Abstract
Background
A knee design with a ball-and-socket articulation of the medial compartment has a femoral rollback profile similar to the native knee. Compared to a conventional, posterior-stabilized knee design, it provides AP stability throughout the entire ROM. However, it is unclear whether this design difference translates to clinical and functional improvement.
Questions/purposes
We asked whether the medially conforming ball-and-socket design differences would be associated with (1) improved ROM; and (2) improved American Knee Society, WOMAC, Oxford Knee, SF-36, and Total Knee Function Questionnaire scores compared to a conventional, fixed-bearing posterior-stabilized TKA.
Patients and Methods
We enrolled 82 patients in a single-center, single-blinded, randomized, controlled trial comparing the medially conforming ball-and-socket design knee prosthesis to a posterior-stabilized total knee prosthesis. Our primary end point was ROM. Our secondary end points were American Knee Society, WOMAC, Oxford Knee, SF-36, and Total Knee Function Questionnaire scores. All patients were followed at 1 and 2 years.
Results
The mean ROM was 100.1° and 114.9° in the posterior-stabilized and medially conforming ball-and-socket groups, respectively. The physical component scores of SF-36 and Total Knee Function Questionnaire were better in the medially conforming ball-and-socket group. We found no difference in American Knee Society, WOMAC, and Oxford Knee scores.
Conclusions
Both implant designs similarly relieved pain and improved function. The medially conforming ball-and-socket articulation provided better high-end function as reflected by the Total Knee Function Questionnaire.
Level of Evidence
Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The total condylar knee prosthesis was first used in 1974 and associated with high functional scores [1, 31, 37]. However, in 1978 an evolution in design was introduced, primarily consisting of a new “cam and post” design, otherwise known as the posterior-stabilized (PS) modification. The intended benefits specifically included improved stair-climbing ability and ROM and prevention of posterior subluxation of the tibia [17]. Further modifications during the 1990s have also included PCL-retaining designs with the intended benefits of optimizing femoral rollback and ROM. Although high Knee Society scores at 5 to 7 years have been reported [5], the ROM and function measured with WOMAC and HSS scores of the PCL-retaining designs are similar to those for the PS design implants [9, 19]. Furthermore, fluoroscopic studies demonstrate paradoxical anterior femoral translation during knee flexion when compared to PS designs [13].
The medially conforming “ball-and-socket” (MCBS) articulation of the medial tibiofemoral compartment is an alternative design concept in providing stability through the complete arc of knee flexion and has been in use since 1994. Kinematic studies of normal knees have demonstrated minimal to no femoral rollback of the medial condyle [7, 15, 28, 36], and consequently it is believed a knee arthroplasty using the MCBS design concept may replicate normal knee movements. Subsequent developments included an increased curvature of the medial femoral condyle to provide improved constraint and lower contact stresses, thereby reducing polyethylene wear [23].
The PS design concept provides high American Knee Society scores (AKSS) with a low incidence of osteolysis (2.2% at a mean followup of 5.2 years) and survivorship of greater than 94% beyond 10 years [10, 30]. Similarly, the MCBS design provides high AKSS, WOMAC, and Oxford Knee scores (OKS), and SF-12 with comparable 10-year survivorship at greater than 94% [2, 18, 23]. While it is evident the two implant designs provide an effective solution with respect to TKA, the question of whether a more natural femoral rollback profile of the MCBS design affords even greater function with particular reference to high-end function in comparison to the PS design remains unanswered. Gait analysis of normal knees suggests forces causing AP femoral translation on the tibia are at their greatest during heel strike in full extension [21]. The increased constraint of the MCBS design through the complete arc of knee motion provides stability to resist these forces in the replaced knee, but it is unclear whether the transfer of these increased forces to the tibial component bone interface predisposes to early loosening.
We therefore asked whether the MCBS design produced better (1) ROM; (2) AKSS, SF-36, OKS, WOMAC, and Total Knee Function Questionnaire (TKFQ) scores; and (3) whether radiographic alignment using the American Knee Society recommendations, posterior condylar offset ratio (PCOR) and the incidence of progressive radiolucent lines were any different compared to a PS implant design.
Patients and Methods
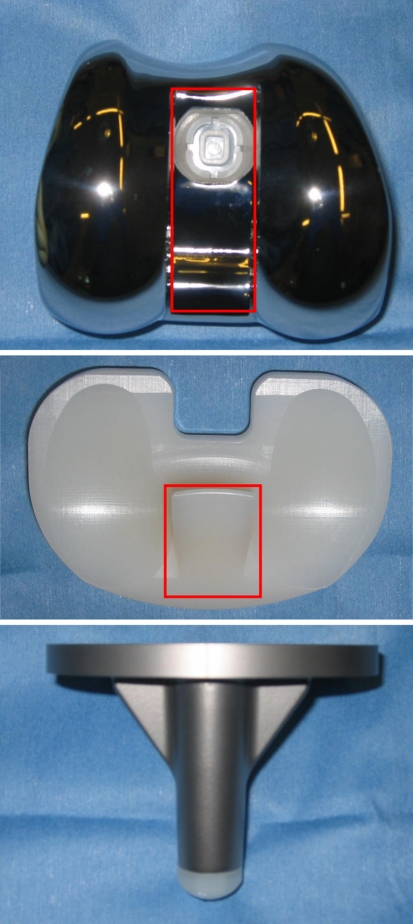
We initiated in February 2006 a prospective, single-center, randomized controlled trial (RCT) comparing the Press Fit Condylar® Sigma™ PS (PFC; DePuy, Warsaw, IN) (Fig. 1) and Medial Rotation Knee™ (MRK; Finsbury Orthopaedics, Leatherhead, Surrey, UK) (Fig. 2) total knee designs. The indications for both implant types included severely painful and/or disabled joints from osteoarthritis, traumatic or rheumatoid arthritis, or progression of disease in joints that have previously undergone unicompartmental arthroplasty or realignment osteotomy. The contraindications for surgery included active, local and systemic infection, loss of musculature, severe osteoporosis, and vascular deficiency of the affected limb. We included patients older than 18 years with symptomatic osteoarthritis of the knee having a primary TKA; patients meeting these criteria with previous arthroscopic meniscectomy or meniscal repair were also included. We excluded patients with cognitive impairment, neurologic deficits affecting mobility, posttraumatic osteoarthritis, previous tibial or femoral osteotomies, previous ACL or PCL reconstruction, and a preexisting TKA in the contralateral limb. Patients were blinded to their treatment arm. Randomization was performed using a computer random number generator on the day of but before surgery by a specialist physiotherapist (JT) who was not involved in the surgery. This study was approved by our institutional ethics committee.
Fig. 1.
Components of the Press Fit Condylar® Sigma™ PS (PFC; DePuy, Warsaw, IN) are shown with a “cam and post” articulation between the femoral component and the tibial insert.
Fig. 2.
Components of the Medial Rotation Knee™ (MRK; Finsbury Orthopaedics, Leatherhead, Surrey, UK) are shown with a “ball-and-socket” articulation of the medial compartment of the femoral component and the tibial insert.
A total of 69 patients were required to have a power of 90%, using a two-sided alpha set to 0.05 to show a difference of greater than 5° in the mean ROM between the two groups. We assumed a common SD of 10° based on a previous pilot series of TKA cases undertaken at our institution. A 5° difference in ROM was chosen as the minimal clinically important difference as it was, in the clinical judgment of the senior author (FSH), considered detectable by patients in daily activities. This concurs with other studies comparing ROM between different TKA implant types [9]. There were no differences in indications or contraindications for the two implant designs. To allow for mortality and patients lost to followup, we aimed to include at least 80 patients. We enrolled a total of 82 patients on an intention-to-treat basis, initially with 40 patients undergoing implantation of a PFC prosthesis (PFC group) and 42 patients with a MRK prosthesis (MRK group). Two patients were lost to followup in the MRK group, leaving a total of 80 patients for final evaluation; one died due to conditions unrelated to knee surgery while the other patient refused to continue due to the time required to complete all the outcome questionnaires. There were 18 men and 22 women with a mean age of 68.9 years in the PFC group and nine men and 31 women with a mean age of 72.5 years in the MRK group (Table 1). We observed no difference in preoperative functional measures between the groups (Table 2). All patients were followed up at 1 and 2 years postoperatively.
Table 1.
Demographic details of the PFC and MRK groups
| Variable | PFC group | MRK group | P value |
|---|---|---|---|
| Gender (male:female) | 18:22 | 9:31 | 0.03 |
| Age (years)* | 68.9 ± 12.1 (44–84) | 72.5 ± 9.7 (53–88) | 0.15 |
| Body mass index (kg/m2)* | 29.5 ± 8.1 (21–41.6) | 28.9 ± 6.2 (18–38.9) | 0.71 |
* Values are expressed as mean ± SD, with range in parentheses; PFC = Press Fit Condylar® Sigma™ posterior-stabilized total knee prosthesis; MRK = Medial Rotation Knee™ total knee prosthesis.
Table 2.
Comparison of preoperative clinical and functional outcomes in the PFC and MRK groups
| Outcome | PFC group | MRK group | P value |
|---|---|---|---|
| ROM | 93.9 ± 19.0 (20–115) | 97.3 ± 15.3 (50–120) | 0.38 |
| AKSS | |||
| Clinical | 48.4 ± 15.9 (11–88) | 43.0 ± 13.6 (14–67) | 0.11 |
| Function | 47.9 ± 20.3 (5–95) | 44.6 ± 15.3 (5–70) | 0.41 |
| WOMAC | |||
| Pain | 11.1 ± 4.5 (2–20) | 11.9 ± 3.7 (3–20) | 0.39 |
| Stiffness | 4.8 ± 1.8 (0–8) | 4.8 ± 2.1 (0–8) | 1 |
| Disability | 37.9 ± 14.2 (9–60) | 39.3 ± 12.7 (6–66) | 0.64 |
| Total | 53.8 ± 19.4 (15–88) | 56.0 ± 17.3 (14–93) | 0.59 |
| OKS | 41.7 ± 8.9 (23–56) | 41.6 ± 7.5 (25–56) | 0.96 |
| SF-36 | |||
| Physical component | 26.7 ± 7.0 (17–46.9) | 26.0 ± 6.8 (8.5–41.4) | 0.65 |
| Mental component | 51.3 ± 10.4 (30.7–70.8) | 49.9 ± 12.5 (27.5–70.4) | 0.59 |
| TKFQ | |||
| Activities of daily living | 3.4 ± 2.0 (0.7–8.2) | 2.8 ± 2.2 (0–7.1) | 0.21 |
| Sport and exercise | 2.9 ± 2.4 (0–6.9) | 2.8 ± 1.9 (0–5) | 0.84 |
| Movement and lifestyle | 3.4 ± 1.8 (0–5.9) | 3.2 ± 1.7 (0–6.1) | 0.61 |
| Composite | 3.1 ± 1.6 (0.6–5.4) | 2.6 ± 1.5 (0–5.6) | 0.15 |
Values are expressed as mean ± SD, with range in parentheses; PFC = Press Fit Condylar® Sigma™ posterior-stabilized total knee prosthesis; MRK = Medial Rotation Knee™ total knee prosthesis; AKSS = American Knee Society score; OKS = Oxford Knee score; TKFQ = Total Knee Function Questionnaire.
All surgery was performed either by the senior author (FSH) or under his supervision both scrubbed and unscrubbed by multiple surgeons. A single intravenous dose of antibiotics was given before skin incision. A midline skin incision was made in all patients with a subsequent medial parapatellar arthrotomy and patellar eversion. The ACL and PCL were excised in all cases. In both groups, tibial preparation was undertaken first with extramedullary alignment. Tibial bone cuts were made perpendicular to the long axis of the tibia in the coronal plane and aiming for a 0° to 3° posterior slope in the sagittal plane. Femoral preparation was undertaken using intramedullary alignment with a valgus angle ranging between 5° to 7° determined preoperatively in reference to the anatomic axis of the tibia on AP radiographs. External rotation of 3° was referenced using either Whiteside’s line or the transepicondylar axis. Femoral bone cuts were made in the sequence as recommended by the respective implants’ operative technique guidelines. Posterior and peripheral osteophytes were removed. Soft tissue balance was assessed in flexion and extension using the implants’ respective supplied spacer device. Flexion and extension gaps were equalized in all cases using a sequential approach [8]. In the PFC group, the patella was measured and cut for resurfacing with the intention to restore the original patellar thickness. Within the MRK group, the patella was prepared so as to facilitate implantation of an inlay component. All components along with tibial inserts were trialled at this stage and final soft tissue and bony adjustments effected. A circumferential thigh tourniquet was subsequently inflated to 300 mm Hg and the knee was thoroughly washed using pulse lavage and dried. In the PFC group, all components were implanted using cement. After the final curing of cement, an appropriately sized tibial insert was placed. In the MRK group, the tibial component and insert were assembled before implantation. Only the tibial and femoral components were implanted with cement; the patellar inlay component was implanted cementless. Refobacin® bone cement (Biomet, Berlin, Germany) was used in all cases. A single intraarticular Redivac drain was placed with closure of the flexed knee undertaken in layers thereafter.
All patients underwent chemical and mechanical thromboprophylaxis unless contraindicated. Two further doses of parenteral antibiotics were given postoperatively. Physiotherapy was started on the first postoperative day in hospital and continued after discharge. On the first postoperative day, patients were encouraged to do flexion-extension exercises of the ankle and isometric quadriceps contraction exercises in bed. Mobilization was supervised by a physiotherapist for 30 minutes per day, aiming at achieving sequential milestones of flexion to 90°, walking with a walker, to walking with crutches by Day 5. Patients were allowed to progress to full weightbearing mobilization and full ROM as tolerated immediately in the postoperative period.
Clinical and radiographic assessment was undertaken preoperatively, at 6 weeks, 6 months, 1 year, and 2 years postoperatively. Clinical assessment undertaken at each followup was recorded and compiled by a specialist physiotherapist (JT) who was blinded to the choice of implant. Radiographic assessment for each followup interval was undertaken by a postdoctoral research assistant (MAF) and an orthopaedic resident (SP) who were blinded to the clinical status of the patients.
Clinical assessment was undertaken using the AKSS [16], WOMAC [3], OKS [12], SF-36 [39], and TKFQ [40]. Passive ROM was measured using a goniometer in a standardized manner with the patient supine. All complications during early and late followup were recorded.
Two of us (MAF, SP) made all radiographic assessments at 2 year followup on standardized AP and lateral views including alignment and position of the components and presence and location of all progressive radiolucent lines at the bone-cement and cement-prosthesis interfaces according to the recommendations of the American Knee Society [14]. In addition, posterior condylar offset was also recorded. To overcome variations in magnification, we modified the method of Bellemans et al. [4] where the posterior condylar offset length was referenced against the diameter of the femur at the termination of the posterior supracondylar flare on the lateral radiograph expressed as a ratio (PCOR). The correlation coefficient for the two observers in measuring PCOR was 0.91.
We calculated descriptive statistics (means, SD, range) for the continuous study variables. A Shapiro-Wilk test was used to identify whether each continuous variable fitted a normal distribution. Depending on this, a two-tailed Student’s t test for parametric data or the Mann-Whitney U test for nonparametric data was undertaken to compare the two cohorts with respect to clinical and radiographic variables. All statistical analyses were performed using XLSTAT (Version 7.0; Addinsoft, New York, NY).
Results
We found greater ROM in the MRK group than in the PFC group at both 1 and 2 years (Table 3). The ROM improved (p = 0.003) in the MRK group at 2 years after surgery compared to preoperatively, however, no difference was seen in the PFC group at 2 years compared to preoperatively. We observed no difference in ROM at 2 years as compared to 1 year postoperatively for either the PFC or MRK groups (p = 0.69 versus 0.89).
Table 3.
Comparison of postoperative ROM and clinical and function outcome scores for PFC and MRK groups at 1- and 2-year followup
| Outcome | 1 year | 2 years | ||||
|---|---|---|---|---|---|---|
| PFC group | MRK group | P value | PFC group | MRK group | P value | |
| ROM | 98.2 ± 14.2 (45–110) | 115.5 ± 11.6 (95–140) | < 0.0001 | 100.1 ± 15.9 (45–110) | 114.9 ± 12.8 (90–140) | < 0.0001 |
| AKSS | ||||||
| Clinical | 66.7 ± 20.4 (40–99) | 73.8 ± 17.0 (52–100) | 0.09 | 68.6 ± 20.4 (40–99) | 76.3 ± 15.5 (52–100) | 0.06 |
| Function | 67.8 ± 23.5 (10–100) | 73.8 ± 17.0 (50–100) | 0.19 | 68.0 ± 24.8 (10–100) | 71.4 ± 15.8 (50–100) | 0.47 |
| WOMAC | ||||||
| Pain | 5.9 ± 5.4 (0–18) | 2.6 ± 1.8 (0–7) | 0.0004 | 5.4 ± 5.2 (0–18) | 2.7 ± 1.8 (0–7) | 0.003 |
| Stiffness | 2.8 ± 2.1 (0–7) | 2.2 ± 2.1 (0–7) | 0.21 | 2.6 ± 2.0 (0–7) | 2.1 ± 2.0 (0–7) | 0.27 |
| Disability | 24.1 ± 17.2 (2–65) | 21.1 ± 12.5 (3–40) | 0.37 | 24.9 ± 17.7 (2–65) | 22.2 ± 11.6 (6–40) | 0.42 |
| Total | 32.9 ± 22.3 (3–90) | 25.9 ± 14.9 (6–50) | 0.10 | 32.9 ± 23.1 (3–90) | 27.1 ± 13.4 (8–50) | 0.17 |
| OKS | 29.5 ± 7.0 (13–41) | 25.9 ± 10.1 (14–44) | 0.07 | 29.1 ± 7.0 (13–41) | 26.2 ± 9.1 (16–44) | 0.11 |
| SF-36 | ||||||
| Physical component | 32.6 ± 12.3 (10.9–55.6) | 40.3 ± 12.9 (15.6–61.4) | 0.008 | 32.8 ± 12.6 (10.9–55.6) | 39.5 ± 12.8 (15.6–61.4) | 0.02 |
| Mental component | 44.4 ± 14.7 (18.4– 65.2) | 55.8 ± 8.7 (28.5–63.1) | < 0.0001 | 43.4 ± 14.1 (18.4–65.2) | 46.3 ± 8.3 (28.5–63.1) | 0.27 |
| TKFQ | ||||||
| Activities of daily living | 4.8 ± 2.1 (0–8.5) | 6.2 ± 2.5 (1.7–9.8) | 0.008 | 5.0 ± 1.7 (1.7–8.3) | 6.2 ± 1.4 (4.5–8.3) | 0.0009 |
| Sport and exercise | 4.9 ± 0.9 (2.8–6.0) | 5.7 ± 2.1 (1.9–10.0) | 0.03 | 5.0 ± 1.6 (1.9–7.0) | 5.6 ± 1.0 (2.8–7.0) | 0.04 |
| Movement and lifestyle | 4.5 ± 1.3 (1.9–6.8) | 5.7 ± 1.5 (3.2–7.6) | 0.0003 | 5.2 ± 1.3 (3.2–7.1) | 5.8 ± 0.9 (3.8–7.1) | 0.02 |
| Composite | 4.7 ± 1.2 (2.6–6.8) | 5.7 ± 2.0 (2.7–8.7) | 0.008 | 5.1 ± 1.5 (2.4–6.9) | 5.9 ± 1.0 (3.9–7.4) | 0.006 |
Values are expressed as mean ± SD, with range in parentheses; PFC = Press Fit Condylar® Sigma™ posterior-stabilized total knee prosthesis; MRK = Medial Rotation Knee™ total knee prosthesis; AKSS = American Knee Society score; OKS = Oxford Knee score; TKFQ = Total Knee Function Questionnaire.
We observed better scores for the WOMAC subscale of pain, physical component of the SF-36, and all components of the TKFQ in the MRK group compared to the PFC group at both 1 and 2 years (Table 3). Better scores in the mental component of the SF-36 and the clinical component of the AKSS were observed in the MRK group at 1 year and 2 years, respectively. There was no difference in all other outcome measures and their respective subscales when comparing the two groups. There was an improvement (p < 0.001) in the AKSS, total WOMAC, OKS, and composite TKFQ scores in both groups 2 years after surgery compared to preoperative scores. The physical component of SF-36 also improved (p = 0.0002) in the MRK group only at 2 years. In contrast, the mental component of SF-36 worsened (p = 0.04) in the PFC group at 2 years after surgery.
There was no difference in radiographic alignment between the two implant groups at 2 years (Table 4). There were no progressive bone-cement or cement-implant interface lucencies observed. There was no difference in pre- and postoperative PCOR for the PFC group (p = 0.40) or the MRK group (p = 0.19).
Table 4.
Radiographic outcomes of the PFC and MRK groups at 2 years
| Outcome | PFC group | MRK group | P value |
|---|---|---|---|
| Femoral valgus angle (°) | 95.5 ± 3.5 (84–101) | 95.6 ± 3.9 (80–100) | 0.90 |
| Tibial valgus angle (°) | 89.2 ± 2.5 (83–93) | 88.4 ± 1.9 (84–92) | 0.11 |
| Total valgus angle (°) | 184.7 ± 3.5 (175–192) | 184.0 ± 4.3 (169–191) | 0.43 |
| Lateral femoral angle (°) | 3.3 ± 4.7 (−5–15) | 2.4 ± 2.7 (−5–6) | 0.30 |
| Lateral tibial angle (°) | 87.4 ± 2.8 (80–93) | 88.7 ± 4.3 (78–99) | 0.11 |
| PCOR | |||
| Preoperative | 0.95 ± 0.16 (0.67–1.39) | 0.90 ± 0.15 (0.64–1.21) | 0.15 |
| Postoperative | 0.99 ± 0.14 (0.72–1.28) | 0.96 ± 0.14 (0.60–1.31) | 0.34 |
| Difference | 0.04 ± 0.14 (−0.33–0.30) | 0.06 ± 0.12 (−0.14–0.30) | 0.49 |
Values are expressed as mean ± SD, with range in parentheses; PFC = Press Fit Condylar® Sigma™ posterior-stabilized total knee prosthesis; MRK = Medial Rotation Knee™ total knee prosthesis; PCOR = posterior condylar offset ratio.
There were two cases of deep vein thrombosis recorded, one in each group. One patient in the MRK group presented with a painful hematoma after a fall 4 weeks after surgery, which was surgically evacuated. There were no revision procedures.
Discussion
The aim of this prospective RCT was to compare the ROM, clinical and functional scores, and radiographic findings of a MCBS design TKA prosthesis with a more natural femoral rollback profile to a conventional PS fixed-bearing TKA implant. We found patients undergoing TKA with a MRK prosthesis had improved ROM, physical component of the SF-36 scores, all components of the TKFQ, and less pain as measured on the WOMAC subscale compared to patients with a PS design TKA at 2-year followup.
There are some limitations to this study. First, although our sample size was appropriately powered for the primary outcome of ROM, it may have been inadequate with respect to the other study variables. Second, there is disparity in gender ratios between the groups. This occurred by chance due to the randomized nature of the study, which in retrospect has allowed all other variables to be well matched. Both groups were homogenous with respect to age and BMI. Furthermore, preoperative ROM, AKSS, WOMAC, OKS and TKFQ scores were similar in the two groups. Third, there was more than one operating surgeon including trainees with varied levels of experience across both PFC and MRK groups, which may have influenced the scores. However, all operations were performed under the direct supervision of the senior author (FSH) using his standardized technique for each implant, and all other protocols were uniform.
We observed higher ROM when using the MCBS prosthesis in comparison to a conventional PS design in a homogenous sample of patients with similar preoperative ROM and function. One RCT including the MCBS design showed worse ROM, clinical scores, and satisfaction and a higher rate of complications when using the Medial Pivot Knee compared to a mobile-bearing TKA prosthesis [20]. While direct comparison with this study cannot be made due to our use of a fixed-bearing implant, a retrospective crossover study reported similar ROM at 12 months between the two fixed-bearing designs [34].
We observed an overall improvement in function after surgery, which is consistent with other single implant reports for both design concepts [11, 18, 23, 30]. The difference between the PFC and MRK group for the clinical component of the AKSS, pain domain of the WOMAC, physical component of the SF-36, and all components of the TKFQ are compatible with findings of a large cohort of patients who underwent bilateral TKAs with a different prosthesis on each side. Subgroup analysis determined 77% of patients preferred a TKA with an MCBS design over a PS design when both were implanted [29]. No difference was observed between the two groups when using the function component of the AKSS, the OKS, or the summative WOMAC score. This may be attributable to the ceiling effect of each measure [24, 25], and while they are successful in the assessment of pain relief and general function, they do not address “high-end” activities such as sports and recreation. In contrast, improvements in the MRK group were observed at both followup intervals using the TKFQ. The TKFQ was designed to assess those activities important to the patient, and it reduces the ceiling effect by including sports and recreation [40]. The increased TKFQ scores in the MRK group may indicate more patients return to these activities, although we have not specifically recorded this. In a longitudinal comparison of both implant designs, there was no further improvement with respect to ROM and further clinical parameters beyond 1 year, which may reflect the time needed for recovery. At 2 years, there is variability in trends of the mental component of the SF-36 in both groups. The SF-36 is a generic quality-of-life measure without disease-specific qualities. The variability suggests the determinants of the mental component are multifactorial.
Radiographic markers of alignment were expectedly similar across both groups without any evidence of lucencies. The specific parameters of lateral tibial angle and posterior condylar offset are, however, reportedly important determinants of postoperative ROM [22, 33]. These specific variables, in addition to preoperative ROM, were similar between the two groups, suggesting other factors such as implant design may be implicated for the increased ROM in the MRK group.
Constraining design elements such as the PS and MCBS aim to reduce paradoxical movement during mobilization [6]. AP stability in PS designs occurs when the post of the tibial insert engages the cam of the femoral component. There is wide variability in the flexion angle at which this occurs, with reports from 20° to more than 90° [6, 35, 38]. In contrast, the MCBS design has a conforming, congruent, medial tibiofemoral articulation with a raised anterior and posterior lip. Consequently, this provides stability throughout the ROM to prevent AP translation. On the lateral side, there is a lack of congruence allowing lateral femoral rollback in a manner close to the normal kinematic profile of the knee [26]. This specific design feature may confer the additional functional benefits seen in the MRK group. The normal knee is a modified hinge joint with a rotational axis medially [7, 15, 28]. The MCBS design emulates this kinematic feature more so than the PS design, which may translate as more efficient kinematics contributing to better ROM.
Patient expectations after TKA go beyond pain relief and return to walking [27]. Furthermore, there are patients who have acceptable radiographic parameters and ROM but are still not satisfied [32]. This may suggest an unmet need for absolute stability through full ROM and a more natural kinematic profile after TKA. The MCBS concept may provide this with consequent improvements in ROM and function measured using validated outcome tools, in particular high-end function as illustrated by our findings. Our observations are nonetheless short-term data and longer followup is needed for confirmation of durability.
Acknowledgments
We thank Jenni Tahmasebbi for her assistance in clinical assessment and data collection. We also thank Miguel A. Fernandez for his assistance in radiographic assessment and data collection.
Footnotes
One or more of the authors (FSH) has received research funding from Smith and Nephew (Memphis, TN), Stryker (Kalamazoo, MI), and Finsbury Orthopaedics (Leatherhead, Surrey, UK).
Each author certifies that his or her institution approved the human protocol for this investigation that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Aglietti P, Rinonapoli E. Total condylar knee arthroplasty: a five-year follow-up study of 33 knees. Clin Orthop Relat Res. 1984;186:104–111. [PubMed] [Google Scholar]
- 2.Amin A, Al-Taiar A, Sanghrajka AP, Kang N, Scott G. The early radiological follow-up of a medial rotational design of total knee arthroplasty. Knee. 2008;15:222–226. doi: 10.1016/j.knee.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 4.Bellemans J, Banks S, Victor J, Vandenneucker H, Moemans A. Fluoroscopic analysis of the kinematics of deep flexion in total knee arthroplasty: influence of posterior condylar offset. J Bone Joint Surg Br. 2002;84:50–53. doi: 10.1302/0301-620X.84B1.12432. [DOI] [PubMed] [Google Scholar]
- 5.Bertin KC. Cruciate-retaining total knee arthroplasty at 5 to 7 years followup. Clin Orthop Relat Res. 2005;436:177–183. doi: 10.1097/01.blo.0000160381.23052.34. [DOI] [PubMed] [Google Scholar]
- 6.Blaha JD. The rationale for a total knee implant that confers anteroposterior stability throughout range of motion. J Arthroplasty. 2004;19:22–26. doi: 10.1016/j.arth.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Blaha JD, Mancinelli CA, Simons WH, Kish VL, Thyagarajan G. Kinematics of the human knee using an open chain cadaver model. Clin Orthop Relat Res. 2003;410:25–34. doi: 10.1097/01.blo.0000063564.90853.ed. [DOI] [PubMed] [Google Scholar]
- 8.Bottros J, Gad B, Krebs V, Barsoum WK. Gap balancing in total knee arthroplasty. J Arthroplasty. 2006;21:11–15. doi: 10.1016/j.arth.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary R, Beaupre LA, Johnston DW. Knee range of motion during the first two years after use of posterior cruciate-stabilizing or posterior cruciate-retaining total knee prostheses: a randomized clinical trial. J Bone Joint Surg Am. 2008;90:2579–2586. doi: 10.2106/JBJS.G.00995. [DOI] [PubMed] [Google Scholar]
- 10.Dalury DF, Barrett WP, Mason JB, Goldstein WM, Murphy JA, Roche MW. Midterm survival of a contemporary modular total knee replacement: a multicentre study of 1970 knees. J Bone Joint Surg Br. 2008;90:1594–1596. doi: 10.1302/0301-620X.90B12.21064. [DOI] [PubMed] [Google Scholar]
- 11.Dalury DF, Gonzales RA, Adams MJ, Gruen TA, Trier K. Midterm results with the PFC Sigma total knee arthroplasty system. J Arthroplasty. 2008;23:175–181. doi: 10.1016/j.arth.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80:63–69. doi: 10.1302/0301-620X.80B1.7859. [DOI] [PubMed] [Google Scholar]
- 13.Dennis DA, Komistek RD, Mahfouz MR, Haas BD, Stiehl JB. Multicenter determination of in vivo kinematics after total knee arthroplasty. Clin Orthop Relat Res. 2003;416:37–57. doi: 10.1097/01.blo.0000092986.12414.b5. [DOI] [PubMed] [Google Scholar]
- 14.Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 15.Freeman MA, Pinskerova V. The movement of the knee studied by magnetic resonance imaging. Clin Orthop Relat Res. 2003;410:35–43. doi: 10.1097/01.blo.0000063598.67412.0d. [DOI] [PubMed] [Google Scholar]
- 16.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 17.Insall JN, Lachiewicz PF, Burstein AH. The posterior stabilized condylar prosthesis: a modification of the total condylar design: two to four-year clinical experience. J Bone Joint Surg Am. 1982;64:1317–1323. [PubMed] [Google Scholar]
- 18.Karachalios T, Roidis N, Giotikas D, Bargiotas K, Varitimidis S, Malizos KN. A mid-term clinical outcome study of the Advance Medial Pivot knee arthroplasty. Knee. 2009;16:484–488. doi: 10.1016/j.knee.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Kim YH, Choi Y, Kwon OR, Kim JS. Functional outcome and range of motion of high-flexion posterior cruciate-retaining and high-flexion posterior cruciate-substituting total knee prostheses: a prospective, randomized study. J Bone Joint Surg Am. 2009;91:753–760. doi: 10.2106/JBJS.H.00805. [DOI] [PubMed] [Google Scholar]
- 20.Kim YH, Yoon SH, Kim JS. Early outcome of TKA with a medial pivot fixed-bearing prosthesis is worse than with a PFC mobile-bearing prosthesis. Clin Orthop Relat Res. 2009;467:493–503. doi: 10.1007/s11999-008-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komistek RD, Dennis DA, Mahfouz M. In vivo fluoroscopic analysis of the normal human knee. Clin Orthop Relat Res. 2003;410:69–81. doi: 10.1097/01.blo.0000062384.79828.3b. [DOI] [PubMed] [Google Scholar]
- 22.Malviya A, Lingard EA, Weir DJ, Deehan DJ. Predicting range of movement after knee replacement: the importance of posterior condylar offset and tibial slope. Knee Surg Sports Traumatol Arthrosc. 2009;17:491–498. doi: 10.1007/s00167-008-0712-x. [DOI] [PubMed] [Google Scholar]
- 23.Mannan K, Scott G. The Medial Rotation total knee replacement: a clinical and radiological review at a mean follow-up of six years. J Bone Joint Surg Br. 2009;91:750–756. doi: 10.1302/0301-620X.91B6.22124. [DOI] [PubMed] [Google Scholar]
- 24.Marx RG, Jones EC, Atwan NC, Closkey RF, Salvati EA, Sculco TP. Measuring improvement following total hip and knee arthroplasty using patient-based measures of outcome. J Bone Joint Surg Am. 2005;87:1999–2005. doi: 10.2106/JBJS.D.02286. [DOI] [PubMed] [Google Scholar]
- 25.Moonot P, Medalla GA, Matthews D, Kalairajah Y, Field RE. Correlation between the Oxford Knee and American Knee Society scores at mid-term follow-up. J Knee Surg. 2009;22:226–230. doi: 10.1055/s-0030-1247753. [DOI] [PubMed] [Google Scholar]
- 26.Moonot P, Mu S, Railton GT, Field RE, Banks SA. Tibiofemoral kinematic analysis of knee flexion for a medial pivot knee. Knee Surg Sports Traumatol Arthrosc. 2009;17:927–934. doi: 10.1007/s00167-009-0777-1. [DOI] [PubMed] [Google Scholar]
- 27.Nilsdotter AK, Toksvig-Larsen S, Roos EM. Knee arthroplasty: are patients’ expectations fulfilled? A prospective study of pain and function in 102 patients with 5-year follow-up. Acta Orthop. 2009;80:55–61. doi: 10.1080/17453670902805007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinskerova V, Johal P, Nakagawa S, Sosna A, Williams A, Gedroyc W, Freeman MA. Does the femur roll-back with flexion? J Bone Joint Surg Br. 2004;86:925–931. doi: 10.1302/0301-620X.86B6.14589. [DOI] [PubMed] [Google Scholar]
- 29.Pritchett JW. Patient preferences in knee prostheses. J Bone Joint Surg Br. 2004;86:979–982. doi: 10.1302/0301-620X.86B7.14991. [DOI] [PubMed] [Google Scholar]
- 30.Rasquinha VJ, Ranawat CS, Cervieri CL, Rodriguez JA. The press-fit condylar modular total knee system with a posterior cruciate-substituting design: a concise follow-up of a previous report. J Bone Joint Surg Am. 2006;88:1006–1010. doi: 10.2106/JBJS.C.01104. [DOI] [PubMed] [Google Scholar]
- 31.Rinonapoli E, Mancini GB, Azzara A, Aglietti P. Long-term results and survivorship analysis of 89 total condylar knee prostheses. J Arthroplasty. 1992;7:241–246. doi: 10.1016/0883-5403(92)90043-P. [DOI] [PubMed] [Google Scholar]
- 32.Robertsson O, Dunbar M, Pehrsson T, Knutson K, Lidgren L. Patient satisfaction after knee arthroplasty: a report on 27,372 knees operated on between 1981 and 1995 in Sweden. Acta Orthop Scand. 2000;71:262–267. doi: 10.1080/000164700317411852. [DOI] [PubMed] [Google Scholar]
- 33.Schurman DJ, Rojer DE. Total knee arthroplasty: range of motion across five systems. Clin Orthop Relat Res. 2005:132–137. [PubMed]
- 34.Shakespeare D, Ledger M, Kinzel V. Flexion after total knee replacement: a comparison between the Medial Pivot knee and a posterior stabilised implant. Knee. 2006;13:371–373. doi: 10.1016/j.knee.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Suggs JF, Hanson GR, Park SE, Moynihan AL, Li G. Patient function after a posterior stabilizing total knee arthroplasty: cam-post engagement and knee kinematics. Knee Surg Sports Traumatol Arthrosc. 2008;16:290–296. doi: 10.1007/s00167-007-0467-9. [DOI] [PubMed] [Google Scholar]
- 36.Duren BH, Pandit H, Beard DJ, Zavatsky AB, Gallagher JA, Thomas NP, Shakespeare DT, Murray DW, Gill HS. How effective are added constraints in improving TKR kinematics? J Biomech. 2007;40(Suppl 1):S31–S37. doi: 10.1016/j.jbiomech.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Vince KG, Insall JN, Kelly MA. The total condylar prosthesis: 10- to 12-year results of a cemented knee replacement. J Bone Joint Surg Br. 1989;71:793–797. doi: 10.1302/0301-620X.71B5.2584249. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Simpson KJ, Chamnongkich S, Kinsey T, Mahoney OM. A biomechanical comparison between the single-axis and multi-axis total knee arthroplasty systems for the stand-to-sit movement. Clin Biomech (Bristol, Avon) 2005;20:428–433. doi: 10.1016/j.clinbiomech.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Weinberger M, Samsa GP, Hanlon JT, Schmader K, Doyle ME, Cowper PA, Uttech KM, Cohen HJ, Feussner JR. An evaluation of a brief health status measure in elderly veterans. J Am Geriatr Soc. 1991;39:691–694. doi: 10.1111/j.1532-5415.1991.tb03623.x. [DOI] [PubMed] [Google Scholar]
- 40.Weiss JM, Noble PC, Conditt MA, Kohl HW, Roberts S, Cook KF, Gordon MJ, Mathis KB. What functional activities are important to patients with knee replacements? Clin Orthop Relat Res. 2002;404:172–188. doi: 10.1097/00003086-200211000-00030. [DOI] [PubMed] [Google Scholar]