was performed. It was initiated in January 2005 and after establishing those patients who had died, data collection began in May and June 2005 and continued for three months. The success or revision of all medial prostheses was established. All living patients were contacted to establish the status of their knees and none was lost to followup; for patients who had died, hospital and local doctors’ records were used to establish the status of every implant at the time of death.
All-cause revision (defined as the removal or exchange of any part of the prosthetic components) was used as the end point for the survival analysis. A life table was constructed (Table 1) and the 1- to 20-year survival rates were determined [8]. The 95% confidence interval was calculated using the Peto method [12]. Comparison of second decade long-term survival between the three phases could not be performed due to the different length of followup times for each phase in this sequential series.
Results
The 16-year all-cause revision cumulative survival rate was 91.0% (CI 6.4, 71 at risk) and this cumulative survival figure was maintained to 20 years, although by this stage the confidence interval was wide (91.0%, CI 36.2, 14 at risk) (Table 1). Worst case scenario survival figures were the same because none were lost to followup.
There had been 29 revision procedures in which the components were removed and revision THA was performed and five cases in which the bearing had been exchanged (Table 2). In 27 cases, the revision was to primary TKA and in two a stemmed revision-type prosthesis was required. Indications for revision surgery were: lateral arthrosis (10), component loosening (nine), infection (five), primary bearing dislocation (two), and unexplained pain (three). There were an additional four cases of bearing dislocation and one of fractured bearing, which were all treated with reoperation and exchange of bearing. One patient sustained a bearing dislocation that was reduced closed and did not require further surgery. In the nine cases in which loosening was the indication for surgery, the majority (seven) involved the femoral component alone with two associated with secondary dislocation of the bearing. In two cases, both femoral and tibial components were loose at surgery.
Table 2.
Details of 34 patients who underwent further surgery
| Number | Indication for primary operation | Phase | Time to further surgery | Cause for further surgery | Procedure | Implant |
|---|---|---|---|---|---|---|
| 1 | OA | 3 | 0.2 | Deep infection | Revision | Primary TKA |
| 2 | OA | 1 | 0.3 | Primary dislocation | Open bearing exchange | 6.5 to 5.5 |
| 3 | PVNS and ACL-deficient | 2 | 0.4 | Primary dislocation | Open bearing exchange | 4.5 to 5.5 |
| 4 | OA | 1 | 0.5 | Deep infection | Revision | Primary TKA |
| 5 | OA and ACL-deficient | 1 | 0.5 | Primary dislocation | Open bearing exchange | 7.5 to 8.5 |
| 6 | OA | 2 | 0.7 | Lateral arthrosis | Revision | Primary TKA |
| 7 | OA | 1 | 0.7 | Unexplained pain | Revision | Primary TKA |
| 8 | OA | 3 | 0.9 | Loose femoral component | Revision | Primary TKA |
| 9 | OA | 3 | 1.0 | Deep infection | Revision | Primary TKA |
| 10 | OA | 3 | 1.0 | Deep infection | Revision | Primary TKA |
| 11 | OA | 2 | 1.1 | Lateral arthrosis | Revision | Primary TKA |
| 12 | OA | 3 | 1.2 | Lateral arthrosis | Revision | Primary TKA |
| 13 | OA | 2 | 1.4 | Lateral arthrosis | Revision | Primary TKA |
| 14 | OA | 1 | 1.6 | Loose femoral component | Revision | Femoral component replaced |
| 15 | OA after HTO | 2 | 1.7 | Unexplained pain | Revision | Revision TKA with stems |
| 16 | OA | 2 | 2.4 | Deep infection | Revision | Revision TKA with stems |
| 17 | OA after HTO | 1 | 2.9 | Unexplained pain | Revision | Primary TKA |
| 18 | OA | 3 | 3.4 | Primary dislocation | Open bearing exchange | 4.5 to 5.5 |
| 19 | OA | 2 | 3.7 | Primary dislocation | Revision | Primary TKA |
| 20 | OA | 1 | 3.9 | Primary dislocation | Revision | Primary TKA |
| 21 | OA | 2 | 4.0 | Lateral arthrosis | Revision | Primary TKA |
| 22 | OA | 2 | 4.5 | Lateral arthrosis | Revision | Primary TKA |
| 23 | OA | 2 | 4.6 | Lateral arthrosis | Revision | Primary TKA |
| 24 | OA | 2 | 5.2 | Loose femoral component and secondary dislocation | Revision | Scan |
| 25 | OA | 2 | 5.4 | Fracture of meniscus | Open bearing exchange | 3.5 to 3.5 |
| 26 | OA | 2 | 5.6 | Loose femoral and tibial components | Revision | Primary TKA |
| 27 | OA | 1 | 5.7 | Loose femoral and tibial components | Revision | Primary TKA |
| 28 | OA | 2 | 5.9 | Loose femoral component and secondary dislocation | Revision | Primary TKA |
| 29 | OA | 2 | 6.8 | Loose femoral component | Revision | Primary TKA |
| 30 | OA | 2 | 7.9 | Lateral arthrosis | Revision | Primary TKA |
| 31 | Psoriatic arthropathy | 1 | 8.5 | Loose femoral component | Revision | Primary TKA |
| 32 | OA | 2 | 11.4 | Lateral arthrosis | Revision | Primary TKA |
| 33 | OA | 2 | 13.5 | Loose femoral component | Revision | Primary TKA |
| 34 | OA | 1 | 15.5 | Lateral arthrosis | Revision | Primary TKA |
OA = osteoarthritis; PVNS = pigmented villonodular synovitis; HTO = high tibial osteotomy; TKA = total knee arthroplasty.
The mean time to revision was 3.3 years (range, 0.3–8.9 years) with three revisions occurring during the second decade. Revision for infection and dislocation tended to occur within the first 2 years of implantation, whereas surgery for lateral arthrosis and loosening occurred later.
Discussion
There remains ongoing debate as to the role of unicompartmental knee arthroplasty in the treatment of medial knee osteoarthritis. Despite the lack of reports of long-term outcome, many believe the procedure represents a pre-total knee replacement as failure is likely in the second decade. The aim of this study was to report for the first time the survival of the Oxford unicompartmental knee arthroplasty through the second decade, commenting on the mode and timing of failure leading to revision.
This study does have weaknesses. First, the relatively small numbers of patients still alive entering the 20th year produces a large confidence interval for the 20-year survival. However, the figure of 14 at risk during this time period falls within the accepted level for survival analysis and suggests the results are likely to be representative [7, 8]. However we report all medial Oxford unicompartmental knee arthroplasties performed at an independent center during the period noted with no exclusions and report a continuous series including all procedures performed within the surgeon’s learning curve period, a fact that may be expected to adversely affect implant survival. We also had no loss to followup. Second, we lacked radiographic assessment, which might potentially identify patients who are at risk of failure. Third, our survival data do not offer any evidence as to the clinical scores of the patients still alive and therefore may be an underestimate of the true failure rate in this series. However, when calculating survival, all-cause revision or bearing exchange was used as the end point and any knees with pending failure were included as failures. Despite these limitations we believe the study does offer important information. Survival analysis is established as a useful tool in reporting the success or failure of specific implants, as highlighted by the joint registers. In this case the data provide some insight as to the actual survival of Oxford implants into the second decade and we believe this adds valuable information in assessing the longevity of the device.
We found 10- and 20-year survival rates of 94% and 91%, respectively, for the Oxford medial unicompartmental knee arthroplasty with few revisions occurring during the period between 10 and 20 years. These data suggest the device is durable in the second decade after implantation and compares well to the only other published series of 20-year followup for UKA. O’Rourke et al. reported results for the fixed bearing Marmor device, with a 20-year all-cause revision survival of 84% and 72% surviving at 25 years [11]. Our report of 91% 20-year survival for the Oxford mobile unicompartmental knee arthroplasty provides more evidence that unicompartmental arthroplasty can have encouraging survival in the long term. The study highlights that low revision rates can be obtained if the correct indications are followed.
The most common cause for revision was progression of arthritis in the lateral compartment, although over the 20-year period, failure from this mechanism occurred in only 10 patients (1.5%). Relatively rapid deterioration of the retained compartment leading to revision, as seen in this study, has been seen in other series of unicompartmental knee arthroplasties and has been attributed to overcorrection of the varus deformity at the time of surgery [2, 3, 15, 16]. The low rate of progressive lateral arthrosis may reflect the success of an implantation method that does not attempt to overcorrect the joint, but rather restores the ligaments to their normal tension. It is also of note that there were no failures attributable to patellofemoral joint problems given that the preoperative state of the patellofemoral joint was not a contraindication to surgery. The survival data imply that after Oxford medial unicompartmental knee arthroplasty, arthritis within the retained compartments uncommonly progresses to the point where TKA might be considered. It is of note there were very few revisions performed for complications relating directly to polyethylene wear, suggesting the low wear design of this fully congruent mobile bearing prosthesis has been successful in the long-term.
We found few revisions in the second decade after surgery, suggesting if the device remains unrevised at 10 years, then survival to 20 years is to be expected. Given the mean age of patients at implantation was 70 years, we believe the device need not be considered a pre-total knee arthroplasty. Where revision surgery has occurred, the majority of patients (96%) were able to have a primary TKA inserted against only two in which a revision TKA was required. This illustrates the Oxford medial unicompartmental knee arthroplasty is a bone-sparing procedure, avoiding the requirement for stemmed revision implants if revision is required. We conclude that, providing correct indications are employed, the Oxford medial unicompartmental knee arthroplasty can have a low revision rate through the second decade after implantation.
Acknowledgments
We thank Dr G. Gudmundsson and Dr B. Tjörnstrand, Consultant Surgeons at the Orthopaedic Department in Skaraborgs Sjukhus Kärnsjukhuset Hospital, for their support of this work.
Footnotes
One or more of the authors (AJP) has received research funding from Biomet Inc. This work was funded by the NHS Biomedical Research Unit at the Nuffield Orthopaedic Centre and the Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Science, Oxford University.
This work was performed at Skaraborgs Sjukhus Kärnsjukhuset, Skövde, Sweden, and the Biomedical Research Unit, Nuffield Orthopaedic Centre, Oxford, UK.
References
- 1.Berger RA, Nedeff DD, Barden RM, Sheinkop MM, Jacobs JJ, Rosenberg AG, Galante JO. Unicompartmental knee arthroplasty. Clinical experience at 6- to 10-year followup. Clin Orthop Relat Res. 1999;367:50–60. doi: 10.1097/00003086-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Insall J, Aglietti P. A five to seven-year follow-up of unicondylar arthroplasty. J Bone Joint Surg Am. 1980;62:1329–1337. [PubMed] [Google Scholar]
- 3.Laskin R. Unicompartmental tibiofemoral resurfacing arthroplasty. J Bone Joint Surg Am. 1978;60:182–185. [PubMed] [Google Scholar]
- 4.Laskin RS. Unicompartmental knee replacement: some unanswered questions. Clin Orthop Relat Res. 2001;392:267–271. doi: 10.1097/00003086-200111000-00034. [DOI] [PubMed] [Google Scholar]
- 5.Marmor L. Unicompartmental arthroplasty of the knee with a minimum ten-year follow-up period. Clin Orthop Relat Res. 1988;228:171–177. [PubMed] [Google Scholar]
- 6.Marmor L. Unicompartmental knee arthroplasty. Ten- to 13-year follow-up study. Clin Orthop Relat Res. 1988;226:14–20. [PubMed] [Google Scholar]
- 7.Murray D. Survival analysis. Assessment methodology. In: Pysent P, Fairbank J, Carr A, eds. Orthopaedics. Oxford, UK: Butterworth-Heinemann; 1997:19–28.
- 8.Murray DW, Carr AJ, Bulstrode C. Survival analysis of joint replacements. J Bone Joint Surg Br. 1993;75:697–704. doi: 10.1302/0301-620X.75B5.8376423. [DOI] [PubMed] [Google Scholar]
- 9.Murray DW, Goodfellow JW, O’Connor JJ. The Oxford medial unicompartmental arthroplasty: a ten-year survival study. J Bone Joint Surg Br. 1998;80:983–989. doi: 10.1302/0301-620X.80B6.8177. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor JJ, Goodfellow JW. Theory and practice of meniscal knee replacement design against wear. Proc Inst Mech Eng [H] 1996;210:217–222. doi: 10.1243/PIME_PROC_1996_210_415_02. [DOI] [PubMed] [Google Scholar]
- 11.O’Rourke MR, Gardner JJ, Callaghan JJ, Liu SS, Goetz DD, Vittetoe DA, Sullivan PM, Johnston RC. The John Insall Award: unicompartmental knee replacement: a minimum twenty-one-year followup, end-result study. Clin Orthop Relat Res. 2005;440:27–37. doi: 10.1097/01.blo.0000185451.96987.aa. [DOI] [PubMed] [Google Scholar]
- 12.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;435:171–180. doi: 10.1097/00003086-200506000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Squire MW, Callaghan JJ, Goetz DD, Sullivan PM, Johnston RC. Unicompartmental knee replacement. A minimum 15 year followup study. Clin Orthop Relat Res. 1999;367:61–72. doi: 10.1097/00003086-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Weale AE, Murray DW, Baines J, Newman JH. Radiological changes five years after unicompartmental knee replacement. J Bone Joint Surg Br. 2000;82:996–1000. doi: 10.1302/0301-620X.82B7.10466. [DOI] [PubMed] [Google Scholar]
- 16.Weale AE, Murray DW, Crawford R, Psychoyios V, Bonomi A, Howell G, O’Connor J, Goodfellow JW. Does arthritis progress in the retained compartments after ‘Oxford’ medial unicompartmental arthroplasty? A clinical and radiological study with a minimum ten-year follow-up. J Bone Joint Surg Br. 1999;81:783–789. doi: 10.1302/0301-620X.81B5.9197. [DOI] [PubMed] [Google Scholar]