Abstract
Background
Synovial fluid white blood cell count is useful for diagnosing periprosthetic infections but the utility of this test in the early postoperative period remains unknown as hemarthrosis and postoperative inflammation may render standard cutoff values inaccurate.
Questions/purposes
We evaluated the diagnostic performance of four common laboratory tests, the synovial white blood cell count, differential, C-reactive protein, and erythrocyte sedimentation rate to detect infection in the first 6 weeks after primary TKA.
Methods
We reviewed 11,964 primary TKAs and identified 146 that had a knee aspiration within 6 weeks of surgery. Infection was diagnosed in 19 of the 146 knees by positive cultures or gross purulence. We compared demographic information, time from surgery, and the laboratory test values between infected and noninfected knees to determine if any could identify infection early postoperatively. Receiver operating characteristic curves were constructed to determine optimal cutoff values for each of the test parameters.
Results
Synovial white blood cell count (92,600 versus 4200 cells/μL), percentage of polymorphonuclear cells (89.6% versus 76.9%), and C-reactive protein (171 versus 88 mg/L) were higher in the infected group. The optimal synovial white blood cell cutoff was 27,800 cells/μL (sensitivity, 84%; specificity, 99%; positive predictive value, 94%; negative predictive value, 98%) for diagnosing infection. The optimal cutoff for the differential was 89% polymorphonuclear cells and for C-reactive protein 95 mg/L.
Conclusions
With a cutoff of 27,800 cells/μL, synovial white blood cell count predicted infection within 6 weeks after primary TKA with a positive predicted value of 94% and a negative predictive value of 98%. The use of standard cutoff values for this parameter (~ 3000 cells/μL) would have led to unnecessary reoperations.
Level of Evidence
Level II, diagnostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
TKA predictably improves pain and function for patients with advanced knee arthropathy [4, 9]. Advancements in knee design, implant manufacturing, perioperative protocols, and surgical techniques have led to improved functional outcomes and longevity of TKA [21, 22, 28]. Unfortunately, periprosthetic infection remains a common mode of failure, occurring in 0.4% to 2% of patients after TKA [2, 11, 19], and is a devastating complication for patients with substantial costs to the patient, caregivers, and healthcare systems [19, 20]. If the current rates of infection are applied to the projected increased number of TKAs that will be performed in the next several decades, the total number of infections will represent a substantial burden to an already strained healthcare system [8, 11, 12].
The diagnosis of infection in the early postoperative period can be particularly difficult as the expected inflammation around the surgical incision and associated edema in the extremity can make it difficult to differentiate an acute postoperative infection from the normal postoperative course. The C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), which are useful as screening tools for identifying a deep chronic infection, are normally elevated in the early postoperative period [1, 13]. Moreover, while the synovial fluid white blood cell (WBC) count and differential are also useful for differentiating septic from aseptic failure in both TKA and THA, it is unclear whether these tests are useful in the early postoperative period as a resolving postoperative hematoma and/or inflammation at the surgical site secondary to wound healing may cause elevations in these values [3, 10, 15, 23].
We determined (1) whether the synovial fluid WBC count, differential, CRP, and/or ESR were different between infected and noninfected knees; (2) the diagnostic testing performance (sensitivity, specificity, positive predictive value [PPV], negative predictive value [NPV]) and values leading to the optimal sensitivity and specificity of these tests; (3) whether any combination of these tests improved diagnostic performance; and (4) whether adjusting synovial WBC count for blood in the aspirate affects test performance in predicting infection of a TKA within 6 weeks of surgery.
Patients and Methods
We reviewed all 11,964 primary TKAs performed in 9826 patients at two institutions by 14 surgeons from April 1999 to December 2008. We retrospectively performed a database and chart review to identify patients who had a knee aspiration with synovial fluid WBC count, differential, and culture (including aerobic, anaerobic, acid-fast bacilli and fungal) within 6 weeks after a primary TKA. Knees were aspirated based on clinical signs of infection, including persistent wound drainage, fever, erythema, effusion, new onset of pain, and/or before any reoperation for a failed TKA where sepsis was suspected. Laboratory values, including CRP and ESR, were recorded. For patients who underwent reoperation within the first 6 weeks, prophylactic antibiotics were routinely withheld until three sets of intraoperative cultures were obtained. Of the 11,964 primary TKAs, 224 knees (1.9%) in 219 patients were identified as having arthrocentesis performed within the first 6 weeks after primary TKA. Seventy-eight aspirations were excluded for the following reasons: clotted specimen (38), inflammatory arthropathy (13), crystals in the synovial fluid (seven), superficial irrigation and débridement (six), insufficient specimen volume (four), antibiotics administered before aspiration (four), interim intraarticular procedure (three), leukemia (two), and history of osteomyelitis (one). These exclusions left 146 knees (142 patients) for analysis, 19 (13.0%) were infected by the criteria below and 127 (87.0%) were uninfected. The mean age at the time of surgery was 66.1 ± 11.7 years (range, 41–95 years), and there were 85 women (58%) and 61 men (42%). Mean time from index procedure to aspiration was longer among infected knees (20.8 ± 7.6 days versus 15.9 ± 10.5 days) (Table 1).
Table 1.
Patient and surgical characteristics
| Characteristic | Infected (n = 19) | Noninfected (n = 127) | P value | Overall (n = 146) |
|---|---|---|---|---|
| Age (years)* | 66.7 ± 10.7 [61.5, 71.8] | 66.0 ± 11.9 [63.9, 68.1] | 66.1 ± 11.7 (41–95) | |
| Aspirated time (days after surgery)* | 20.8 ± 7.6 [17.2, 24.5] | 15.9 ± 10.5 [14.1, 17.7] | 16.5 ± 10.3 (2–42) | |
| Gender (number of patients) | 0.3038 | |||
| Female | 9 (47%) | 76 (60%) | 85 (58%) | |
| Male | 10 (53%) | 51 (40%) | 61 (42%) |
* Values are expressed as mean ± SD, with 95% confidence Interval in brackets and range in parentheses.
Patients were diagnosed with a deep periprosthetic infection if they had positive cultures on solid media or if grossly purulent material was identified intraoperatively. Of the 19 knees in the infected group, 17 had positive cultures on solid media. One patient with negative cultures had gross purulence intraoperatively and was considered infected. One patient was included in the infected group for two separate broth cultures for the same coagulase-negative Staphylococcus species and a positive Gram stain. In the culture-positive group, there were nine Staphylococcus aureus (three methicillin resistant), three S epidermidis, one Enterobacter cloacae, and three unspecified coagulase-negative Staphylococcus species. The remaining two patients both grew Proteus mirabilis and had intraoperative cultures with multiple species (P mirabilis, Enterococcus faecalis, Bacteroides fragilis, and nonhemolytic Streptococci spp in one; P mirabilis and Coryneform spp in the second).
All 19 patients in the infected group underwent subsequent surgery at an average of 22 days (range, 14–40 days) after primary TKA. Reoperations in the infected group included deep irrigation and débridement with polyethylene liner exchange in 12, of whom seven (58%) had no further surgery and the other five underwent repeat irrigation and débridement (two) or resection arthroplasty (three). Four patients had an initial resection arthroplasty and three patients underwent an irrigation and débridement in addition to the revision of at least one fixed component.
There were 14 patients in the uninfected group who underwent reoperation at a mean of 168 days (range, 14 days to 2.4 years). Reoperations included repair for extensor mechanism problems (six patients), single component revision (two patients), and open reduction with fixation for fracture (two patients). An irrigation and débridement with polyethylene liner exchange was performed in four patients suspected of having sepsis but subsequently having no intraarticular purulence or positive cultures.
In an attempt to correct for postoperative hemarthrosis that would be expected after primary TKA, an “adjusted WBC count” was calculated for those with peripheral blood draws [6]. The formula utilized attempted to predict the expected WBC count in the synovial fluid due to hemarthrosis based on the synovial red blood cell (RBC) count and the ratio of WBC count to RBC count in the peripheral blood. This predicted value was then subtracted from the absolute synovial WBC count observed at the time of aspiration to calculate the “adjusted WBC count.” Thus, the formula is: adjusted WBC = aspirated WBC – ([WBCblood/RBCblood] × RBCfluid).
Synovial fluid WBC count, percentage of polymorphonuclear cells (%PMN), CRP levels, ESR values, culture results, organisms, and sensitivities were evaluated within this cohort. Normally distributed univariate data were analyzed using t tests or ANOVA. Categorical data were analyzed using the chi square test for dichotomous data. Predictive models were fit using a stepwise logistic regression process to determine optimal variable selection. Youden’s J statistic was used to select sets of optimal cutoff values, with final judgment based on clinically acceptable levels of sensitivity and specificity. Receiver operating characteristic (ROC) curves and the associated area under the curve (AUC) measure were used to further examine the fit and clinical applicability of the predictive models. SAS® Version 9.1.3 (SAS Institute Inc, Cary, NC) was used for all analysis.
Results
The synovial WBC count, %PMN, and CRP values were all elevated in the infected group compared to the noninfected group. The ESR values were not different between the two groups (Table 2). All patients diagnosed with infection had a synovial WBC count of 10,700 cells/μL or more, except for one patient. All patients diagnosed as noninfected had a synovial WBC count of less than 27,800 cells/μL, except for one patient.
Table 2.
Summary statistics for the diagnostic test measures
| Diagnostic test measure | Infected | Noninfected | P value |
|---|---|---|---|
| ESR (mm/hour) | 80 ± 29 (38–140) | 75 ± 30 (1–140) | 0.4626 |
| CRP (mg/dL) | 171 ± 127 (29–490) | 88 ± 75 (4–382) | 0.0042* |
| Synovial fluid %PMN | 89.6 ± 20.6 (6–99) | 76.9 ± 21.2 (5–100) | 0.0314* |
| Synovial fluid WBC count (cells/μL) | 92,600 ± 127,000 (3520–570,000) | 4200 ± 5700 (0–41,000) | < 0.0001* |
Values are expressed as mean ± SD, with range in parentheses; the step-down Bonferroni method was used to control for error inflation; * significantly different at the 0.05 level with step-down Bonferroni correction method; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; %PMN = percentage of polymorphonuclear cells; WBC = white blood cell.
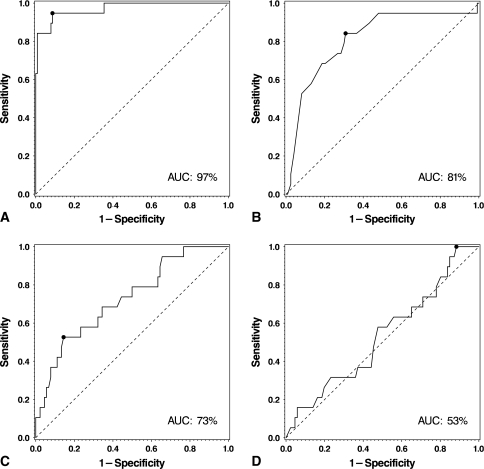
ROC curve analysis demonstrated the synovial fluid WBC count was the best test for diagnosis of acute infection (AUC = 97%; Fig. 1). We identified two potential cutoff values for the synovial fluid WBC count. With the threshold set at 10,700 cells/μL, acute infection could be diagnosed with a sensitivity, specificity, PPV, and NPV of 95%, 91%, 62%, and 99%, respectively. With the threshold set at 27,800 cells/μL, the sensitivity, specificity, PPV, and NPV were 84%, 99%, 94%, and 98%, respectively (Table 3).
Fig. 1A–D.
(A) A ROC curve for the synovial WBC count is shown, with an AUC of 97%. A cutoff value of 10,700 cells/μL demonstrates a 95% sensitivity, 91% specificity, 62% PPV, and 99% NPV. A cutoff value of 27,800 cells/μL would demonstrate an 84% sensitivity, 99% specificity, 94% PPV, and 98% NPV. (B) A ROC curve for %PMN is shown, with an AUC of 81%. A cutoff value of 89% demonstrates an 84% sensitivity, 69% specificity, 29% PPV, and 97% NPV. (C) A ROC curve for serum CRP levels is shown, with an AUC of 73%. A cutoff value of 166 mg/L demonstrates a 53% sensitivity, 86% specificity, 43% PPV, and 90% NPV. (D) A ROC curve for ESR is shown, with an AUC of 51%. This test had no strength in diagnosing acute infection postoperatively.
Table 3.
Evaluation of diagnostic test measures
| Diagnostic test measure | Sensitivity* | Specificity* | Positive predictive Value* | Negative predictive Value* |
|---|---|---|---|---|
| ESR | ||||
| Threshold ≥ 120 mm/hour | 16% [9%, 23%] | 94% [90%, 99%] | 38% [28%, 47%] | 84% [76%, 91%] |
| Threshold ≥ 38 mm/hour | 100% [100%, 100%] | 12% [5%, 18%] | 20% [12%, 28%] | 100% [100%, 100%] |
| CRP | ||||
| Threshold ≥ 166 mg/dL | 53% [43%, 62%] | 86% [79%, 92%] | 43% [34%, 53%] | 90% [84%, 95%] |
| Threshold ≥ 95 mg/dL | 68% [60%, 77%] | 66% [57%, 74%] | 30% [21%, 38%] | 91% [85%, 96%] |
| Synovial fluid %PMN | ||||
| Threshold > 89% | 84% [78%, 90%] | 69% [62%, 77%] | 29% [22%, 37%] | 97% [94%, 100%] |
| Synovial fluid WBC count | ||||
| Threshold ≥ 10,700 cells/μL | 95% [91%, 98%] | 91% [87%, 96%] | 62% [54%, 70%] | 99% [98%, 100%] |
| Threshold ≥ 27,800 cells/μL | 84% [78%, 90%] | 99% [98%, 100%] | 94% [90%, 98%] | 98% [95%, 100%] |
| Adjusted synovial fluid WBC count | ||||
| Threshold ≥ 10,536 cells/μL | 94% [90%, 99%] | 92% [87%, 97%] | 70% [61%, 78%] | 99% [97%, 100%] |
*95% confidence interval in brackets; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; %PMN = percentage of polymorphonuclear cells; WBC = white blood cell.
There did not appear to be any improvement in diagnostic performance if these tests were used in combination. The synovial WBC count provided the greatest sensitivity, specificity, PPV, and NPV and these parameters were not enhanced when combined with the differential or CRP data.
Adjusting the synovial fluid WBC count to correct for hemarthrosis during a “bloody aspirate” did not provide any improvement in diagnostic accuracy (Table 3).
Discussion
With no gold standard test to diagnose a periprosthetic knee infection, the evaluation of a potentially infected TKA in the acute postoperative period is often a difficult process. The aspirated synovial WBC count, differential, CRP, and ESR have proven reliable diagnostic tools in evaluating acute hematogenous and chronic infections [3, 7, 10, 15, 26]. We determined whether these four tests were different in infected compared to noninfected TKAs in the early postoperative period, and if so, the optimal values for each of these tests in diagnosing infection.
One of the limitations of this study is its retrospective nature; however, relatively rare events such as TKA infection in the first 6 weeks after surgery (0.16% incidence in this series) are difficult to study in a prospective manner and are often better suited for retrospective analyses. Second, the multisurgeon patient population may introduce inconsistency in determining which patients were deemed potentially infected when deciding to aspirate the knee. High-volume surgeons at high-volume centers cared for the majority of these patients, all with substantial experience managing complications after TKA. Moreover, the multisurgeon and multicenter design may mitigate single surgeon or institution biases. Finally, the criteria used to diagnose infection (positive culture on solid media and/or gross purulence) may have introduced bias as alterations in the definition of infection will inevitably alter the diagnostic performance of a given test. We chose to define infection in our cohort by a set of particularly stringent criteria. As a result, it is possible some cases of occult infection in our cohort were incorrectly classified as uninfected, but our goal in developing the present criteria was to ensure our diagnosis of infection was unequivocal and highly specific.
In an attempt to stratify the risk of infection for specific synovial WBC count and %PMN, several studies have aimed to establish a cutoff range for the normal values after TKA. Della Valle et al. [3] demonstrated a WBC count of greater than 3000 cells/μL yielded a sensitivity of 100%, specificity of 98%, and accuracy of 99% in diagnosing infection. Mason et al. [15] recommended a WBC count of greater than 2500 cells/μL and %PMN of greater than 60% were highly suggestive of infection. More recently in a multicenter review of 429 knees revised for multiple reasons including infection, Ghanem et al. [7] observed the optimal cutoff values for WBC count and %PMN were more than 1100 cells/μL and more than 64%, respectively. This study is consistent with this previous work in demonstrating elevated synovial WBC, differential, and CRP in the infected TKA but failed to demonstrate different values in the ESR (Table 4). Traditional serum markers of inflammation, such as the ESR and CRP levels, are expected to be elevated in the postoperative period [3, 13]. We found elevated serum CRP levels in the infected group, while the ESR values were not. This may correspond to the observation that CRP levels tend to normalize at a faster rate after an inflammatory-generating insult than ESR [24].
Table 4.
Previously reported cutoff values for diagnosis of periprosthetic joint infection
| Study | WBC (cells/μL) | %PMN | CRP (mg/L) | ESR (mm/hour) | Population | Time from index surgery (years)* |
|---|---|---|---|---|---|---|
| Spangehl et al. [25] | 50,000† | 80† | 10† | 30† | 202 hips (35 infected) | Mentioned anecdotally, up to 11 years |
| Mason et al. [15] | 2500 cells/mL‡ | 60 | 86 knees (36 infected) | Not mentioned | ||
| Trampuz et al. [26] | 1700 | 65 | 133 knees (34 infected) | > 6 months | ||
| Parvizi et al. [17] | 1760 | 73 | 145 knees (78 infected); | Not mentioned | ||
| Trampuz et al. [27] | 1700† | 65† | 10† | 30† | 331 joints (207 knees, 124 hips) (79 infected) | Not mentioned |
| Della Valle et al. [3] | 3000 cells/mL‡ | 65† | 10† | 30† | 94 knees (41 infected) | Not mentioned |
| Nilsdotter-Augustinsson et al. [16] | 1700† | 10† | 30† | 85 knees (25 infected) | Uninfected: 9 (1–22) Infected: 3 (0.2–16) | |
| Ghanem et al. [7] | 1100 | 64 | 10† | 30† | 429 knees (161 infected) | 1.2 (0.1 –7.8) |
| Schinsky et al. [23] | 4200 cells/mL‡ | 80 | 10† | 30† | 201 hips (55 infected) | Uninfected: 8 Infected: 4.5 (including 7 with < 6 weeks) |
| Parvizi et al. [18] | 1100 | 64 | 10† | 30† | 296 knees (116 infected) | Not mentioned |
| Ghanem et al. [5] | 20.5 | 31 | 479 hips (127 infected) | Not mentioned | ||
| Bedair et al. [current study] | 27,800 | 89 | 95 | 146 knees (19 infected) | < 6 weeks |
Tabulated results from previous studies that have investigated various cutoff points for differentiating between septic and aseptic failure of hip or knee arthroplasty based on serologic and synovial fluid analyses; not included are studies performed in populations without an implant in place or in which infected patients were excluded; we include here several studies which, although they do not report independently derived cutoff values, have described their own institutional experience with previously defined thresholds; *values are expressed as mean or mean, with range in parentheses; †values not based on independent receiver operating characteristic analysis performed for the purpose of the study in question but on thresholds set by prior studies; in the case of Spangehl et al. [25], thresholds for WBC counts and neutrophil percentages were established empirically by earlier authors for the differentiation of septic arthritis, rheumatoid arthritis, crystalline arthritis, and osteoarthritis in the absence of a prosthetic implant; ‡units given in these cases are as reported in source literature; WBC = white blood cell; %PMN = percentage of polymorphonuclear cells; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate.
The analysis of this group of patients demonstrates the synovial WBC count remains an important test even in the early postoperative period, albeit at a threshold higher than previously reported for patients with late infections. During the healing process after a primary TKA, the normal inflammatory cascade and postoperative hemarthrosis will likely create an elevated level of WBCs and perhaps altered differential in the synovial fluid. If previously described criteria were applied to these patients, unnecessary procedures would likely have been performed [7, 10, 15, 26]. In our cohort of uninfected patients, the mean WBC count and %PMN in the early postoperative period were 4200 cells/mL and 76.9%, respectively, much higher than values reported in septic knees at later time periods after arthroplasty. Kersey et al. [10] found, in knees undergoing revision for aseptic failure, the mean WBC count and %PMN were 782 cells/μL and 13%, respectively. It is important to note many of the WBC counts observed in patients who were not infected in this study were higher than standard cutoff values for chronic infection.
In this group of patients, knees with a synovial WBC count of greater than 27,800 cells/μL could be diagnosed with infection with a certainty of 94%; this cutoff value had an even stronger NPV of 98% (ie, 98% of knees with WBC counts of less than 27,800 cells/μL were uninfected). If the threshold for diagnosing infection was decreased to greater than 10,700 cells/μL, the NPV increased to 99%, but the PPV was reduced to 62%. Interestingly, the WBC counts we observed in the acute postoperative period were nearer to those in septic arthritis of native knees [14]. Tests for %PMN and CRP were less valuable in diagnosing early infection, but both tests’ optimal thresholds demonstrated high NPV (97% and 91%, respectively) and could potentially be used to rule out infection but are less useful in diagnosing infection.
In an attempt to improve diagnostic performance, the four laboratory tests were combined in differing permutations as has been demonstrated in previous studies [7]. There was, however, no benefit to combining these tests, which is likely due to the synovial WBC count demonstrating very high values of sensitivity and specificity that were not improved upon by the addition of other tests. Because of the paucity of data points of synovial WBC count of less than 10,700 cells/μL in the infected group (n = 1) and greater than 27,800 cells/μL in the noninfected group (n = 1), no statistical conclusions could be drawn when combining %PMNs and CRP in this range.
We expected many patients would still have large hemarthroses, as is observed in many knee aspirations in the early postoperative period, and this could confound the data obtained with regard to the WBC count. The formula described by Ghanem et al. [6] applied to the joint fluid analysis in an attempt to account for the WBCs in a “bloody tap” does not appear to offer superior diagnostic performance over the absolute number of WBCs observed in this case (Table 3). While the reason for this finding is unclear, it may be in part due to the large difference in average WBC counts between the infected and noninfected groups. The inflammatory response due to infection may generate an extremely high concentration of WBCs that would proportionally overwhelm the normal concentration of RBCs in the joint fluid.
Based on the observations of this study, we suggest a synovial WBC count of greater than 27,800 cells/μL is the single best predictor of periprosthetic knee infection in the early postoperative period. The use of secondary laboratory results such as %PMN (< 89%) in the synovial fluid and serum CRP levels (< 95 mg/L) will likely help confirm the absence of infection in cases in which the synovial WBC count is inconclusive. Establishing high specificity thresholds for these values should eliminate unnecessary surgery. If none of these parameters assist in generating a definitive diagnosis, the treating physician may always rely on synovial fluid cultures, although this may delay treatment.
Footnotes
Dr. Parvizi is a consultant for Stryker Orthopaedics (Mahwah, NJ). Dr. Della Valle is a consultant for Smith and Nephew Inc (Memphis, TN), Biomet Inc (Warsaw, IN), and Kinamed Inc (Camarillo, CA) and receives research/institutional support from Zimmer Inc (Warsaw, IN).
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Rush University Medical Center and Thomas Jefferson University School of Medicine.
References
- 1.Bilgen O, Atici T, Durak K, Karaeminogullari, Bilgen MS. C-reactive protein values and erythrocyte sedimentation rates after total hip and total knee arthroplasty. J Int Med Res. 2001;29:7–12. doi: 10.1177/147323000102900102. [DOI] [PubMed] [Google Scholar]
- 2.Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688–691. doi: 10.1302/0301-620X.86B5.14887. [DOI] [PubMed] [Google Scholar]
- 3.Della Valle CJ, Sporer SM, Jacobs JJ, Berger RA, Rosenberg AG, Paprosky WG. Preoperative testing for sepsis before revision total knee arthroplasty. J Arthroplasty. 2007;22((6 Suppl 2)):90–93. doi: 10.1016/j.arth.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86:963–974. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Ghanem E, Antoci V, Jr, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis. 2009;13:e444–e449. doi: 10.1016/j.ijid.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Ghanem E, Houssock C, Pulido L, Han S, Jaberi FM, Parvizi J. Determining “true” leukocytosis in bloody joint aspiration. J Arthroplasty. 2008;23:182–187. doi: 10.1016/j.arth.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Ghanem E, Parvizi J, Burnett RS, Sharkey PF, Keshavarzi N, Aggarwal A, Barrack RL. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am. 2008;90:1637–1643. doi: 10.2106/JBJS.G.00470. [DOI] [PubMed] [Google Scholar]
- 8.Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111–122. [PubMed] [Google Scholar]
- 9.Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27:1745–1752. [PubMed] [Google Scholar]
- 10.Kersey R, Benjamin J, Marson B. White blood cell counts and differential in synovial fluid of aseptically failed total knee arthroplasty. J Arthroplasty. 2000;15:301–304. doi: 10.1016/S0883-5403(00)90578-3. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Karrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(Suppl 3):144–151. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 13.Larsson S, Thelander U, Friberg S. C-reactive protein (CRP) levels after elective orthopedic surgery. Clin Orthop Relat Res. 1992;275:237–242. [PubMed] [Google Scholar]
- 14.Li SF, Cassidy C, Chang C, Gharib S, Torres J. Diagnostic utility of laboratory tests in septic arthritis. Emerg Med J. 2007;24:75–77. doi: 10.1136/emj.2006.037929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason JB, Fehring TK, Odum SM, Griffin WL, Nussman DS. The value of white blood cell counts before revision total knee arthroplasty. J Arthroplasty. 2003;18:1038–1043. doi: 10.1016/S0883-5403(03)00448-0. [DOI] [PubMed] [Google Scholar]
- 16.Nilsdotter-Augustinsson A, Briheim G, Herder A, Ljunghusen O, Wahlstrom O, Ohman L. Inflammatory response in 85 patients with loosened hip prostheses: a prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta Orthop. 2007;78:629–639. doi: 10.1080/17453670710014329. [DOI] [PubMed] [Google Scholar]
- 17.Parvizi J, Ghanem E, Menashe S, Barrack RL, Bauer TW. Periprosthetic infection: what are the diagnostic challenges? J Bone Joint Surg Am. 2006;88(Suppl 4):138–147. doi: 10.2106/JBJS.F.00609. [DOI] [PubMed] [Google Scholar]
- 18.Parvizi J, Ghanem E, Sharkey P, Aggarwal A, Burnett RS, Barrack RL. Diagnosis of infected total knee: findings of a multicenter database. Clin Orthop Relat Res. 2008;466:2628–2633. doi: 10.1007/s11999-008-0471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. doi: 10.1097/00003086-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 21.Rand JA, Ilstrup DM. Survivorship analysis of total knee arthroplasty: cumulative rates of survival of 9200 total knee arthroplasties. J Bone Joint Surg Am. 1991;73:397–409. [PubMed] [Google Scholar]
- 22.Rand JA, Trousdale RT, Ilstrup DM, Harmsen WS. Factors affecting the durability of primary total knee prostheses. J Bone Joint Surg Am. 2003;85:259–265. doi: 10.2106/00004623-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90:1869–1875. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 24.Shah K, Mohammed A, Patil S, McFadyen A, Meek RM. Circulating cytokines after hip and knee arthroplasty: a preliminary study. Clin Orthop Relat Res. 2009;467:946–951. doi: 10.1007/s11999-008-0562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 28.Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, Varin F. A multimodal analgesia protocol for total knee arthroplasty: a randomized, controlled study. J Bone Joint Surg Am. 2006;88:282–289. doi: 10.2106/JBJS.E.00173. [DOI] [PubMed] [Google Scholar]