Abstract
Background
Multidrug resistant Acinetobacter baumannii (MDR AB) with and without Staphylococcus aureus (SA) is a commonly isolated organism in infected segmental bone defects in combat-related trauma in Iraq and Afghanistan. Although MDR AB in visceral infections is a therapeutic challenge, control of infection appears more common for combat-related osteomyelitis.
Questions/purposes
Using a rat model, we explored the virulence of MDR AB in segmental bone defects alone and in combination with SA.
Methods
Segmental defects in 60 rat femurs were created, stabilized, and inoculated with MDR AB alone and 60 with MDR AB and SA. We performed qualitative and quantitative bacteriology and radiographic assessments at 2, 4, and 8 weeks for MDR AB and at 1, 2, and 3 weeks for MDR AB and SA.
Results
Quantitative bacteriology revealed a 3- to 5-log decrease in MDR AB from the initial inoculum. After polymicrobial inoculation, only 10 of 60 animals had positive cultures for MDR AB, whereas 59 of 60 animals had positive cultures for SA. Recovered SA were 2 to 5 log greater than the initial inoculum, while there again was a 3- to 5-log decrease in MDR AB. MDR AB alone did not cause bony lysis, but there was radiographic evidence of new bone formation in 67% of the segmental defects. Osteolysis was noted with MDR AB and SA.
Conclusions
MDR AB did not appear to cause or contribute to clinically apparent osteomyelitis in this pilot study.
Clinical Relevance
Resolution of infections in combat-related segmental bone defects inoculated with MDR AB may be attributable to low virulence. Additional studies are needed to confirm low virulence and bone formation with MDR AB.
Introduction
War wounds encountered in soldiers deployed to Iraq and Afghanistan have underscored the need for improved therapy of infected segmental bone defects [1, 8, 15]. Infection of segmental bone defects is problematic because it usually inhibits bone healing and increases bone resorption [13, 16]. Primary and nosocomial infections are not easily prevented or eradicated in the presence of foreign bodies and a poor vascular supply. The presence of graft material and fixation devices provides excellent surfaces for bacterial biofilm formation that are less susceptible to antibiotics and host immune defenses [9, 10].
The majority of organisms isolated in war wounds of the extremities in soldiers returning from Iraq and Afghanistan are Gram-positive cocci such as coagulase-negative Staphylococci, SA, and to a lesser extent, Streptococcus species [15]. These bacteria colonize the skin and are commonly implicated in soft tissue infections and osteomyelitis. Gram-negative organisms such as Klebsiella pneumoniae, MDR AB, and Pseudomonas aeruginosa also are found in blood and wound samples of US soldiers with extremity injuries [15, 22]. Some studies suggest that A. baumannii rarely is present at the time of bone and soft tissue injury, but rather is hospital-acquired [12, 15, 18]. Infections involving MDR AB, such as pneumonia, meningitis, urinary tract infections, wound infections, and bacteremia can be difficult to treat with few consistently effective antimicrobials [2, 19] and often are associated with high mortality, high rates of mechanical ventilation, and the need for considerable assistance in activities of daily living after discharge [1]. However, several studies suggest infections with this organism may be easier to control in combat-related osteomyelitis [8, 17, 20, 21].
This screening pilot study endeavored to answer the following two questions: (1) Does MDR AB introduced into a plated segmental defect of femurs in a rat model result in increased bacteria compared with the initial inoculum and radiographically evident osteolysis? (2) Using the same rat model, does a combination of MDR AB and SA, a common copathogen with MDR AB, cause synergy or antagonism of these organisms as measured by bacteriology and radiography?
Materials and Methods
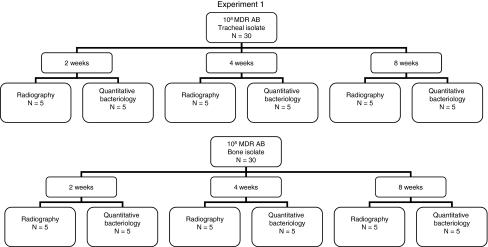
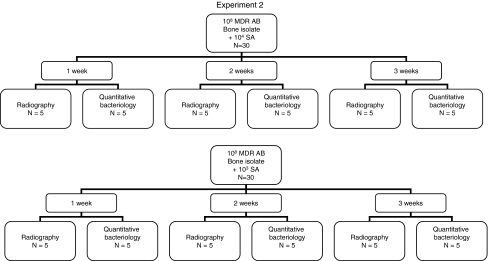
In Experiment 1, a segmental bone defect created in 60 rat femurs was inoculated with MDR AB with qualitative and quantitative bacteriology and radiographic assessment performed at 2, 4, and 8 weeks to characterize the course of these infections (Fig. 1). In Experiment 2, these experiments were repeated on another 60 rats with MDR AB and SA using the same assessments at 1, 2, and 3 weeks to analyze what, if any, interaction occurred between MDR AB and SA in this model (Fig. 2). In previously published work, one of the authors (DTT) performed these experiments using the same rat model with SA alone [6], permitting analysis of interactions between MDR AB and SA together and each of the pathogens alone.
Fig. 1.
A flow diagram of Experiment 1 with Acinetobacter baumannii is shown.
Fig. 2.
A flow diagram of Experiment 2 with Acinetobacter baumannii and Staphylococcus aureus is shown.
We obtained approval for all procedures involving animals through the Institutional Animal Care and Use Committee in accordance with the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care. Each animal was anesthetized via intraperitoneal injection of ketamine (80–120 mg/kg) in combination with xylazine (2–3 mg/kg). Using aseptic technique, a longitudinal incision was made over the anterolateral femur. The entire length of the femoral shaft was exposed by separating the vastus lateralis and biceps femoris muscles. All attached muscles and periosteum were stripped from the shaft. A polyacetal plate with six threaded Kirschner wires stabilized a 6-mm surgically created segmental defect in the left femur of 120 adult male Sprague-Dawley rats. An absorbable collagen sponge (Medtronic Sofamor Danek, Memphis, TN, USA) was wetted with various inocula of MDR AB alone and MDR AB with SA suspended in 0.1 mL of normal saline and packed into the defect. The analgesic buprenorphine was administered intramuscularly (0.013–0.026 mg/kg) immediately after surgery and continued at one dose per day for up to 2 postoperative days. The animals were allowed full activity in their cages postoperatively. Activity level, food intake, condition of the surgical wound, and clinical signs of systemic infection were monitored daily, and the animals were weighed twice a week.
We experimentally determined suitable bacterial inocula and time from contamination in preliminary work to guide the parameters of this project. Our goal was to achieve osteomyelitis without sepsis or seriously compromising defect fixation, and we based our inocula on knowledge we gained in previously published work that established a monomicrobial chronically infected defect [6]. The isolate of SA used was obtained from a patient with an infected total hip arthroplasty at Hennepin County Medical Center, Minneapolis, MN. The bone isolate of A. baumannii was a patient isolate obtained from Brooke Army Medical Center, Fort Sam Houston, TX. The tracheal isolate of A. baumannii was a patient isolate from Hennepin County Medical Center. Both A. baumannii strains were multidrug-resistant with full susceptibility only to amikacin. No local or systemic antibiotics were administered in any experiments.
As we were never able to establish a chronic infection with MDR AB, we used the largest inoculum that we could prepare, and the longest interval that was feasible. After the initial surgery and contamination with 108 colony-forming units (CFUs) of MDR AB alone, 60 animals were allowed to recover to permit the contamination to progress to chronic infection. At 2, 4, and 8 weeks, rats were euthanized by inhalant anesthesia followed by intraperitoneal injection of 100 mg/kg of euthanasia solution. We then harvested the femurs. We processed the defects for qualitative and quantitative bacteriology to confirm the presence and extent of the intended infection in 30 rats (15 with the bone isolate and 15 with the tracheal isolate). High-resolution Faxitron radiographic lysis assessments and qualitative bacterial cultures were performed at 2, 4, and 8 weeks in the remaining 30 rats contaminated with MDR AB alone (15 with the bone isolate and 15 with the tracheal isolate) (Fig. 1).
In a second experiment, we used SA and MDR AB to contaminate defects in 60 rats. In preliminary work we found the suitable bacterial inocula and time required to establish chronic infection without sepsis or compromise of defect fixation. First, 30 defects were contaminated with 104 CFU of SA and 108 CFU of MDR AB (bone isolate). We then contaminated defects in 30 additional rats with a reduced SA inoculum (103 CFUs) coupled with 108 CFU MDR AB (bone isolate) to evaluate a potential dose-related synergism or antagonism of SA on MDR AB. Ten rats of each group were euthanized at Weeks 1, 2, or 3. Five of each group and at each time were used for qualitative and quantitative bacterial cultures. We used the remaining five of each group and at each time for high-resolution Faxitron radiographic lysis assessments and qualitative bacterial cultures (Fig. 2).
Previous studies with SA alone had established that 3 weeks of infection was sufficient for bone lysis to be evident on radiographs and that bony lysis first becomes radiographically evident where the Kirschner wires cross the cortical bone [6]. Three observers (CAC, CGL, WDL) determined bony lysis by counting the number of locations where lysis occurred (12 possible sites of lysis where the six Kirschner wires cross the cortical bone twice). Each observer evaluated each radiograph independently. A final determination of the presence or absence of lysis was made by consensus agreement. A previous study investigating bone lysis using the same rat model and SA as the infecting pathogen was used as a reference [6].
The plate, Kirschner wires, and all soft tissues were removed, and each femur was weighed, snap-frozen in liquid nitrogen, and ground to a fine powder under sterile conditions. The resulting bone powder was suspended in 2 mL of tryptic soy broth and serially diluted in tryptic soy broth. For specimens with MDR AB alone, aliquots of each dilution were plated onto the surfaces of 5% sheep blood agar and MacConkey agar and incubated at 37°C for 48 hours in 5% CO2. For the polymicrobial specimens with MDR AB and SA, aliquots of each dilution were plated onto the surface of 5% sheep blood agar, MacConkey agar, and colistin nalidixic acid agar and incubated at 37°C for 48 hours in 5% CO2. Results were expressed as the log10 number of recovered CFUs of bacteria per gram of bone.
Additional pilot studies using 20 rats with P. aeruginosa alone (104 and 106 CFUs) and 64 rats with P. aeruginosa and SA (103 and 104 CFUs) in the same model were conducted to verify that the model could produce clinical infection with a different clinically relevant Gram-negative organism (Appendix 1).
Results
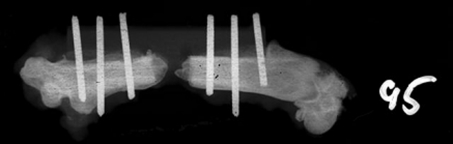
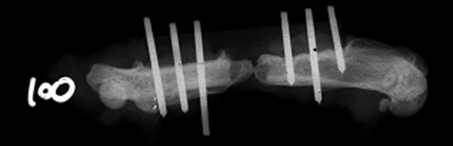
We observed no increase in the number of CFUs of MDR AB in Experiment 1 in rats whose fractures had been contaminated with MDR AB alone. Bacterial swabs of the defects after euthanasia revealed the presence of very few colonies of MDR AB. The number of CFUs of MDR AB recovered from bone was reduced more than 1000-fold compared with the initial contaminating inoculum of 108 organisms (Table 1). To address the possibility of an inadequate model, pilot experiments using the same model were replicated using P. aeruginosa alone and with SA (Appendix 1). Recovered P. aeruginosa at 2 and 3 weeks were larger than initial inocula. Moreover, 106 CFUs of P. aeruginosa combined with 103 and 104 CFUs of SA resulted in dyspnea and death in several animals. In addition to the surprising reduction in bacterial counts with MDR AB, no bony lysis was radiographically evident at Weeks 2, 4, and 8 using 108 CFU of tracheal and bone isolates of MDR AB (Table 2). Twenty of 30 femurs (67%), distributed equally between tracheal and bone isolates of MDR AB (108 CFUs) showed radiographic evidence of bone formation at the ends of the defect (Fig. 3). This new bone formation nearly connected the ends of the defect in two of these 20 animals (Fig. 4). Bone formation was radiographically apparent in the defects of three of 10 femurs at 2 weeks, nine of 10 femurs at 4 weeks, and eight of 10 femurs at 8 weeks.
Table 1.
Monomicrobial contamination: log 10 CFU of bacteria recovered from infected defects
| Bacterial inoculum log CFUs | 2 weeks | 4 weeks | 8 weeks |
|---|---|---|---|
| 8 (tracheal isolate) | 4.3 ± 1.5 (n = 5) |
4.7 ± 0.9 (n = 4*) |
3.4 ± 3.1 (n = 5) |
| 8 (bone isolate) | 4.1 ± 1 (n = 5) |
4.8 ± 0.6 (n = 5) |
1.4 ± 2.2 (n = 5) |
Data shown as mean ± standard deviation of the mean; *one rat contaminated with another type of bacteria with no A. baumannii isolated.
Table 2.
Monomicrobial contamination: number of sites of bony lysis
| Intervention | Time from contamination | ||
|---|---|---|---|
| 2 weeks | 4 weeks | 8 weeks | |
| A. baumannii from tracheal isolate: 108 CFUs | 0 (0.50) | 0 (0.25) | 0 (0) |
| A. baumannii from bone isolate: 108 CFUs | 0 (1.25) | 0 (0.25) | 0 (1.25) |
Data shown as median (interquartile range).
Fig. 3.
An example of bone capping the end of the defect contaminated with MDR AB is shown.
Fig. 4.
This is an example of bone nearly connecting the ends of the defect contaminated with MDR AB.
Adding SA to MDR AB in Experiment 2 produced local infection without apparent synergy or antagonism compared with previously published work using SA alone in the same rat model by one of the authors (DTT) [6]. Bacterial swabs of the polymicrobial-contaminated defects taken after euthanasia revealed very little MDR AB at the defect site while SA was present. The numbers of recovered A. baumannii remained at a level 3 to 4 logs less than the contamination level of 108 CFUs (Table 3) and were not substantially different than levels without SA (Table 1). The numbers of recovered SA were 2 to 5 logs greater than their contamination levels of 103 and 104 CFUs (Table 3). Greater levels of both bacteria were recovered when the polymicrobial contamination was 103 CFUs SA + 108 CFUs MDR AB compared with 104 CFUs SA + 108 CFUs MDR AB. In contrast to Experiment 1, 103 or 104 CFU of SA and 108 CFU of MDR AB produced bony lysis (Table 4) that was radiographically evident (Fig. 5). Two of 30 femurs (6.7%) in Experiment 2 showed radiographic evidence of bone formation. This occurred in two of five femurs infected with 104 CFUs SA and 108 CFUs MDR AB at 3 weeks.
Table 3.
Polymicrobial contamination: log CFU of bacteria recovered from infected defects
| Polymicrobial infection | Time after contamination (weeks) | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Log CFU | |||
| SA (103) | 7.7 ± 0.5 | 7.3 ± 0.4 | 6.8 ± 0.5 |
| AB (108) | 5.3 ± 0.6 | 4.9 ± 0.6 | 5.1 ± 0.5 |
| Number of rats | 5 | 4† | 3† |
| SA (104) | 6.8 ± 0.3 | 6.9 ± 0.3 | 5.5 ± 2.3 |
| AB (108) | 4.3 ± 0.82 | 4.5 ± 0.2 | 3 ± 2.3 |
| Number of rats | 5 | 5 | 4* |
Data shown as mean ± standard error of the mean; †one of the 2-week rats and two of the 3-week rats were contaminated with other bacteria, *one of the 3-week rats was contaminated with other bacteria.
Table 4.
Polymicrobial contamination: number of sites of bony lysis from high resolution radiographs.
| Intervention | 1 week | 2 weeks | 3 weeks |
|---|---|---|---|
| S. aureus: 104 CFUs A. baumannii: (bone isolate): 108 CFUs |
2 (3.75) | 0 (.25) | 2 (2.25) |
| S. aureus: 103 CFUs A. baumannii: (bone isolate): 108 CFUs |
0 (0.25) | 0 (0) | 2 (2.25) |
Data shown as median (interquartile range).
Fig. 5.
This radiograph of a femur shows a defect 1 week after contamination with 104 colony-forming units (CFUs) of SA and 108 CFUs of MDR AB from the bone isolate with sites of lysis.
Discussion
Internally stabilized bone defects infected with MDR AB alone and with SA are common in combat-related trauma. We wished to determine possible reasons why outcomes with MDR AB-related osteomyelitis in soldiers often are favorable while MDR AB infections at other sites generally carry a poor prognosis. Can bacteriologic and radiographic studies in plated segmental bone defects using two distinct strains of MDR AB in a previously defined rat model explain the generally favorable outcomes in osteomyelitis seen in soldiers (Experiment 1)? Is there synergy or antagonism between MDR AB and SA, a common copathogen with MDR AB (Experiment 2)?
Our observations suggesting that MDR AB may have low virulence in chronic osteomyelitis have several limitations. First, this is a pilot study that may not have sufficient power to establish statistically significant conclusions. More definitive studies with appropriate power could be performed to confirm our findings. Second, we performed no histologic studies. Histologic analysis may have revealed a degree of chronic inflammation. However, there were no clinical or radiographic signs of active infection at 2, 4, and 8 weeks and we observed spontaneous microbiologic improvement without therapy in Experiment 1. Given these findings, we presume that histologic changes alone would not have created a compelling argument in favor of a clinically relevant infection. Histology also may have shown mineralized callus or more organized bone to support radiographic evidence of bone formation in the defect. Third, evaluation of the host response through serologies, such as rat immunoglobulins to specific MDR AB antigens, was not performed. Positive specific immunoglobulins could help describe the host response that led to a spontaneous decrease in bacterial load and no evidence of osteolysis seen in our model with MDR AB. Fourth, the lack of virulence found with A. baumannii might be attributable to the experimental model used. To address this possibility, similar experiments using the same model were replicated using P. aeruginosa (Appendix 1). These Gram-negative bacteria frequently contaminate and infect war wounds of the extremities and can lead to osteomyelitis (22). We interpret our findings as suggesting the lack of pathogenicity of A. baumannii described here cannot be attributed to an inadequate model. Fifth, despite using two separate strains of MDR AB, we were unable to create chronic osteomyelitis with MDR AB alone. Other authors have been able to create osteomyelitis with some, but not all strains of MDR AB [7], suggesting that the role of MDR AB in osteomyelitis is highly dependent on strains. Sixth, we performed no experiments without bacteria or collagen carriers. These controls may have shown that no bone formation occurs in the absence of MDR AB. Bone formation has not been observed in this model of infection without some form of osteogenic treatment [3–5]. One explanation could be that the newly formed bone may be the result of the presence of the absorbable collagen sponge used to retain the bacteria in the defect in the short term. However, the presence of the collagen sponge in an uninfected defect is not osteogenic: we chose this defect model because it is critical, meaning no bone forms unless an osteogenic treatment is applied [3–5]. Seventh, the virulence of SA was such that the polymicrobial experiments were not performed past 3 weeks. In contrast, the monomicrobial experimental arm with MDR AB was performed to 8 weeks to ascertain that classic osteomyelitis was not occurring with a longer incubation period. It is possible bone formation with a mixed MDR AB/SA infection might occur more frequently with a longer-lasting infection. However, in 2/3 of the defects contaminated with MDR AB alone, radiographic evidence of bone formation capped the ends of the defect and in some animals nearly connected the ends of the defect beginning at 2 weeks of incubation. In addition, it is conceivable that some form of synergy or antagonism between the two organisms may become apparent with a longer observation period. Finally, these findings need to be interpreted with the limitations inherent in extrapolating animal data to the human setting.
The literature supports a limited role for A. baumannii in clinical osteomyelitis [8, 17, 20, 21]. In one study, clinical improvement of osteomyelitis, including MDR AB, occurred without recurrence in 18 of 18 patients after a mean of 9 months, often despite the presence of internal stabilization and lack of extended continuation therapy with oral antibiotics. These findings were attributed to expert débridement, 6 weeks of directed antibiotics, and to the general good health of the patients [8]. Another study revealed Gram-negative rods like A. baumannii were widely present in first episodes of osteomyelitis but more rarely present in recurrences or treatment failures despite drug resistance and orthopaedic devices [21]. In two case reports of patients with A. baumannii, osteomyelitis resolved uneventfully with débridement and 6 weeks of antibiotics [17, 20]. These findings are consistent with low or reduced virulence of A. baumannii, as suggested by our experiments. Furthermore, a recent study using a murine model revealed the lack of biofilm formation and osteoblastic rather than osteolytic lesions with MDR AB [7]. Additionally, one strain used could not be used to establish bone infection [7]. Our findings are consistent with these results.
We are not aware of any experimental studies that investigate synergy or antagonism of MDR AB and SA in osteomyelitis. Observational reports identify these two pathogens in combat-related trauma of the extremities. Initial deep wound cultures of open tibial fractures at admission revealed that A. baumannii was the most frequent organism isolated in 27 soldiers (13/27). On repeat culture, A. baumannii was not isolated in any patients after 6 to 8 weeks of broad spectrum antibiotics targeting MDR AB. In at least four of these patients, SA, but no A. baumannii was isolated when deep cultures were performed 2 to 15 months later [11]. A retrospective chart review concluded that initial episodes of combat-related osteomyelitis from the upper and lower extremities were more likely to be polymicrobial than monomicrobial. A. baumannii was more likely to be recovered during original episodes of osteomyelitis than during recurrences, whereas SA was more likely to be isolated during recurrences than during the initial episode of osteomyelitis [21]. Another retrospective chart review again highlighted the polymicrobial nature of combat-related osteomyelitis, but found Gram-negative rods like A. baumannii were more common than Gram-positive organisms such as SA during early and late infections [14]. These observations shed no light on a potential interaction between these two organisms. Sufficiently powered well-designed studies could be done to confirm or reject the lack of interaction between MDR AB and SA in osteomyelitis suggested by our pilot data.
Despite the limitations, our pilot data suggest MDR AB may have reduced virulence in bone compared with other pathogens such as SA and P. aeruginosa. If confirmed, this reduced virulence may explain the relative ease of treatment of soldiers with MDR AB osteomyelitis. Our observations reveal a possible association with bone formation in bone defects inoculated with MDR AB compared with bone defects coinfected with SA. Additional studies are needed to more completely evaluate the role of MDR AB in osteomyelitis.
Acknowledgments
We thank William D. Lew MS (formerly at the Minneapolis Medical Research Foundation, Minneapolis, MN, currently at Nerites Corporation, Madison, WI) for contributions to study design, data analysis, and editing. We also thank David W. Polly Jr MD (Department of Orthopaedic Surgery, University of Minnesota, Minneapolis, MN) for participation in the study design; LTC Clinton K. Murray MD (Brooke Army Medical Center, Fort Sam Houston, TX) for providing the bone isolate of A. baumannii; and Joseph R. Kalugdan MD (Minneapolis Medical Research Foundation, Minneapolis, MN) for assistance during the initial phases of this work.
Appendix 1: Pilot Experiment Using 103 or 104 CFUs of SA and/or 104 or 106 CFUs of P. aeruginosa
Two experimental arms using P. aeruginosa alone and with SA in the same model were conducted to verify that the model could produce clinical infection with a different clinically relevant Gram-negative organism. The bone isolate of P. aeruginosa was obtained from a patient with tibial osteomyelitis at Hennepin County Medical Center, Minneapolis, MN. The experimental arms using P. aeruginosa in the same model revealed that the substitution of Acinetobacter baumannii with P. aeruginosa, when used alone or in combination with SA reliably led to recovery of high amounts of this pathogen from bone (Tables 1, 2). Moreover, the combined virulence of SA and P. aeruginosa led to respiratory distress and a mortality rate of 30% with 106 CFUs of P. aeruginosa and 103 or 104 CFUs of SA. These concentrations did not result in systemic effects leading to death in monomicrobial experiments using each of these pathogens independently. These results suggest an additive or synergistic relationship between SA and P. aeruginosa in bone infections.
Table 1.
Qualitative bacteriology of P. aeruginosa at 2 weeks
| P. aeruginosa inoculum | Culture results | No growth | Rare | Few | Moderate | Many |
|---|---|---|---|---|---|---|
| 104 | 4 | 4 | 2 | |||
| 106 | 6 | 4 |
Table 2.
Results of qualitative bacteriology in the polymicrobial model PA + SA
| PA | SA | Time (weeks) | Pathogen | No growth | Few | Moderate | Many | Clinical observation |
|---|---|---|---|---|---|---|---|---|
| 104 | 103 | 2 | SA | 4 | 2 | 3 | ||
| PA | 2 | 4 | 3 | |||||
| 3 | SA | 3 | 3 | 3 | ||||
| PA | 2 | 4 | 3 | |||||
| 104 | 2 | SA | 2 | 2 | 3 | 3 | ||
| PA | 1 | 4 | 4 | 1 | ||||
| 3 | SA | 2 | 1 | 4 | 2 | |||
| PA | 2 | 3 | 3 | 1 | ||||
| 106 | 103 | 2 | SA | 3 | 6 | 3 rats developed respiratory failure and died | ||
| PA | 5 | 4 | ||||||
| 3 | SA | 2 | 2 | 2 | 1 | |||
| PA | 2 | 4 | 1 | |||||
| 104 | 2 | SA | 6 | 5 rats developed respiratory failure and died | ||||
| PA | 1 | 2 | 3 | |||||
| 3 | SA | 5 | ||||||
| PA | 1 | 1 | 3 |
PA = Pseudomonas aeruginosa; SA = Staphylococcus aureus.
Footnotes
One or more of the authors (DTT) received funding from the US Army Medical Research and Material Command under Contract No. W81XWH-07-1-0195; one of the authors (SC-A) receives research support through the National Institutes of Health grant 2 T32 AI007329-16; one of the authors (CGTL) receives research support from Medtronic, the Scoliosis Research Society, and the Spinal Research Foundation.
The views, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation.
Approval for all procedures involving animals was obtained through the Institutional Animal Care and Use Committee in accordance with the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care.
This work was performed at the Minneapolis Medical Research Foundation, Minneapolis, MN, USA.
References
- 1.Abbo A, Carmeli Y, Navon-Venezia S, Siegman-Igra Y, Schwaber MJ. Impact of multi-drug-resistant Acinetobacter baumannii on clinical outcomes. Eur J Clin Microbiol Infect Dis. 2007;26:793–800. doi: 10.1007/s10096-007-0371-8. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. MMWR Morb Mortal Wkly Rep. 2004;53:1063–1066. [PubMed]
- 3.Chen X, Kidder LS, Lew WD. Osteogenic protein-1 induced bone formation in an infected segmental defect in the rat femur. J Orthop Res. 2002;20:142–150. doi: 10.1016/S0736-0266(01)00060-2. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Schmidt AH, Mahjouri S, Polly DW, Jr, Lew WD. Union of a chronically infected internally stabilized segmental defect in the rat femur after débridement and application of rhBMP-2 and systemic antibiotic. J Orthop Trauma. 2007;21:693–700. doi: 10.1097/BOT.0b013e31815a7e91. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Schmidt AH, Tsukayama DT, Bourgeault CA, Lew WD. Recombinant human osteogenic protein-1 induces bone formation in a chronically infected, internally stabilized segmental defect in the rat femur. J Bone Joint Surg Am. 2006;88:1510–1523. doi: 10.2106/JBJS.E.01136. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Tsukayama DT, Kidder LS, Bourgeault CA, Schmidt AH, Lew WD. Characterization of a chronic infection in an internally-stabilized segmental defect in the rat femur. J Orthop Res. 2005;23:816–823. doi: 10.1016/j.orthres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Crane DP, Gromov K, Li D, Søballe K, Wahnes C, Büchner H, Hilton MJ, O’Keefe RJ, Murray CK, Schwarz EM. Efficacy of colistin-impregnated beads to prevent multidrug-resistant A. baumannii implant-associated osteomyelitis. J Orthop Res. 2009;27:1008–1015. doi: 10.1002/jor.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis KA, Moran KA, McAllister CK, Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis. 2005;11:1218–1224. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK. Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis. 2007;45:409–415. doi: 10.1086/520029. [DOI] [PubMed] [Google Scholar]
- 12.Kaspar RL, Griffith ME, Mann PB, Lehman DJ, Conger NG, Hospenthal DR, Murray CK. Association of bacterial colonization at the time of presentation to a combat support hospital in a combat zone with subsequent 30-day colonization or infection. Mil Med. 2009;174:899–903. doi: 10.7205/milmed-d-04-3908. [DOI] [PubMed] [Google Scholar]
- 13.Meghji S, Crean SJ, Hill PA, Sheikh M, Nair SP, Heron K, Henderson B, Mawer EB, Harris M. Surface-associated protein from Staphylococcus aureus stimulates osteoclastogenesis: possible role in S. aureus-induced bone pathology. Br J Rheumatol. 1998;37:1095–1101. doi: 10.1093/rheumatology/37.10.1095. [DOI] [PubMed] [Google Scholar]
- 14.Mody RM, Zapor M, Hartzell JD, Robben PM, Waterman P, Wood-Morris R, Trotta R, Andersen RC, Wortmann G. Infectious complications of damage control orthopedics in war trauma. J Trauma. 2009;67:758–761. doi: 10.1097/TA.0b013e3181af6aa6. [DOI] [PubMed] [Google Scholar]
- 15.Murray CK, Roop SA, Hospenthal DR, Dooley DP, Wenner K, Hammock J, Taufen N, Gourdine E. Bacteriology of war wounds at the time of injury. Mil Med. 2006;171:826–829. doi: 10.7205/milmed.171.9.826. [DOI] [PubMed] [Google Scholar]
- 16.Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996;64:2371–2380. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafer JJ, Mangino JE. Multidrug-resistant Acinetobacter baumannii osteomyelitis from Iraq. Emerg Infect Dis. 2008;14:512–514. doi: 10.3201/eid1403.070128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, Mancuso J, Milstrey E, Bautista CT, Patel J, Ewell A, Hamilton T, Gaddy C, Tenney M, Christopher G, Petersen K, Endy T, Petruccelli B. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 19.Urban C, Segal-Maurer S, Rahal JJ. Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii. Clin Infect Dis. 2003;36:1268–1274. doi: 10.1086/374847. [DOI] [PubMed] [Google Scholar]
- 20.Volpin G, Krivoy N, Stein H. Acinetobacter sp. osteomyelitis of the femur: a late sequel of unrecognized foreign body implantation. Injury. 1993;24:345–346. doi: 10.1016/0020-1383(93)90063-C. [DOI] [PubMed] [Google Scholar]
- 21.Yun HC, Branstetter JG, Murray CK. Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J Trauma. 2008;64(2 suppl):S163–S168. doi: 10.1097/TA.0b013e318160868c. [DOI] [PubMed] [Google Scholar]
- 22.Yun HC, Murray CK, Roop SA, Hospenthal DR, Gourdine E, Dooley DP. Bacteria recovered from patients admitted to a deployed US military hospital in Baghdad, Iraq. Mil Med. 2006;171:821–825. doi: 10.7205/milmed.171.9.821. [DOI] [PubMed] [Google Scholar]