Abstract
Background
Solitary bone cysts (SBC), nonossifying fibromas (NOF), and fibrous dysplasia (FD) create benign intramedullary lytic bone lesions. They are typically asymptomatic and treated conservatively. We present a series of lesions that caused performance-limiting pain in young athletes, a symptom phenomenon and possible treatment indication that has been poorly described in the literature.
Questions/purposes
We asked whether intralesional curettage and defect grafting of these lesions would alleviate pain in young athletes and permit their return to unrestricted athletic activities.
Patients and Methods
We retrospectively identified 29 patients (30 lesions) who underwent curettage and grafting for SBC (12 patients), NOF (nine), or FD (eight). All patients had pain predominantly with athletic involvement. The mean age of the patients was 18 years (range, 12–31 years). Tumor locations were the femur (eight lesions), humerus (seven), tibia (six), fibula (five), pubic ramus (two), ulna (one), and calcaneus (one). Signs/symptoms were pain alone (24 patients) and pain plus fracture (five). Surgery involved curettage and packing with allograft cancellous chips, bone substitute, or demineralized bone matrix. Two patients required internal fixation. The mean followup was 21 months (range, 2–114 months).
Results
Twenty-four patients had no pain and five had occasional mild pain at last followup. All patients resumed full activity at a mean of 3.3 months (range, 1.5–8.3 months), excluding two who required repeat surgery.
Conclusions
Our observations suggest curettage and packing with bone graft/substitute can provide pain relief and allow full athletic recovery for young athletes with benign lytic bone lesions.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Benign intramedullary lytic lesions of bone such as SBC, NOF, and FD occur most commonly during the first through third decades of life [7]. These lesions often are asymptomatic [2, 9] and found incidentally on imaging for separate complaints, but they also can present with mild pain and localized swelling of short duration [8]. They reportedly are self-limited, ultimately stabilizing (SBC, FD) or spontaneously resolving (NOF) after skeletal maturity is achieved [2, 7]. Numerous authors therefore suggest that surgery is not required for these benign processes unless the size of the lesion or the proportion of bone involved predisposes to pathologic fracture or if a fracture already has occurred [1, 2, 5, 8, 9]. The exact lesion size or percentage of bone involvement that demands treatment is controversial, however, and varies by anatomic site [1, 2, 5, 8, 9].
An overlooked issue regarding benign bone lesions such as SBC, NOF, and FD is their occurrence in young athletes. As a result of their typical incidence in younger populations, the lesion’s development can coincide with competitive or recreational athletic participation. Although typically asymptomatic in the general population, we found several lesions that caused severe, performance-limiting pain in young adults during athletic activities but minimal to no pain during sedentary activities. This phenomenon of pain with athletic activity and the necessary degree of intervention for these patients is not well understood from the current literature.
We describe performance-limiting pain in young athletes with benign lytic bone lesions and asked whether (1) curettage and grafting alleviates pain in young athletes with SBC, NOF, or FD; and (2) curettage and grafting, followed by a graduated return to activities, lead to a painless and an unrestricted return to athletics.
Patients and Methods
We retrospectively investigated the use of curettage and grafting for benign bone lesions in young athletes. We searched our institution’s surgical pathology database for all patients with diagnoses of NOF, FD, or SBC treated from 1999 to 2009. Inclusion criteria were (1) histologically confirmed diagnosis of one of the three previously mentioned conditions; (2) treatment by curettage and placement of any combination of allograft cancellous chips, synthetic bone graft, or demineralized bone matrix; (3) age younger than 40 years and older than 12 years; (4) daily or weekly participation in athletic activities; (5) pain with athletic activities but little to no pain with sedentary activities; (6) no previous surgical treatment at the site of the lesion; and (7) at least 8 weeks of followup. We identified 112 patients who underwent curettage and grafting for one of the three diagnoses. We excluded 37 of these patients for lack of documented consistent, measurable athletic activity; 33 for age older than 40 years, five for incomplete records, four for having prior surgery on the lesion, and four for lack of postoperative followup. This left 29 patients who met all inclusion criteria (Table 1); these 29 patients had a total of 30 lesions. The mean followup was 21 months (range, 2–114 months). None of these patients were lost to followup. The mean age of the patients was 18 years (range, 12–31 years). Twelve patients had a diagnosis of SBC, nine had NOF (one patient had two NOF lesions), and eight had FD. Tumor locations were the femur (eight patients), humerus (seven), tibia (six), fibula (five), pubic ramus (two), ulna (one), and calcaneus (one). Each lesion initially had been identified by radiographic examination, with plain radiographs, CT, or MRI. Pathologic diagnosis was confirmed later by histologic analysis at the time of definitive treatment. We had prior Institutional Review Board approval.
Table 1.
Patient characteristics, diagnoses, and treatment outcomes
| Patient number | Age (years) and gender | Diagnosis | Location | Sport or activity causing pain | History of pathologic fracture | Return to full activity (months) | Pain at last followup |
|---|---|---|---|---|---|---|---|
| Curettage and bone grafting | |||||||
| 1 | 17 M | SBC | Calcaneus | Basketball | N | 1.9 | None |
| 2† | 16 M | SBC | Humerus | Football | N | 2.7 | None |
| 4 | 19 M | SBC | Humerus | Weightlifting | Y | 8.3 | Mild, occasional |
| 6† | 13 M | SBC | Femur | Football‡ | N | 2.7 | None |
| 7 | 14 M | FD | Ulna | Ice hockey | N | 46.4|| | None |
| 8 | 12 F | FD | Fibula | Softball | N | 2.5 | None |
| 9† | 18 M | SBC | Humerus | Baseball | Y | 3.2 | None |
| 11† | 19 M | NOF | Tibia | Basketball | N | 3.8 | None |
| 13† | 15 M | SBC | Fibula | Basketball | N | 3.0 | None |
| 14 | 17 F | NOF | Femur | Weightlifting | N | 14.1** | Mild with prolonged activity |
| 16 | 17 M | SBC | Humerus | Soccer | Y | 1.6 | None |
| 19 | 13 F | NOF | Tibia | Running | N | 1.6 | None |
| 20 | 14 F | NOF | Femur (R) | Track and field | N | 2.9 | None |
| 20 | 14 F | NOF | Femur (L) | Track and field | N | 2.2 | None |
| 21 | 16 F | NOF | Tibia | Basketball | N | 2.7 | None |
| 22† | 30 M | FD | Femur | Racquetball | N | 4.2 | Occasional on maximal exertion |
| 23 | 14 F | NOF | Fibula | Basketball | Y | 3.7 | None |
| 24 | 15 F | SBC | Fibula | Soccer | N | 1.6 | Mild with prolonged activity |
| 25 | 18 M | SBC | Femur | Lacrosse | N | 3.4 | None |
| 26 | 19 F | FD | Tibia | Running | N | 3.0 | None |
| 27 | 15 M | NOF | Fibula | Wrestling | N | 3.0 | None |
| 28†† | 31 F | FD | Femur | Running | Y | 5.8 | Mild with prolonged activity |
| 29 | 31 M | FD | Pubic ramus | Running | N | 4.2 | None |
| Curettage and bone substitute | |||||||
| 3 | 14 M | SBC | Humerus | Weightlifting | Y | 2.8 | None |
| 5 | 20 M | NOF | Tibia | Running | N | 4.6 | None |
| 12 | 18 M | SBC | Tibia | Running | N | 1.5 | None |
| 15 | 15 F | NOF | Femur | Soccer | N | 2.0 | None |
| 18 | 15 M | SBC | Humerus | Weightlifting | N | 3.0 | None |
| Curettage and demineralized bone matrix alone | |||||||
| 17 | 27 F | FD | Pubic ramus | Running | Y | 5.0 | None |
| Curettage, bone grafting, and autologous marrow placement | |||||||
| 10†† | 20 M | FD | Humerus | Weightlifting | Y | 4.2 | None |
SBC = simple bone cyst; FD = fibrous dysplasia; NOF = nonossifying fibroma; †patient also received demineralized bone matrix; ‡patient had minimal pain; lesion was incidental finding after injury of contralateral femur; ||patient had later distal ulna resection for further injury to and progression of FD lesion; **patient had later repeat procedure for incomplete allograft and stress fracture of lesion; ††patient also underwent internal fixation during primary treatment; M = male; F = female; R = right; L = left; N = no; Y = yes.
The primary symptoms were pain in 24 patients (one patient had pain in two distinct lesions) and fracture with pain in five patients. Pain was presumed to be attributable to the lesion when the reported location of pain coincided anatomically with a radiographic abnormality. Three patients (Patients 3, 4, and 16) had at least one pathologic fracture in the distant past, 7-months to 7-years before current presentation. These had been allowed to heal before biopsy and definitive treatment, not identified as pathologic at the treating facility, or not treated appropriately owing to social issues. Three patients in this study, two with humerus lesions and one with a fibular lesion, had localized swelling. One patient with a humeral lesion had limited ROM and decreased strength, and one with a fibular lesion also had intermittent numbness.
Surgery was recommended to all patients for their athletics-limiting pain and/or pathologic fracture. The typical surgical approach involved curettage and packing of the lesion with cancellous allograft (18 lesions), allograft plus demineralized bone matrix (DBM) (Grafton, Osteotech, Inc., Shrewsbury, NJ) (six), synthetic bone substitute (five), or DBM alone (one). The choice of packing material used was based on surgeon preference. Internal fixation generally was avoided but available if judged important. One patient (Patient 10) underwent an open reduction internal fixation with a humerus plate for a displaced humerus fracture through a FD lesion. He also had autologous bone marrow harvested and implanted in conjunction with cancellous allograft. Another patient (Patient 28) underwent open reduction and internal fixation with a dynamic hip screw, in addition to curettage and packing, for a proximal femur fracture through a FD lesion.
Postoperatively, all patients were asked to remain nonweightbearing or limited weightbearing, depending on the location of the lesion. Patients were allowed to mobilize joints immediately. Lower extremity patients were given crutches to use for the nonweightbearing period. Upper extremity patients were treated with a sling initially for comfort but encouraged to maintain active motion. About 2 weeks postoperatively, at the first postoperative clinic visit, physiotherapy was initiated if restriction of motion was clinically significant, but strengthening was withheld until full weightbearing was permitted.
Patients were seen in the office 2 weeks postoperatively for a wound check and physical examination and again at 6 and 12 weeks. Particular attention was given to any tenderness and any decreased or painful passive/gentle active ROM of the joints above and below the surgical site. Orthogonal radiographs and another clinical examination were performed 6 weeks postoperatively. Radiographs were used to qualitatively determine whether there was any recurrence of the lytic lesion and whether the radiopaque graft material was retained. Sedentary activity was permitted 6 weeks postoperatively after radiographs showed there was no fracture, there was continued good filling of the lesion, and the patient had minimal-to-no pain. Orthogonal radiographs and clinical examination were completed again 12 weeks postoperatively. Patients were released to full activity at 12 weeks postoperatively if radiographs continued to show appropriate filling and there was no pain (Figs. 1–2) . If symptoms persisted, weightbearing restrictions were maintained and the patient was rechecked with additional imaging and clinical examinations at 6- to 12-week intervals. Patients with FD subsequently were followed yearly. Patients with SBC and NOF subsequently were followed on an as-needed basis.
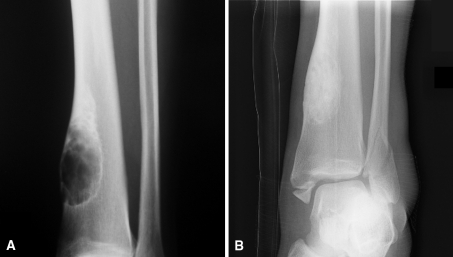
Fig. 1A–B.
(A) This preoperative AP radiograph of Patient 11 shows a large tibial nonossifying fibroma with possible cortical disruption at the inferomedial pole. (B) This AP radiograph was taken 36 months after curettage and placement of cancellous allograft chips and DBM. It shows substantial healing of the lesion, which is uninvolved in the trauma-related bimalleolar fracture also seen in the image. The patient had been asymptomatic and had returned to playing basketball before the fracture.
Fig. 2A–B.
(A) This preoperative AP radiograph of the humerus of Patient 3 shows a large metadiaphyseal bone cyst, which was painful with weightlifting. (B) An AP radiograph of the patient’s humerus taken 29 months after curettage and placement of bone substitute shows substantial healing and cortical remodeling of the bone. The patient had returned to full athletic participation without restrictions, and no limb-length discrepancy was seen.
Outcome measures for this study included pain status at last followup, time until return to full activity, and repeat/additional surgical interventions. Pain was measured clinically, with patient surveys and analog pain scales. Time until return to full activity was measured from the date of initial curettage and packing to the date the patient reported first resuming full athletic participation.
Results
Twenty-four patients (83%) had no pain and five (17%) had residual pain. All patients reporting ongoing pain described it as mild and occasional not requiring any analgesic treatment. Although one patient was unable to note a pattern to the occurrence of pain, the remaining four specifically reported pain occurring only with either prolonged activity or maximal exertion.
All patients returned to full athletic participation without physician- or self-imposed restrictions. Patients resumed full activity at a mean of 3.3 months after surgery (range, 1.5–8.3 months), excluding those requiring repeat surgery, as discussed subsequently. Thirteen (45%) of the 29 patients returned to full activity before the end of the advised 12-week recovery period. One patient (Patient 9), a baseball player with a pathologic fracture through a SBC of the humerus, reported an inability to throw at full speed at the time of his return, 3.2 months after surgery. We presumed this was a result of muscle atrophy and since it later resolved over the course of a few weeks.
Two of the 29 patients (7%) had repeat surgery. One patient (Patient 14), a multisport athlete with a NOF of the distal femur measuring 3 × 2.5 × 1.5 cm, experienced sudden-onset severe pain while walking 3 months after her initial surgery. She returned to our office as a result of inability to bear weight secondary to pain. Radiography revealed no obvious fracture, and a stress fracture was presumed. The pain did not improve after 4 weeks of nonweightbearing. CT later revealed incomplete allograft filling of the NOF lesion and slight cortical disruption. The patient subsequently underwent a second curettage and grafting 23 weeks after her initial procedure and returned to full painless activity 8.8 months later. A second patient, an ice hockey player with FD of the ulna (Patient 7), had repeat curettage and grafting 19 months after his initial procedure for a large, expanding, primarily cystic lesion that measured 11.0 × 4.0 × 3.0 cm. He was able to return to full asymptomatic activity after both curettage procedures, but rapid expansion of the FD and its fragile cystic nature eventually necessitated distal ulna resection 24 months after this second grafting procedure. The patient returned to full painless activity 3.8 months after the definitive resection and was the only patient in our study who experienced lesion recurrence.
Discussion
Benign lytic bone lesions such as SBC, NOF, and FD most often affect younger individuals [7]. Generally asymptomatic [2, 9], these lesions typically stabilize or resolve after skeletal maturity [2, 7]. Because of this, surgery usually is not required unless the lesion’s size predisposes to pathologic fracture, at which point curettage and grafting are standard treatment [2, 8, 18]. We noted a phenomenon of these lesions causing severe pain in young athletes during strenuous activity and minimal to no pain otherwise. This pattern of pain and the degree of intervention necessary for benign lytic lesions in this specialized population are poorly documented in the literature. We therefore sought to investigate whether standard-of-care curettage and grafting would eliminate pain and allow full return to sports.
Our study was limited by several aspects of the design. First, the sample size was limited by the relative rarity of these diseases. However, we believe these few cases do provide some answers to the questions. Second, a control group was not feasible because all patients with these lesions were treated identically. We merely sought to report whether standard treatment, namely curettage and bone grafting, provided adequate stability for young adults participating in athletic activities. Establishing improvement over other treatment options was not our intent, although this question should be pursued in future studies. Third, the retrospective design resulted in the inclusion of heterogeneous packing materials used during grafting procedures. Different packing materials could conceivably considerably affect patient outcomes. Because it is difficult to radiographically judge incorporation of graft material into bone, distinguishing the clinical performance of one material from another is challenging. Fourth, we included athletes with a broad spectrum of performance/intensity levels. Only a minimal activity level of weekly sports participation was required for inclusion in the study. Further documentation or categorization was not performed, which limited our ability to complete any subgroup analysis based on performance/intensity level (collegiate athlete versus recreational athlete). Similarly, no comparisons were made between high impact/contact sports and other athletic activities.
Differences can be seen in the amount of time taken to return to full activity when the different diagnoses and ages are compared. Excluding the two patients who required additional procedures after curettage and grafting, the 12 patients with SBC took a mean 3.0 months (range, 1.5–8.3 months) to return to full activity, the eight remaining patients with NOF took a mean 3.0 months (range, 1.6–4.6 months), and the seven remaining patients with FD took a mean 4.1 months (range, 2.5–5.8 months). Similarly, the 23 remaining patients younger than 25 years took a mean 3.0 months (range, 1.5–8.3 months) to return to full activity while the four patients older than 25 years took a mean 4.8 months (range, 4.2–5.8 months). However, firm conclusions cannot be drawn from these data owing to the small sample size.
Many authors consider observation appropriate for most asymptomatic SBC, NOF, and FD lesions [2, 5, 6, 8–10, 12, 15]. The exception is when lesion size predisposes to pathologic fracture [1, 2, 5, 8, 9]. In such cases, surgery usually is indicated. The treatment of symptomatic lesions without fracture, however, only recently has been reported [3, 5, 6, 15]. Older studies included impending pathologic fracture in their criteria for surgical intervention [2, 10, 14, 18], but did not mention treatment for stable and painful lesions. Some studies better acknowledge that pain may occur with these lesions [5, 8, 15, 18], and some authors include it as a condition warranting surgery [3, 5, 6, 15]. Although the exact cause of pain with SBC, NOF, and FD lesions is not yet known, some authors suggest that these painful lytic lesions represent mechanical insufficiency and therefore correlate to patients at risk for pathologic fracture [6, 8, 15]. Given the high demands placed on the body during physical activity, mechanical insufficiency in benign lytic lesions may manifest itself more readily in athletic populations. If given adequate time without intervening trauma, the body is normally able to surround weakened areas in benign lytic lesions with structurally sound bone. Strenuous activity might traumatize the weakened areas before natural stabilization, exceeding a mechanical threshold for the lesion and causing performance-limiting pain. Saglik et al. proposed a similar pain-generation model for benign bone lesions from radiographically negative microfractures [15].
Only a few authors have considered activity level when determining the appropriate degree of intervention for benign bone lesions [8, 12, 17]. In a series of 104 patients with various lesions, Shih et al. [17] used internal fixation along with curettage and grafting for all “active” patients with lower extremity lesions. Drennan et al. [8] similarly suggested prophylactic curettage and grafting for lower extremity lesions in “active adolescents.” Literature addressing treatment strategies for young athletes, however, consists of only isolated case reports using various treatments. One report described a pathologic femur fracture through a NOF in a 12-year-old runner [16]. Treated with 5 weeks of nonweightbearing, the patient returned to running 3 months after injury. Another report discussed the management of a painful FD lesion in the femur of a collegiate soccer player [13]. A pathologic fracture occurred after unsuccessful treatment with injections of autologous bone marrow and grafting. The patient then was treated by internal fixation and intravenous pamidronate, but no subsequent followup was reported. Other cases describe benign aggressive tumors [4, 11], which may require more aggressive surgical bone removal. However, these authors did find patients treated with curettage or curettage and grafting could return to full athletic activity, whereas a patient treated with bony resection had permanent functional limitations. We prefer curettage and grafting for treatment of symptomatic benign lytic bone lesions. Based on our observations, curettage and grafting leaves adequate mechanical stability for graduated return to athletic activity without the cost of internal fixation or the extended recovery time or possible permanent deficits of larger resections. Surgery must be determined on a case-by-case basis, however, because the lesion’s location, the quality and amount of remaining bone, or the occurrence of fracture may warrant additional stabilization.
Although most benign lytic bone lesions from SBC, NOF, and FD are asymptomatic, some may cause severe, performance-limiting pain in young athletes. We used curettage and packing with any combination of allograft cancellous chips, synthetic bone graft, and DBM to treat such lesions in young athletes. The approach allowed patients to return to full, painless activity relatively quickly in most cases.
Footnotes
One or more of the authors (RLS, VMM, EAC) received funding in the form of a research fellowship from Stryker Orthopaedics, Mahwah, NJ. One of the authors (RDL) is a consultant for Stryker Orthopaedics, Mahwah, NJ.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the University of Pennsylvania, Philadelphia, PA, USA.
References
- 1.Ahn JI, Park JS. Pathological fractures secondary to unicameral bone cysts. Int Orthop. 1994;18:20–22. doi: 10.1007/BF00180173. [DOI] [PubMed] [Google Scholar]
- 2.Arata MA, Peterson HA, Dahlin DC. Pathological fractures through non-ossifying fibromas: review of the Mayo Clinic experience. J Bone Joint Surg Am. 1981;63:980–988. [PubMed] [Google Scholar]
- 3.Bahk WJ, Kang YK, Rhee SK, Chung YG, Lee AH, Bahk YW. Cystic fibrous dysplasia in the long bone. Orthopedics. 2007;30:871–873. doi: 10.3928/01477447-20071001-05. [DOI] [PubMed] [Google Scholar]
- 4.Berry DC, Barton J, Deivert RG. Aneurysmal bone cyst in a female collegiate field hockey player: a case report. J Athl Train. 2000;35:86–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Betsy M, Kupersmith LM, Springfield DS. Metaphyseal fibrous defects. J Am Acad Orthop Surg. 2004;12:89–95. doi: 10.5435/00124635-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 6.DiCaprio MR, Enneking WF. Fibrous dysplasia: pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005;87:1848–1864. doi: 10.2106/JBJS.D.02942. [DOI] [PubMed] [Google Scholar]
- 7.Dorfman HD, Czerniak B. Bone Tumors. St Louis, MO: Mosby; 1998. [Google Scholar]
- 8.Drennan DB, Maylahn DJ, Fahey JJ. Fractures through large non-ossifying fibromas. Clin Orthop Relat Res. 1974;103:82–88. doi: 10.1097/00003086-197409000-00047. [DOI] [PubMed] [Google Scholar]
- 9.Easley ME, Kneisl JS. Pathologic fractures through nonossifying fibromas: is prophylactic treatment warranted? J Pediatr Orthop. 1997;17:808–813. doi: 10.1097/00004694-199711000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Hase T, Miki T. Autogenous bone marrow graft to nonossifying fibroma with a pathologic fracture. Arch Orthop Trauma Surg. 2000;120:458–459. doi: 10.1007/s004029900100. [DOI] [PubMed] [Google Scholar]
- 11.Maffulli N, Pintore E, Petricciuolo F. Tumours mimicking sports injury in two young athletes. Br J Sports Med. 1990;24:207–208. doi: 10.1136/bjsm.24.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makley JT, Joyce MJ. Unicameral bone cyst (simple bone cyst) Orthop Clin North Am. 1989;20:407–415. [PubMed] [Google Scholar]
- 13.Maldonado I, Catalano E, Reginato AJ. Pathologic fracture of the femoral neck in a female soccer player. J Clin Rheumatol. 2002;8:30–34. doi: 10.1097/00124743-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Marks KE, Bauer TW. Fibrous tumors of bone. Orthop Clin North Am. 1989;20:377–393. [PubMed] [Google Scholar]
- 15.Saglik Y, Atalar H, Yildiz Y, Basarir K, Erekul S. Management of fibrous dysplasia: a report on 36 cases. Acta Orthop Belg. 2007;73:96–101. [PubMed] [Google Scholar]
- 16.Sakamoto A, Tanaka K, Yoshida T, Iwamoto Y. Nonossifying fibroma accompanied by pathological fracture in a 12-year-old runner. J Orthop Sports Phys Ther. 2008;38:434–438. doi: 10.2519/jospt.2008.2655. [DOI] [PubMed] [Google Scholar]
- 17.Shih HN, Chen YJ, Huang TJ, Hsu KY, Hsu RW. Semistructural allografting in bone defects after curettage. J Surg Oncol. 1998;68:159–165. doi: 10.1002/(SICI)1096-9098(199807)68:3<159::AID-JSO5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Tiedeman JJ, Huurman WW, Connolly JF, Strates BS. Healing of a large nonossifying fibroma after grafting with bone matrix and marrow: a case report. Clin Orthop Relat Res. 1991;265:302–305. [PubMed] [Google Scholar]