Abstract
Background
Patellar crepitus (PC) is reported in up to 14% of subjects implanted with cruciate-substituting total knee arthroplasty (TKA). Numerous etiologies of PC have been proposed.
Questions/purposes
We determined when painful PC typically occurs postoperatively and compared patients undergoing primary TKA who developed painful PC requiring subsequent surgery with a matched group without this complication to identify clinical, radiographic, and surgical variables associated with this complication.
Methods
From the databases of two institutions (greater than 4000 TKAs), we identified 60 patients who required surgery for painful PC from 2002 to 2008. This group was then compared with an identified control group of 60 TKA subjects without PC who were matched for the key variables of age, gender, and body mass index to determine clinical, radiographic, and surgical factors associated with the development of PC.
Results
The mean time to presentation of PC was 10.9 months. The incidence of PC correlated with a greater number of previous knee surgeries, decreased patellar component size, decreased composite patellar thickness, shorter preoperative and postoperative patellar tendon length, increased posterior femoral condylar offset, use of smaller femoral components and thicker tibial polyethylene inserts, and placement of the femoral component in a flexed posture.
Conclusions
Many of the factors associated with an increased incidence of postoperative PC such as shortened patellar tendon length, use of smaller patellar components, decreased patellar composite thickness, and increased posterior femoral condylar offset may all increase quadriceps tendon contact forces against the superior aspect of the intercondylar box, increasing the risk of fibrosynovial proliferation and entrapment within the intercondylar region of the femoral component. Based on these findings, the authors recommend use of larger patellar components when possible, avoid oversection of the patella or increasing posterior femoral condylar offset, and advising patients preoperatively who have had previous knee surgery or demonstrate a shortened patellar tendon length of an increased risk of development of postoperative patellar crepitus.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
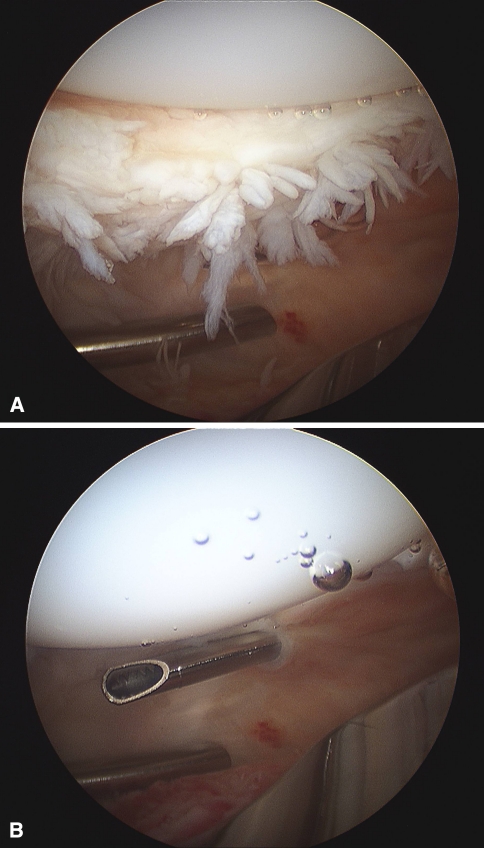
Long-term reports of subjects implanted with posterior-stabilized (PS) total TKA devices have demonstrated excellent pain relief and high functional scores [4]. One notable complication unique to implantation of a PS TKA is patellar crepitus or clunk syndrome. The incidence of this phenomenon ranges from 0% to 14% [1, 3, 6, 7, 9–12, 14–16]. The symptoms of patellar crepitus are secondary to peripatellar fibrosynovial hyperplasia at the junction of the superior pole of the patella and the distal quadriceps tendon. This fibrosynovial hyperplasia may become entrapped within the intercondylar box of the PS femoral component and cause painless or painful crepitation or clunk. Patellar crepitus is defined as a palpable, continued grinding sensation detected in the region of the distal quadriceps tendon and most commonly observed during terminal knee extension. Patellar clunk occurs when the fibrosynovial hyperplasia becomes transiently entrapped within the intercondylar box, limiting patellar excursion until the fibrosynovial hyperplasia escapes the intercondylar box, resulting in a sudden superior motion (clunk) of the patellar component, which may be audible in some cases. In some cases, patellar crepitus is symptomatic enough to merit surgical treatment with either arthroscopic or open débridement of the hyperplasia [1, 9, 10, 12–14] (Fig. 1).
Fig. 1A–B.
The intraoperative arthroscopic photograph demonstrates peripatellar fibrosynovial proliferation on the posterior aspect of the quadriceps tendon in (A) a subject with symptomatic patellar crepitus and (B) after arthroscopic débridement of the fibrosynovial tissue (B).
One reported cause of patellar crepitus has been femoral component design [1, 3, 6, 10, 12–14], particularly those with a “boxy” sagittal geometry, a sharp transition zone at the superior aspect of the intercondylar box, and a higher intercondylar box ratio [6]. Others suggest patellar crepitus occurs as a result of intraoperative technical errors such as anterior placement of the tibial tray [5, 14, 16], elevation of the joint line [1, 5, 9], altering patellar thickness [5, 9], proximal overhang of the patellar component [9], postoperative patellar baja [5, 16], or increased postoperative knee flexion [15].
The purpose of this study was to conduct a retrospective matched case-control analysis to identify when patellar crepitus typically occurs postoperatively as well as to determine the patient clinical, radiographic, and surgical variables (Table 1) that increase the risk to patients of developing painful, symptomatic patellar crepitus after PS TKA.
Table 1.
Variables analyzed
| Patient clinical variables* | Radiographic variables* | Surgical variables† |
|---|---|---|
| Gender | Preoperative alignment | Previous knee surgeries |
| Age | Postoperative alignment | Tibial component size |
| Height | Preoperative patellar tendon length | Femoral component size |
| Body weight | Postoperative patellar tendon length | Polyethylene thickness |
| Body mass index | Preoperative Insall-Salvati ratio | Patellar component size |
| Preoperative knee motion | Postoperative Insall-Salvati ratio | Patellar component shape |
| Postoperative knee motion | Femoral component flexion | Lateral release required |
| Posterior femoral offset | Polyethylene bearing type | |
| Posterior tibial slope | ||
| Posterior tibial offset | ||
| Anterior tibial offset | ||
| Preoperative patellar thickness | ||
| Postoperative patellar thickness | ||
| composite patellar thickness | ||
| Patellar tilt | ||
| Joint line |
* Patient clinical and radiographic variables were treated as continuous variables; †surgical variables were treated as categorical variables.
Patients and Methods
We searched the surgical databases of two institutions to identify all patients who had surgery (knee arthroscopy or arthrotomy) for symptomatic patellar crepitus or clunk after TKA from 2002 to 2008. During this time period, greater than 4000 TKA procedures were performed at the two institutions. From the database and the medical records we identified 60 patients meeting these criteria. Sixteen of these subjects demonstrated patellar clunk, whereas the remaining 44 exhibited patellar crepitus. All subjects were implanted with the same femoral component design (PFC Sigma Posterior Cruciate Substituting; DePuy, Warsaw, IN). Subjects were entered into the study group based on the time of diagnosis of patellar crepitus, which ranged from 4 to 27 months. No patients were recalled specifically for this study; all data were obtained from office records and the research databases of the two participating institutions. We obtained Institutional Review Board approval before the initiation of this case-control study.
We then searched the databases to identify 60 patients with a well-functioning TKA matched for age (± 3 years), gender, and body mass index (± 3 points) that served as a matched control group against which the study group subjects were compared (Table 2). Minimum followup required for inclusion into the control group was 12 months (mean, 31.3 months; range, 12–89 months). Any time a patient with patellar crepitus requiring operative treatment had a well-functioning contralateral TKA without patellar crepitus and had at least 12 months followup, their contralateral limb was used as their control. Determination of a well-functioning TKA was based on patient response to questions and physical examination findings routinely collected in the authors’ research database and was defined as one that was implanted into a subject who reported they were satisfied with their operative result, did not report residual pain or stiffness, exhibited a Knee Society score in the excellent category, and lacked evidence of instability or patellar crepitus during physical examination.
Table 2.
Patient demographics
| Parameter | Patellar crepitus group | Control group | |
|---|---|---|---|
| Gender (male/female) | 18/42 | 18/42 | |
| Age (years) | Mean | 62.3 | 62.2 |
| SD | 10.8 | 10.6 | |
| Range | 23–84 | 36–82 | |
| 95% confidence interval | 59.50–65.07 | 59.51–64.96 | |
| Height (inches) | Mean | 66.3 | 66.5 |
| SD | 3.9 | 3.4 | |
| Range | 59–76 | 60–74 | |
| 95% confidence interval | 65.25–67.28 | 65.65–67.39 | |
| Body weight (pounds) | Mean | 186.2 | 187.4 |
| SD | 45.5 | 41.5 | |
| Range | 109–295 | 109–290 | |
| 95% confidence interval | 174.44–197.96 | 176.66,198.20 | |
| Body mass index (kg/m2) | Mean | 29.8 | 29.9 |
| SD | 7.1 | 7.0 | |
| Range | 19.9–51.4 | 19.9–49.0 | |
| 95% confidence interval | 27.96–31.63 | 28.10–31.71 | |
After matching, we reviewed clinical records and operative reports and conducted a radiographic analysis of all subjects in both groups. Data collected from the clinical charts and research database, aside from demographic data, were history of previous surgeries, preoperative and postoperative ROM, time to presentation of symptoms, and whether the subject’s symptoms were patellar crepitus or clunk. Surgical data collected from the operative reports were tibial, femoral, and patellar component size; patellar button shape (oval versus round); thickness and type (fixed versus mobile) of tibial polyethylene bearing; and the need for lateral retinacular release.
One person at each contributing institution (DRJ, BDS) reviewed all pre- and postoperative radiographs. Identical instruction was provided to each evaluator of the radiographs in an attempt to lessen interobserver variability of measurements. Radiographs were measured to determine femoral-tibial alignment, modified Insall-Salvati ratio [7], modified patellar tendon length/patellar height [7], patellar component thickness, and postoperative patellar tilt [15]. The composite patellar thickness was also calculated by adding the postoperative patellar thickness to the thickness of the patellar component. Also measured were the posterior femoral condylar offset [2], flexion or extension of the femoral component, anterior or posterior tibial tray offset [5], posterior tibial slope, and level of the joint line. Changes in the modified Insall-Salvati ratio, modified patellar tendon length/patellar height, posterior condylar offset, and joint line [1] were also calculated. All radiographic measurements were performed using a goniometer for celluloid images for radiographs taken before 2006 and digital measurements using preinstalled software on a picture archiving communication system for images obtained in 2006 and later. Alignment was measured from the angle created between the long axes of the tibia and femur on an AP long-leg film. All other radiographic measurements were performed on 18-inch films. Modified patellar tendon length was measured on a lateral radiograph measuring from the most proximal point of the tibial tubercle to the most distal point of the articular facet of the patella. Modified patellar height was measured on a lateral radiograph from the most distal point of the articular facet to the most proximal point of the articular facet. The modified Insall-Salvati ratio was calculated by dividing the modified tendon length by the modified patellar height. The distance between the line paralleling the posterior femoral cortex and the most posterior aspect of the femoral condyle on the lateral radiograph was used to determine the posterior femoral condylar offset.
Univariate statistical analysis was initially conducted to identify the possible variables for a multivariate logistic regression model. For categorical variables, this was accomplished by analyzing the differences in proportions using a two-tailed Fisher’s exact test and checking for the strength of association by calculation of an odds ratio at a 95% confidence level. For the odds ratio calculations, the referent group was always the one with the highest frequency of occurrence in the control group. The continuous variables were first checked for normality using the Shapiro-Wilk W test. Significant differences in the continuous variables across the case and control groups were identified using either the Student’s t-test or the Mann-Whitney U test at a 0.05 level of significance depending on the results of the normality tests. Variables included in the multivariate models consisted of those previously reported in the literature to be associated with an increased risk of patellar crepitus, those with clinical and/or surgical plausibility, those with a correlation magnitude of less than 0.6 with other variables, and those with p values of < 0.25 obtained from the univariate analyses. The multivariate logistic regression was performed using a mixed backward and forward elimination scheme. After identification of the main effects of the logistic regression models, all meaningful two-way interactions were tested in the models. The final model(s) were checked for goodness of fit with the Hosmer and Lemeshow test [8] and by collinearity and residuals diagnostics to ensure they were well specified and fit the data. Only the best multivariate model has been reported here. All statistical tests were carried out in the commercially available JMP® Statistical DiscoveryTM software (SAS Institute Inc, Cary, NC).
Results
The average time to presentation of symptoms in the patellar crepitus group was 10.9 months (range, 4–27 months), whereas the average followup in the control group was 31.3 months (range, 12–89 months).
Patient and radiographic variables found to increase the risk of developing patellar clunk or crepitus included a reduced preoperative (54.54 mm versus 57.90 mm; p = 0.0089) or postoperative (54.56 mm versus 58.10 mm; p = 0.0089) patellar tendon length, a thinner postoperative composite patellar component thickness (22.33 mm versus 23.24 mm; p = 0.0219), and an increase in posterior femoral condylar offset (1.27 mm versus 0.17 mm; p = 0.023; Table 3). No differences were noted in other continuous variables, including preoperative ROM or alignment, postoperative ROM or alignment, preoperative or postoperative Insall-Salvati ratio, degree of femoral component flexion, tibial component slope or offset, preoperative or postoperative patellar thickness, postoperative patellar component tilt, or change in joint line level (Table 3).
Table 3.
Univariate analysis of surgical variables
| Parameter | Cases (N = 60) | Control subjects (N = 60) | Odds ratio (95% confidence interval) | p Value |
|---|---|---|---|---|
| Number of previous surgeries | ||||
| None (0) | 22 (37%) | 42 (70%) | 1.0 (referent) | |
| 1 | 21 (35%) | 13 (22%) | 3.1 (1.3, 7.3) | 0.0111 |
| > 1 | 17 (28%) | 5 (8%) | 6.5 (2.1, 19.9) | 0.0005 |
| Tibial component size | ||||
| < 3 | 18 (30%) | 14 (23%) | 1.5 (0.6, 3.4) | 0.5185 |
| 3 to 4 | 31 (52%) | 35 (58%) | 1.0 (referent) | |
| > 4 | 11 (18%) | 11 (18%) | 1.1 (0.4, 3.0) | 0.9999 |
| Femoral component size | ||||
| < 3 | 14 (23%) | 6 (10%) | 2.8 (1.0, 8.1) | 0.0777 |
| 3 to 4 | 35 (58%) | 42 (70%) | 1.0 (referent) | |
| > 4 | 11 (18%) | 12 (20%) | 1.1 (0.4, 2.8) | 1.0 |
| Polyethylene thickness (mm) | ||||
| < 12.5 | 28 (47%) | 28 (47%) | 1.0 (referent) | |
| 12.5 | 20 (33%) | 27 (45%) | 0.7 (0.3, 1.6) | 0.5525 |
| > 12.5 | 12 (20%) | 4 (7%) | 3. (0.9, 10.4) | 0.0930 |
| Patellar component size (mm) | ||||
| 32 | 14 (23%) | 4 (7%) | 5.44 (1.5, 19.1) | 0.0111 |
| 35 | 24 (40%) | 19 (32%) | 1.96 (0.8, 4.6) | 0.1395 |
| 38 | 18 (30%) | 28 (47%) | 1.0 (referent) | |
| 41 | 4 (7%) | 9 (15%) | 0.69 (0.2, 2.6) | 0.7488 |
| Patella component shape | ||||
| Oval | 52 (87%) | 57 (95%) | 1.0 (referent) | |
| Round | 8 (13%) | 3 (5%) | 2.92 (0.7, 11.6) | 0.2043 |
| Lateral release | ||||
| No | 50 (83%) | 55 (92%) | 1.0 (referent) | |
| Yes | 10 (17%) | 5 (8%) | 2.2 (0.7–6.9) | 0.2693 |
| Bearing type | ||||
| Fixed | 9 (15%) | 6 (10%) | 1.0 (referent) | |
| Mobile | 51 (85%) | 54 (90%) | 0.63 (0.2–1.9) | 0.4076 |
Surgical variables that increased the risk of development of patellar crepitus included a history of previous knee surgery (p = 0.011) and reduced patellar component size (p = 0.011) (Table 4). Patients in the patellar crepitus group had experienced 1.18 (95% confidence interval [CI], 0.77–1.60) previous surgical procedures compared with 0.42 (95% CI, 0.23–0.60) previous knee operations in the control group. Sixty-three percent of patients with patellar crepitus had at least one previous surgery compared with 30% of patients in the control group (relative risk [RR] = 2.11, 95% CI = 1.37–3.25; OR = 4.03, 95% CI = 1.88–8.63). The average patellar component size for patients with crepitus was 35.60 mm (95% CI = 34.93–36.27), whereas the average patellar component size in the control group was 37.10 mm (95% CI = 36.49–37.71). In the patellar crepitus group, 63.3% (38 of 60) of patients were implanted with a size 32 mm or 35 mm patellar component implanted compared with 39% in the control group (RR = 1.65, 95% CI = 1.14–2.40; OR = 2.78, 95% CI = 1.33–5.82). A patellar button size of 32 mm was present in 23.3% of the crepitus group compared with only 7% of the control group (RR = 3.50, 95% CI = 1.22–10.02; OR = 4.26, 95% CI = 1.31–13.83). No differences were noted in other surgical variables analyzed, including femoral or tibial component size, patellar component shape (round versus oval), the incidence of lateral retinacular release, or polyethylene bearing type (fixed versus mobile; Table 4).
Table 4.
Univariate analysis of patient clinical and radiographic variables
| Parameter | Patellar crepitus | Control | p value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (range) | Mean ± SD | Median (range) | ||
| Preoperative extension (°) | 4.5 ± 4.0 | 5 (0 to 15) | 6.2 ± 6.2 | 5 (0 to 20) | 0.0843 |
| Preoperative flexion (°) | 117.3 ± 11.5 | 120 (85 to 140) | 117.0 ± 14.1 | 120 (70 to145) | 0.9030 |
| Preoperative alignment (°) | 0.0 ± 8.6 | −3 (−15 to 28) | 1.2 ± 8.6 | −3 (−10 to 21) | 0.4742 |
| Postoperative extension (°) | 0.3 ± 1.0 | 0 (0 to 5) | 0.4 ± 1.2 | 0 (0 to 6) | 0.8135 |
| Postoperative flexion (°) | 122.7 ± 9.1 | 120 (90 to 140) | 122.1 ± 10.6 | 120 (85 to150) | 0.7122 |
| Postoperative alignment (°) | 4.9 ± 1.0 | 5 (3 to 7) | 5.0 ± 1.2 | 5 (2 to 7) | 0.4236 |
| Preoperative patellar tendon length (mm) | 54.5 ± 6.6 | 55.0 (40.0 to 70.0) | 58.0 ± 7.4 | 57 (44 to 82) | 0.0089 |
| Preoperative Insall-Salvati ratio | 1.5 ± 0.2 | 1.5 (0.9 to 1.9) | 1.5 ± 0.2 | 1.5 (1.2 to 2.0) | 0.1493 |
| Postoperative patellar tendon length (mm) | 54.6 ± 6.7 | 53.5 (39.6 to 71.0) | 58.1 ± 7.8 | 58 (43 to 82) | 0.0089 |
| Postoperative Insall-Salvati ratio | 1.5 ± 0.2 | 1.5 (0.8 to 2.2) | 1.5 ± 0.2 | 1.5 (1.1 to 2.0) | 0.2142 |
| Femoral component flexion (°) | 1.5 ± 2.0 | 1.1 (−3.0 to 7.0) | 0.8 ± 3.0 | 0 (−5 to10) | 0.1262 |
| Posterior femoral offset (mm) | 1.3 ± 2.7 | 1 (−5 to 10) | 0.2 ± 2.3 | 0 (−5 to 4) | 0.0230 |
| Posterior tibial slope (°) | 2.8 ± 2.3 | 3 (−4 to 8) | 3.2 ± 2.2 | 3 (−2 to 9) | 0.343 |
| Posterior tibial offset (mm) | 1.8 ± 1.6 | 2 (0 to 6) | 2.0 ± 1.7 | 2 (0 to 6) | 0.3889 |
| Anterior tibial offset (mm) | 0.9 ± 1.6 | 0 (−2 to 8) | 0.8 ± 1.3 | 0 (−1 to 4) | 0.7406 |
| Preoperative patellar thickness (mm) | 20.9 ± 2.8 | 21 (14 to 28) | 21.4 ± 2.1 | 21 (18 to 27) | 0.4118 |
| Postoperative patellar thickness (mm) | 13.6 ± 1.7 | 14 (9 to 17) | 14.1 ± 1.8 | 10 (14 to17) | 0.2710 |
| Composite patellar thickness (mm) | 22.3 ± 2.1 | 22.5 (16.8 to 28.4) | 23.2 ± 2.4 | 23.1 (18.5 to 28.4) | 0.0219 |
| Patellar tilt (°) | 0.5 ± 5.4 | 0 (−12 to 18) | 1.6 ± 4.4 | 0 (−10 to 15) | 0.2456 |
| Change in joint line (mm) | 0.6 ± 3.4 | 0 (−7 to 13) | 0.0 ± 3.5 | 0 (−7 to 9) | 0.4025 |
The best multivariate logistic regression model also indicated patients at increased risk of patellar crepitus include those with previous knee surgery (p = 0.0009), a reduced preoperative tendon length (p = 0.0098), and an increase in postoperative posterior femoral condylar offset (p < 0.0001; Table 5). It also indicated an increased risk of patellar crepitus in those implanted with smaller femoral components (less than size 3; p = 0.0253), thicker polyethylene bearings (greater than 12.5 mm; p = 0.0015), and femoral components positioned in flexion (p = 0.0282). Preoperative patellar tendon length was highly correlated with postoperative tendon length and preoperative and postoperative patellar height. A moderate amount of correlation was seen between the femoral component size and the patellar component size with smaller femoral sizes incorporating smaller patellar components. However, alternate models using the correlated variables performed poorer in the goodness-of-fit tests when compared with the current reported model.
Table 5.
Multivariate logistic regression model for the development of patellar crepitus
| Parameter | Odds ratio | 95% confidence level | p value | |
|---|---|---|---|---|
| Previous surgeries | 1 | 6.2 | 5.9–6.4 | 0.0009 |
| > 1 | 18.2 | 18.0–18.3 | < 0.0001 | |
| Femoral component size < 3 | 4.6 | 4.4–4.7 | 0.0253 | |
| Polyethylene bearing thickness > 12.5 | 12.4 | 12.2–12.6 | 0.0015 | |
| Preoperative patellar tendon length (mm) | 0.9 | 0.8–1.0 | 0.0098 | |
| Femoral component flexion (°) | 1.2 | 1.0–1.5 | 0.0282 | |
| Change in posterior condylar offset (mm) | 1.5 | 1.2–1.9 | < 0.0001 | |
Discussion
Patellar crepitus is a complication unique to PS TKA with an incidence 0% to 14% [1, 3, 6, 9–12, 14–16]. Although the severity of symptoms is highly variable, for some patients, the symptoms can be substantial. The operative treatment options for those deemed disabled enough to merit surgical intervention are arthroscopic or open débridement of the fibrosynovial hyperplasia on the articular surface of the quadriceps tendon. Many factors in the literature have been implicated as a cause of patellar crepitus [1, 3, 6, 9–12, 14–16]. The purpose of the current report was to determine when patellar crepitus occurs postoperatively in addition to evaluating a myriad of clinical, radiographic, and surgical variables that may affect the incidence of postoperative patellar crepitus requiring operative intervention in a control-matched clinical investigation.
There are several important limitations to this study. First, this is a retrospective study that only included analysis of patients with patellar crepitus symptomatic enough to warrant surgery. Consequently, subjects with patellar crepitus symptoms of lesser magnitude were excluded. This method was selected by the authors to allow a focused analysis of those truly having substantial disability from this condition. Second, the operative procedures were performed by multiple surgeons, although all were high-volume, fellowship-trained arthroplasty specialists. Third, only a single femoral component design was evaluated and therefore the current findings may not be applicable to all PS femoral component designs, which may have differing patellofemoral and femoral-tibial condylar geometry and exhibit differing patellofemoral kinematic motion patterns. Finally, a power analysis of each variable was not performed before initiation of the study. Therefore, the volume of subjects analyzed may be insufficient to provide sufficient power for all variables. We therefore cannot dispute other risk factors for patellar crepitus and clunk reported in the literature might not be important.
Femoral component design has been implicated as a cause of patellar clunk [1, 3, 6, 10, 12–14]. One series showed an incidence of patellar clunk of 4% in the Insall-Burstein II PS TKA (Zimmer, Warsaw, IN) with an incidence of 0% in the NexGen Legacy PS TKA (Zimmer) [12]. A 0% incidence of patellar clunk was reported in another larger series of patients implanted with the NexGen Legacy PS TKA at 48-month followup [3]. Another report showed an incidence of 13.8% with the AMK Congruency TKA (DePuy), a 3.8% incidence with the AMK PS TKA (DePuy), and 0% with the PFC Sigma PS TKA (DePuy) [13]. A recent study [6] reported an incidence of patellar clunk to be 13.3% in a cohort of 93 patients (113 TKA) implanted with a PFC Sigma PS RP TKA (DePuy). They compared the intercondylar box height ratio (ratio of the length of the intercondylar box to the AP width of the femoral component; Fig. 2) of this particular femoral component (0.85–0.87) with designs with a historically lower reported incidence of patellar crepitus and clunk and found those with a lower incidence demonstrated intercondylar box ratios of less than 0.7, suggesting femoral components with a higher intercondylar box height are at greater risk for this complication.
Fig. 2A–B.
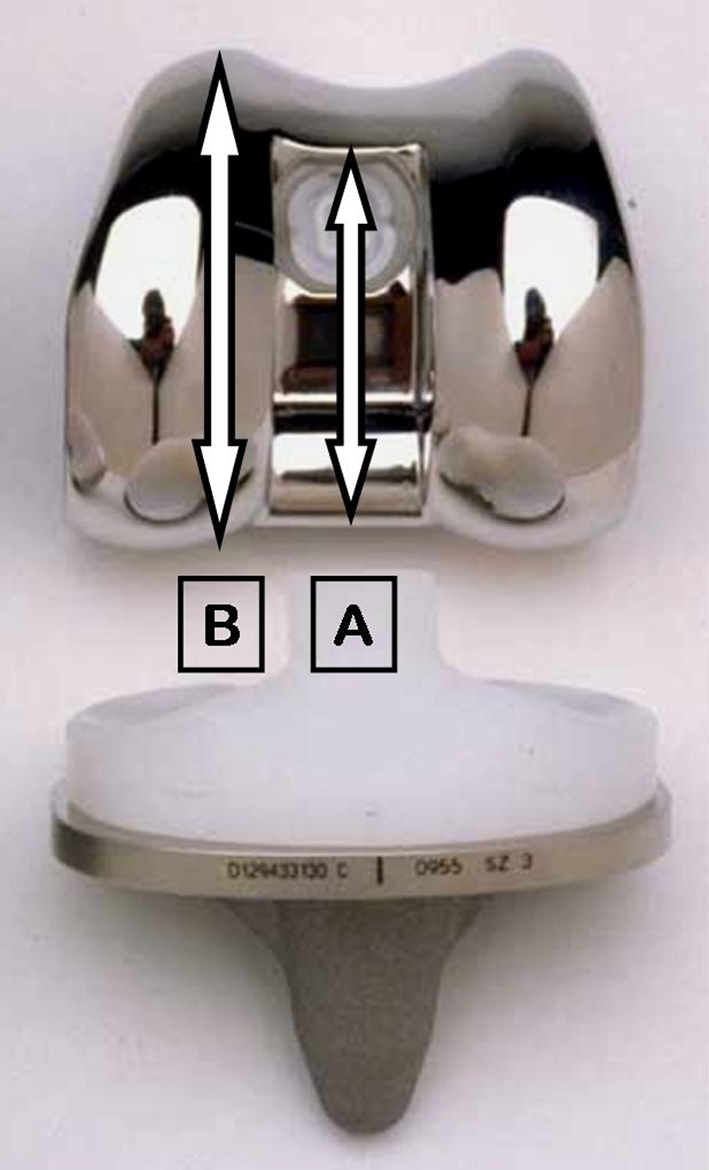
The photograph demonstrates the intercondylar box ratio (A/B) comprised from measurement of the intercondylar box height (A) versus the anteroposterior width (B) of the femoral component.
Decreased postoperative patellar tendon length or Insall-Salvati ratio have been implicated as a cause of patellar crepitus by several authors [1, 5, 9, 14, 16]. Many of these reports also implicate raising the joint line greater than 8 mm resulting in patellar baja increases the risk of patellar crepitus. Our data confirm decreased postoperative patellar tendon length is associated with an increased risk in patellar crepitus. However, unlike other reports, the current analysis determined decreased patellar tendon length preoperatively also increases the risk of developing patellar crepitus. Additionally, use of thicker tibial polyethylene inserts (greater than 12.5 mm) was associated with an increased incidence of patellar crepitus, possibly secondary to the reduction in patellar height typically associated with use of thicker polyethylene inserts. A difference in joint line level between the two groups was not observed, although the joint line was elevated more than 8 mm in only two patients (3%) in those with patellar crepitus.
Other patella and patellar component factors reported in the literature to increase the incidence of patellar crepitus and clunk include patellar tilt [5], increased patellar thickness [1, 5, 9, 14], patellar component placement too superiorly beyond the superior border of the native patella [9], and failure to totally cover the patella during resurfacing with uncovered patellar bone exposed [9, 14]. In contrast, we found patients with patellar crepitus had a nonsignificant trend toward less lateral patellar tilt (0.5° versus 1.6°) and decreased composite patellar thickness (22.33 mm versus 23.24 mm) was present in the crepitus group when compared with the control group. No incidence of patellar component overhang or substantial uncoverage of the lateral patellar facet was noted in the patellar crepitus group.
Anterior femoral component placement and use of an undersized femoral component, thus decreasing the posterior femoral condylar offset, have also been implicated in the development of patellar crepitus [5]. The authors of this study indicate decreasing the posterior femoral offset creates a need to raise the joint line to balance a subsequently loose flexion gap to the extension gap. Contrary to this suggestion, our data indicated no difference in anterior femoral placement of the components (0.92 mm versus 0.85 mm) between the crepitus and control groups and found an increase in posterior condylar offset (1.27 mm versus 0.17 mm) in the patellar crepitus group. Multivariate analysis did show a correlation between use of smaller femoral components (less than size 3) and placement of the femoral component in a flexed posture with the subsequent development of patellar crepitus. We presume femoral component flexion may irritate the distal aspect of the quadriceps tendon as it traverses the proximal aspect of the anterior flange of the femoral component.
In addition to femoral component placement, anterior placement of the tibial component in the sagittal plane [5, 14, 16] has been implicated as a cause of patellar crepitus. In the current series, in both the patellar crepitus (1.71 mm) and control (2.07 mm) groups, the mean tibial component placement was posterior to the center axis of the tibia. The control group did show more posterior placement of the tibial tray, but this trend was not statistically significant.
Lastly, increased postoperative knee flexion has been observed by some to increase the risk of developing patellar crepitus. Schroer et al. [15] compared two cohorts of patients undergoing TKA performed using a traditional surgical exposure versus a minimally invasive subvastus approach and observed higher knee flexion and an increased incidence of patellar clunk in the minimally invasive group. They found patients with crepitus had a mean flexion of 124° compared with 117° in those without crepitus. We found a slight increase in flexion in the patellar crepitus group (122.72° versus 122.00°), although the trend was not statistically significant.
Patellar crepitus is multifactorial in nature. We found previous knee surgery associated with this complication. Although we are unsure of the exact reason for this association, we speculate previous surgery results in increased intraarticular scar formation, reduced soft tissue flexibility, and possibly reduced patellar height in some cases, which may increase tissue tension, increase the risk of soft tissue irritation, and the subsequent risk of development of fibrosynovial proliferation within the knee. For this reason, the authors currently alert patients preoperatively who have had previous knee surgeries of an increased risk for this complication. The association of reduced patellar component size and composite patellar thickness with an increased incidence of patellar crepitus may be secondary to a reduction in offset of the distal aspect of the quadriceps tendon from the superior aspect of the intercondylar notch resulting in irritation of the posterior aspect of the quadriceps tendon in deeper flexion and subsequent proliferation of fibrosynovial tissue, which can become entrapped within the intercondylar notch in deeper knee flexion. For this reason, it is our practice and recommendation to avoid overresection of the native patella and use the largest patellar component available that will not result in component overhang.
Our report confirms decreased patellar height and shorter patellar tendon length, both preoperatively and postoperatively, increases the risk of patellar crepitus. We suggest this condition increases contact forces between the distal quadriceps tendon and superior aspect of the intercondylar notch, resulting in tendon irritation and proliferation of the fibrosynovial tissue classically present in cases of patellar crepitus. Preoperative counseling regarding an increased risk of postoperative patellar crepitus is therefore wise in subjects with reduced preoperative patellar height. Finally, we found increased posterior condylar offset as a potential cause of patellar crepitus. We speculate increasing the posterior condylar offset results in a relative anterior shift of the intercondylar notch of the femoral component in flexion, consequently leading to increased quadriceps tendon contact forces, the subsequent development of tendon irritation, and fibrosynovial tissue posteriorly on the distal quadriceps tendon. Although we realize the importance of achieving a balanced flexion gap and flexion stability, the authors caution against “overstuffing” the flexion space and increasing the posterior femoral condylar offset.
In summary, numerous factors have been found to be associated with the development of patellar crepitus after TKA. We hypothesize that many of these factors are associated with either increased irritation or contact forces in the distal quadriceps tendon as it traverses the superior aspect of the intercondylar box of a PS femoral component. Presently, data are lacking in the literature to prove this hypothesis and therefore basic science analyses are currently ongoing at our institution to further evaluate the many etiologic factors observed in this report to be associated with the development of patellar crepitus after PS TKA. As previously stated, the authors cannot dispute causes of postoperative patellar crepitus previously reported in the literature such as anterior tibial component placement or joint line elevation that were not found to be associated factors in this report because the number of subjects evaluated may not provide the statistical power to determine statistical significance of these variables. Given the size of our study, we believe the findings provide valuable insight into our understanding of patellar clunk and crepitus after PS TKA.
Acknowledgments
We thank the research personnel of Colorado Joint Replacement and OrthoCarolina as well as Joshua Carothers, MD, for their help in collecting and organizing the data analyzed in this report.
Footnotes
The Rocky Mountain Musculoskeletal Research Laboratory has received funding for clerical support in preparation of this manuscript. No authors have received any financial support directly related to the preparation of this manuscript, although one or more of the authors (DAD, TKF) serve as consultants to the manufacturing company (Depuy, Inc) of the implant studied in this review.
The current analysis has received approval from the Institutional Review Boards of the participating institutions.
This work was performed at Colorado Joint Replacement, Denver, CO, USA, and OrthoCarolina, Charlotte, NC, USA.
References
- 1.Beight JA, Yao B, Hozack WJ, Hearn SL, Booth RE. The patellar ‘clunk’ syndrome after posterior stabilized total knee arthroplasty. Clin Orthop Relat Res. 1994;299:139–142. [PubMed] [Google Scholar]
- 2.Bellemans J, Banks S, Victor J, Vandenneucker H, Moemans A. Fluoroscopic analysis of the kinematics of deep flexion in total knee arthroplasty: influence of posterior condylar offset. J Bone Joint Surg Br. 2002;84:50–53. doi: 10.1302/0301-620X.84B1.12432. [DOI] [PubMed] [Google Scholar]
- 3.Clarke HD, Fuchs R, Scuderi GR, Mills EL, Scott WN, Insall JN. The influence of femoral component design in the elimination of patellar clunk in posterior-stabilized total knee arthroplasty. J Arthroplasty. 2006;21:167–171. doi: 10.1016/j.arth.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Colizza WA, Insall JN, Scuderi GR. The posterior stabilized total knee prosthesis: assessment of polyethylene damage and osteolysis after a ten-year-minimum follow-up. J Bone Joint Surg Am. 1995;77:1713–1720. doi: 10.2106/00004623-199511000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Figgie HE, Goldberg VM, Heiple KG, Moller HS, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Joint Surg Am. 1986;68:1035–1040. [PubMed] [Google Scholar]
- 6.Fukunaga K, Kobayashi A, Minoda Y, Iwaki H, Hashimoto Y, Takaoka K. The incidence of the patellar clunk syndrome in a recently designed mobile-bearing posterior stabilized total knee replacement. J Bone Joint Surg Br. 2009;91:463–468. doi: 10.1302/0301-620X.91B4.21494. [DOI] [PubMed] [Google Scholar]
- 7.Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;282:170–176. [PubMed] [Google Scholar]
- 8.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York, NY: John Wiley and Sons; 2000. [Google Scholar]
- 9.Hozack WJ, Rothman RH, Booth RE, Balderston RA. The patellar clunk syndrome: a complication of posterior stabilized total knee arthroplasty. Clin Orthop Relat Res. 1989;241:203–208. [PubMed] [Google Scholar]
- 10.Ip D, Ko PS, Lee OB, Wu WC, Lam JJ. Natural history and pathogenesis of the patella clunk syndrome. Arch Orthop Trauma Surg. 2004;124:597–602. doi: 10.1007/s00402-003-0533-9. [DOI] [PubMed] [Google Scholar]
- 11.Larson CM, Lachiewicz PF. Patellofemoral complications with the Insall-Burstein II posterior-stabilized total knee arthroplasty. J Arthroplasty. 1999;14:288–292. doi: 10.1016/S0883-5403(99)90053-0. [DOI] [PubMed] [Google Scholar]
- 12.Lonner JH, Jasko JG, Bezwada HP, Nazarian DG. Incidence of patellar clunk with a modern posterior-stabilized knee design. Am J Orthop. 2007;36:550–553. [PubMed] [Google Scholar]
- 13.Pollock DC, Ammeen DJ, Engh GA. Synovial entrapment: a complication of posterior stabilized total knee arthroplasty. J Bone Joint Surg Am. 2002;84:2174–2178. [PubMed] [Google Scholar]
- 14.Ranawat AS, Ranawat CS, Slamin JE, Dennis DA. Patellar crepitation in the PFC Sigma total knee system. Orthopedics. 2006;29(Suppl):S68–S70. [PubMed] [Google Scholar]
- 15.Schroer WC, Diesfeld PJ, Reedy ME, LeMarr A. Association of increased knee flexion and patella clunk syndrome after mini-subvastus total knee arthroplasty. J Arthroplasty. 2009;24:281–287. doi: 10.1016/j.arth.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Yau WP, Wong JWK, Chiu KY, Ng TP, Tang WM. Patellar clunk syndrome after posterior stabilized total knee arthroplasty. J Arthroplasty. 2003;18:1023–1028. doi: 10.1016/S0883-5403(03)00447-9. [DOI] [PubMed] [Google Scholar]