Abstract
Background
The tibial post in posterior-stabilized total knees is a potential source of polyethylene wear debris, but the relationship between the shape and location of the tibial post in relation to the tibiofemoral bearing surfaces and the subsequent wear damage patterns remains unknown.
Questions/purposes
We used observations made on retrieved implant components from three contemporary posterior-stabilized knee designs to examine how differences in tibial post design affected wear damage on the post.
Methods
We examined 113 retrieved Zimmer NexGen®, 103 Exactech Optetrak®, and 58 Smith and Nephew Genesis® II posterior-stabilized inserts using a subjective scale to grade post damage.
Results
All 274 inserts demonstrated wear damage. Total wear scores and scores for wear damage on the anterior post differed among designs: Optetrak® 20 ± 4 and 5 ± 1, NexGen® 13 ± 4 and 3 ± 1, and Genesis® II 8 ± 3 and 1 ± 1, respectively. The Optetrak® had predominantly anterior wear damage, the NexGen® had more global wear damage, and the Genesis® II had predominantly posterior wear damage. Tibial post wear damage and anterior post wear damage were primarily determined by implant design and to a lesser extent by length of implantation and revision diagnosis.
Conclusions
Although tibial post wear damage is multifactorial, the primary determinant of wear damage, and specifically anterior wear damage, is implant design.
Clinical Relevance
The constraint provided by the posterior-stabilized post-cam contact in modern knee arthroplasties is reflected in the wear damage patterns that occur during in vivo use. Unintended constraint such as anterior impingement should be addressed through design modifications for future posterior-stabilized knee arthroplasties.
Introduction
The tibial polyethylene post in posterior-stabilized (PS) TKA designs is a source of substantial wear debris, and retrieval analyses demonstrated evidence of post wear on 100% of the implants examined [5, 13, 22]. In severe cases, this wear can lead to fracture of the tibial post, resulting in instability and revision knee arthroplasty.
The intent of the tibial post in a PS knee is to articulate with the femoral cam as the knee flexes, providing a mechanical constraint to prevent anterior translation of the femur on the tibia during flexion [17]. Thus, wear on the posterior surface of the tibial post should be expected from contact with the femoral cam. However, retrieval studies suggest additional wear damage on the other three surfaces of the tibial post that were not intended to articulate with the femoral box [2, 22]. The anterior tibial post reportedly impinges on the femoral box in hyperextension and low flexion angles [2, 18]. A recent study by our group comparing retrieved Insall-Burstein I and II knee arthroplasties showed tibial post wear damage, and more specifically anterior post wear damage, changed by altering the position of the post relative to the bearing surfaces by less than 2 mm within a single design [9]. Thus, tibial post wear damage in PS knees is design-dependent, suggesting the overall wear burden might be as well.
The purpose of this study was to further investigate the relationship between implant design and tibial post wear damage in three different contemporary PS knee designs by (1) comparing total wear damage and anterior wear damage of the tibial post among designs; (2) determining the impact of design, demographic, and clinical factors on wear damage of the entire tibial post and specifically on the anterior surface of the post; and (3) comparing the relative location of the tibial post with the observed patterns of wear damage among designs.
Patients and Methods
We retrieved 274 PS polyethylene inserts as part of an ongoing Institutional Review Board-approved implant retrieval system at our institution. The group consisted of 113 NexGen® (Zimmer, Inc, Warsaw, IN), 103 Optetrak® (Exactech, Inc, Gainesville, FL), and 58 Genesis® II PS (Smith and Nephew, Inc, Memphis, TN) components. These three designs were selected because they represent the largest numbers of contemporary PS knee designs in our retrieval collection. The NexGen® and Optetrak® were gamma-irradiated in an inert environment and the Genesis® II implants were sterilized by ethylene oxide gas. Retrievals were obtained at the time of revision surgery performed at our institution but represent primary PS TKAs done by multiple surgeons at various institutions. Of the 274 retrieved PS polyethylene inserts, 163 (59%) were removed from women and 111 (41%) from men (Table 1). The average age of patients at the time of revision surgery was 65 years, and the average patient body mass index (BMI) was 31 kg/m2. The most common reasons for revision were infection (35% of the cases) followed by instability (23%), stiffness (18%), aseptic loosening (16%), and other miscellaneous causes (8%). Age, BMI, and reason for revision did not differ among groups. The average length of implantation was similar for the Optetrak® (2.6 ± 2.6 years) and NexGen® (2.1 ± 2 years) designs; however, the Genesis® II inserts were implanted for less (p = 0.004) time (1.5 ± 1.5 years) than the Optetrak® components. The majority of retrieved implants had a length of implantation of less than 5 years with only 11% (30 of 274) being in vivo for longer than 5 years.
Table 1.
Patient demographics
| Variable | Genesis® II | NexGen® | Optetrak® | p value |
|---|---|---|---|---|
| Total number of patients | 58 | 113 | 103 | |
| Number of females | 28 (49.1%) | 66 (58.4%) | 67 (65.0%) | 0.144 |
| Age (years)* | 65.7 (± 12.2) | 65.2 (± 10.4) | 64.4 (± 11.8) | 0.778 |
| Length of implantation (years)* | 1.44 (± 1.48) | 2.14 (± 2.05) | 2.60 (± 2.58) | 0.006 |
| Range 0.02–8.01 | Range 0.04–11.00 | Range 0.03–10.13 | ||
| Side (number of patients) | ||||
| Left | 28 (48.3%) | 49 (43.4%) | 53 (51.5%) | 0.488 |
| Right | 30 (51.7%) | 64 (56.6%) | 50 (48.5%) | |
| Body mass index* | 30.9 (± 7.0) | 30.1 (± 6.7) | 32.0 (± 6.4) | 0.111 |
| Reason for revision (number of patients) | ||||
| Infection | 19 (34%) | 39 (35%) | 34 (35%) | 0.331 |
| Loosening | 7 (12%) | 21 (19%) | 15 (15%) | |
| Instability | 11 (20%) | 28 (25%) | 22 (23%) | |
| Stiffness | 11 (20%) | 19 (17%) | 14 (14%) | |
| Miscellaneous | 8 (14%) | 4 (4%) | 13 (13%) | |
* Values are expressed as mean (± SD).
Patient demographic data gathered for the study included age, BMI, reason for revision, and length of implantation. Radiographic evaluation was performed by a single observer (MMD) who was blinded to the demographic and retrieval data at the time of evaluation. The Knee Society radiographic evaluation system was used to assess the coronal and sagittal alignment of the femoral and tibial components [7]. Measurements included the femoral and tibial varus-valgus angle, femoral component flexion-extension angle, and tibial posterior slope. In the AP view, the femoral joint line angle was measured from a line drawn parallel to the femoral condyles to a line drawn along the axis of the femoral shaft. The tibial angle was measured between a line drawn parallel to the base of the tibial tray and a line drawn along the axis of the tibial shaft. These values were referenced to neutral and reported as varus or valgus angles. On the lateral view, the femoral component flexion-extension angle was measured from a line perpendicular to the metallic femoral box to a line parallel to the axis of the femoral shaft. The femoral box in the Optetrak® design is in 8° of flexion relative to the distal femoral osteotomy, so this angle was subtracted from the femoral component flexion-extension angle to provide an accurate comparison of alignment among designs. Anterior and posterior slope of the tibial component was measured from a line parallel to the base of the tibial tray to a line drawn along the axis of the tibial shaft. On average, the components were implanted in good alignment other than slight varus positioning of the tibial component. We observed no differences in radiographic component positioning among designs (Table 2).
Table 2.
Component orientation determined radiographically
| Design | Femoral varus-valgus | Tibial angle | Femoral flexion-extension | Tibial posterior slope |
|---|---|---|---|---|
| NexGen® | 5.3° valgus ± 2.7º | 1.5° varus ± 2.57º | 2.9° flexion ± 3.3º | 5.1° ± 3.0º |
| Optetrak® | 5.9° valgus ± 2.4º | 2.2° varus ± 3.2º | 2.7° flexion ± 3.8º | 5.2° ± 2.8º |
| Genesis® II | 5.8° valgus ± 1.9º | 1.2° varus ± 3.2º | 2.2° flexion ± 3.3º | 4.1° ± 2.2º |
Values are expressed as mean ± SD.
All inserts were examined for evidence of surface wear damage to the anterior, medial, lateral, posterior, and superior surfaces of the tibial post. The previously described method of Hood et al. [14] was used to grade the extent and mode of wear damage involving each face of the post. Grading was performed by two independent observers (MMD, RL) who were blinded to the demographic and radiographic data at the time of grading. Before analyzing wear damage scores for the individual designs, the results of the two independent observers were compared. These demonstrated a very high interrater reliability (interclass correlation > 0.9) with respect to wear damage scores in the three designs. Because of this, the scores of the more senior observer (MMD) were used for all further analyses. The wear damage scores were analyzed to determine the most prevalent mode and location of wear for each design.
Descriptive data are presented as means ± SDs for continuous variables and frequencies as percentages for categorical or discrete variables. To determine if differences existed in the wear damage of the tibial post among the three designs, the total wear damage scores and dominant wear damage locations were compared using one-way analysis of variance (ANOVA). Patient demographics and radiographic alignment among designs were also compared using one-way ANOVA. A Kolmogorov-Smirnov (KS) goodness-of-fit test was conducted to assess the distribution of the post wear outcome variable and found to be 1.26 with a p value of 0.084. Therefore, the nonsignificant p value of the KS statistic allowed us to assume that wear score came from a normal distribution and use a linear regression model to further analyze the data. Using the entire sample, separate multivariate linear regression models were developed to predict total tibial post wear damage and anterior tibial post wear damage based on the demographic and clinical data: age, gender, length of implantation, BMI, reason for revision, and design. The lateral radiographic alignments of the femoral and tibial components were also included in the model of anterior post wear to determine the effect of femoral component flexion and posterior tibial slope on anterior wear damage. All variables were considered as eligible candidates for evaluation in the regression model. To control for any possible confounding effects from other variables, a forward stepwise procedure was used. Variables that maintained a significance level of a p value ≤ 0.15 were maintained in the model. Regression coefficients (β) were calculated and are reported with significant predictors expressed as p < 0.05. Post hoc power analysis of the total and anterior wear damage scores determined a sample size of 274 implants achieves 81.5% power to detect an R2 increase of 0.05 with the addition of six independent variables into the model with significance level set at alpha equal to 0.05. All statistical analyses were performed using SAS® Version 9 (SAS Institute, Inc, Cary, NC).
To obtain objective measurements of the tibial post design for comparisons, a virgin implant of a similar size from each design was analyzed using a three-dimensional (3D) scanner (NextEngine, Santa Monica, CA). The overall width of the implant and the width of the tibial post were measured in millimeters. The AP location of the tibial post was determined based on measurements made at a point on the anterior and posterior aspects of the tibial post that were 10 mm above the dwell point of the particular implant design. The dwell point was defined as the lowest point on the condylar surface of the tibial insert.
Results
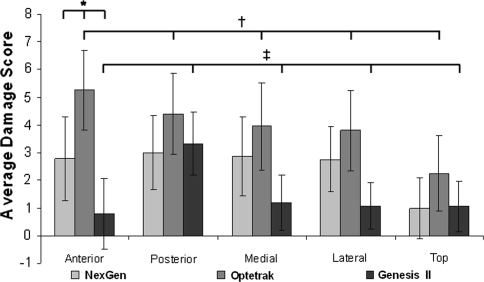
Average total wear damage scores were 20 ± 4 for the Optetrak®, 13 ± 4 for the NexGen®, and 8 ± 3 for the Genesis® II (p < 0.001). The primary location of post wear damage also differed among the three designs (Fig. 1). The Optetrak® had more wear damage on the anterior surface (27% of the total wear damage) than the other surfaces of the post. In the NexGen®, a global pattern of wear damage was observed with a relatively equal distribution of wear damage among the anterior (22%), posterior (24%), medial (23%), and lateral (22%) faces of the post. In the Genesis® II, the posterior surface of the post was the predominant location of wear damage (44% of total wear). When analyzing anterior post wear damage specifically, we observed differences in both the amount and type of wear damage among designs. The mean total anterior wear damage scores were 5.3 ± 1.4 for the Optetrak®, 2.8 ± 1.5 for the NexGen®, and 0.8 ± 1.4 for the Genesis® II (p < 0.001). The NexGen® had predominantly burnishing (41%) and surface deformation (40%) on the anterior surface of the post (Fig. 2A). In the Optetrak®, anterior wear damage was predominantly burnishing (37%) along with surface deformation (22%) and pitting (18%) (Fig. 2B). The anterior wear damage for the Genesis® II design was predominantly burnishing (50%) along with scratching (20%) and surface deformation (15%) (Fig. 2C).
Fig. 1.
A graph shows average wear scores by location for the three contemporary posterior-stabilized knee designs. *The mean total anterior wear damage scores were 5.3 ± 1.4 for the Optetrak®, 2.8 ± 1.5 for the NexGen®, and 0.8 ± 1.4 for the Genesis® II. †The Optetrak® design had predominantly anterior post wear damage, ‡the Genesis® II design had predominantly posterior wear damage, and the NexGen® design demonstrated a more global wear damage pattern (p < 0.001 for all comparisons).
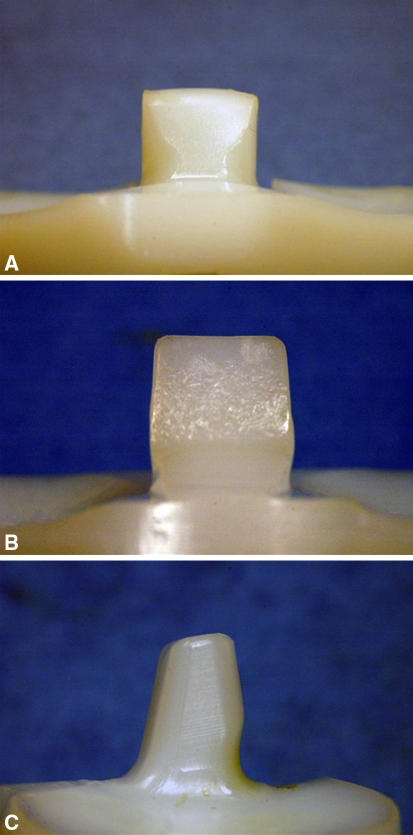
Fig. 2A–C.
Photographs demonstrate representative tibial post wear of the three designs evaluated in this study. (A) Characteristic surface deformation on the anterior tibial post of the NexGen® design is shown. (B) Surface deformation and pitting on the anterior surface of the Optetrak® design is shown. (C) Posterior tibial post wear damage in the Genesis® II design is shown.
The multivariate regression models predicting total and anterior post wear damage had R2 values of 0.611 and 0.668, respectively, thus accounting for approximately two-thirds of the total variance in wear scores. After controlling for other variables, regression analysis demonstrated design, reason for revision, and length of implantation were independent variables associated with both total post wear damage and anterior post wear damage (Tables 3, 4). The variable most affecting total wear damage score and anterior wear damage score was the implant design. After adjusting for the other variables, the NexGen® and Genesis® II implants were associated with less (p < 0.001) total wear damage than the Optetrak® design (β [NexGen®] = −7.02; β [Genesis® II] = −11.57). The same relationship was found with respect to anterior wear damage score with a smaller effect (β [NexGen®] = −2.08; β [Genesis® II] = −4.20; p < 0.001). Compared with infection, revision for loosening or instability was associated with a higher total wear damage score (β = 2.74; p < 0.001) and anterior wear damage score (β = 0.85; p < 0.001). Longer implantation was associated with a higher total wear damage score (β = 0.32; p = 0.006) and anterior wear damage score (β = 0.24; p < 0.001) but to a lesser degree than design and reason for revision. Female gender was associated with a higher total wear damage score (β = 1.15; p = 0.0272) but not with anterior wear damage score. Sagittal radiographic alignment of the femoral and tibial components did not affect anterior post wear damage (p = 0.226 and 0.912, respectively).
Table 3.
Multivariable linear regression analysis for total damage score (R2 = 0.611)
| Variable | Regression coefficient | 95% confidence interval | Standard error | p value |
|---|---|---|---|---|
| Intercept | 14.66 | 11.54 to 20.07 | 2.22 | < 0.001 |
| Age | 0.02 | 0.02 | 0.406 | |
| Length of implantation | 0.32 | 0.09 to 0.55 | 0.11 | 0.006 |
| Body mass index | 0.04 | 0.03 | 0.224 | |
| Reason (reference = infection) | 0.06 | 0.35 | < 0.001 | |
| Loosening/instability | 2.74 | 1.58 to 3.90 | 0.59 | < 0.001 |
| Stiffness/miscellaneous | −0.45 | −1.76 to 0.85 | 0.66 | 0.495 |
| Design (reference = Optetrak®) | 5.91 | 0.36 | < 0.001 | |
| NexGen® | −7.02 | −8.12 to −5.93 | 0.55 | < 0.001 |
| Genesis® II | −11.57 | −12.93 to −10.21 | 0.69 | < 0.001 |
| Female | 1.15 | −2.17 to 0.13 | 0.52 | 0.0272 |
Table 4.
Multivariable linear regression analysis for anterior damage score (R2 = 0.668)
| Variable | Regression coefficient | 95% confidence interval | Standard error | p value |
|---|---|---|---|---|
| Intercept | 3.77 | 0.73 | < 0.001 | |
| Age | 0.01 | 0.01 | 0.831 | |
| Length of implantation | 0.24 | 0.15 to 0.29 | 0.04 | < 0.001 |
| Body mass index | 0.02 | 0.01 | 0.228 | |
| Reason (reference = infection) | 0.02 | 0.11 | 0.829 | |
| Loosening/instability | 0.85 | 0.54 to 1.29 | 0.19 | < 0.001 |
| Stiffness/miscellaneous | −0.11 | −0.59 to 0.25 | 0.22 | 0.608 |
| Design (reference = Optetrak®) | 2.09 | 0.12 | < 0.001 | |
| NexGen® | −2.08 | −2.69 to −1.99 | 0.18 | < 0.001 |
| Genesis® II | −4.20 | −4.54 to −3.67 | 0.23 | < 0.001 |
| Female | 0.15 | 0.17 | 0.376 | |
| Femoral component flexion | 0.03 | 0.02 | 0.226 | |
| Tibial posterior slope | −0.01 | 0.03 | 0.912 |
The measurements obtained from the 3D scanner of the sample implants demonstrated the different positions of the tibial post in the three designs relative to the dwell point of the polyethylene insert (Table 5). The Optetrak® design had the most anteriorly placed tibial post with the anterior aspect of the post located 14.4 mm anterior to the dwell point and the posterior surface located 1.3 mm anterior to the dwell point. In the NexGen® design, the anterior and posterior surfaces of the post were 9.6 mm anterior and 1.7 mm posterior to the dwell point, respectively. The Genesis® II design had the most posteriorly positioned tibial post with the anterior surface 4.7 mm anterior and the posterior surface 8.5 mm posterior to the dwell point. The tibial post of the NexGen® was wider (14.9 mm) than the Genesis® II (13.5 mm) and the Optetrak® (13.2 mm) implants. The Optetrak® design demonstrated predominantly anterior post wear damage, consistent with the more anteriorly placed tibial post relative to the other two designs. The NexGen® implant had a wider tibial post compared with Optetrak® and Genesis® II of a similar size and demonstrated a more global pattern of wear damage distributed to all surfaces of the tibial post compared with the other two designs. The Genesis® II design, with its more posteriorly positioned post compared with the other designs, displayed wear damage primarily on the posterior surface of the tibial post.
Table 5.
Relative post location for the three contemporary PS knee designs
| Implant | Measurement (mm) | |||
|---|---|---|---|---|
| A | B | C | D | |
| Optetrak® | 13.2 | 14.4 | 1.3 | 68 |
| NexGen® | 14.9 | 9.6 | −1.7 | 65 |
| Genesis® II | 13.5 | 4.7 | −8.5 | 68 |
PS = posterior stabilized; A = overall width of the tibial post; B = distance of the anterior surface of the tibial post relative to the dwell point; C = distance of the posterior surface of the tibial post relative to the dwell point; and D = overall width of the tibial insert.
Discussion
Previous retrieval studies of the tibial post in PS TKAs focused on gross fracture of the post and the wear damage mechanisms experienced by the post because it acts as a constraint to tibiofemoral translation and rotation [1, 4, 5, 9, 13]. Few studies included design as a factor influencing post wear damage. Our group previously established subtle changes in post design, including the dimensions of the post and its location relative to the tibiofemoral bearing surfaces, markedly influenced post damage, particularly the tendency for the post to experience anterior wear damage in hyperextension [9]. However, that retrieval study involved older designs that are no longer in clinical use. In the current study, we sought to further investigate this relationship between implant design and tibial post wear damage in three different contemporary PS knee designs by (1) comparing total wear damage and anterior wear damage of the tibial post among designs; (2) determining the impact of design, demographic, and clinical factors on wear damage of the entire tibial post and specifically on the anterior surface of the post; and (3) comparing the relative location of the tibial post with the observed patterns of wear damage among designs.
Our study has several limitations. The primary limitation is that it is a retrieval analysis of failed implants that were obtained after a short time in vivo and thus may not accurately reflect the behavior of well-functioning TKAs. Thirty-nine percent of the implants in the study were revised for instability or aseptic loosening, factors that could have resulted in abnormal kinematics and thus increased tibial post wear damage. Although the indications for revision surgery were similar for the three implants, the regression model demonstrated reason for revision did indeed influence post wear with revision for instability and aseptic loosening having a stronger association with wear damage than revision for infection or stiffness. Compared with design, however, revision diagnosis had a smaller effect on tibial post wear damage. This relationship is supported by other studies suggesting unintended tibial post wear occurs in vivo regardless of revision diagnosis [2, 5, 9, 12, 22, 23]. Second, we did not examine all the design features of these three implant systems. Other differences, especially the location of the post relative to the tibiofemoral articulating surfaces, could explain differences in tibial post wear damage. We created a 3D model to make measurements of the relative tibial post location in the three implant designs with the intention of providing objective differences among designs in terms of post position, but the tibial post cannot be considered in isolation. A more complete analysis of the post-box and bearing surface relationship requires in vitro cadaveric biomechanical testing [18], in vivo fluoroscopic studies [2, 11, 12, 21], or finite element analyses [15]. Although the temptation in a study such as ours is to use the results in isolation to condemn one design over another, this was not our intention, and such comparisons are inappropriate given the limitation in emphasizing post wear over other design differences. For example, increased anterior impingement may indeed lead to more polyethylene damage, but could also reflect the need for treating anterior contact between the post and the anterior margin of the femoral component as a necessary constraint provided by the implant. As such, design changes to the anterior contacting surfaces might be considered to transform an unintended consequence into a positive feature of the implant system. On the other hand, we had a large number of implants we systematically analyzed for tibial post wear damage. The addition of demographic and radiographic data allowed for the development of a regression model to determine the influence of different variables on post wear damage.
Previous studies focused on tibial post wear were unable to demonstrate a clear relationship between wear and implant design. These studies were limited in that they examined a single implant design [1, 20] or had relatively small numbers of retrieved implants available for analysis [4, 22]. For example, Puloski et al. [22] reviewed 23 implants from four different manufacturers, and although they found variability in the pattern of wear among implants, they did not find a difference in wear scores among the different designs. Conversely, we were able to demonstrate a clear relationship between implant design and tibial post wear damage in our study of 48 Insall-Burstein PS I and 186 Insall-Burstein PS II inserts, two implants with very similar bearing surface designs [9]. The more anterior placement of the post on the Insall-Burstein PS II components led to increased anterior tibial post wear damage. Although anterior tibial post impingement clearly occurs in vivo, from a design perspective, it has often been considered an aberrant articulation. The posterior aspect of the tibial post is designed to articulate with the femoral cam to induce femoral rollback, and accordingly, this is designed to be a low contact stress articulation with a high contact area. However, unintended anterior tibial post impingement between metallic and polyethylene surfaces not designed for articulation can result in high contact stresses occurring within a low contact area with the edge of the tibial post contacting the edge of the femoral box during extension and rotation. Using a finite element model, Huang et al. [16] demonstrated, compared with a flat-on-flat design, a curve-on-curve anterior post and box design can reduce edge loading and contact stresses on the tibial post in the setting of hyperextension and tibial rotation. This design feature essentially treats the anterior post and box as an articulation to mitigate the effects of anterior post impingement.
Factors other than design influence post wear. For example, Banks et al. [2] used fluoroscopic measurements in patients with TKA to demonstrate anterior post impingement occurred secondary to hyperextension of the knee during the early and late stance phases of gait. Li et al. [18] investigated the biomechanical characteristics of anterior post impingement in a cadaver model and found impingement occurs in both hyperextension and low flexion angles with the tibial post contributing to anterior knee stability. Component position has also been implicated in anterior post wear. Callaghan et al. [4] demonstrated a tendency to place femoral components in flexion using intramedullary instrumentation, and Banks et al. [2] showed neutral surgical placement is biased by 10º of hyperextension secondary to the anterior bow of the femur and posterior slope of the tibia. In an in vivo fluoroscopic study, Hanson et al. [12] reported knees with anterior post contact in hyperextension had increased femoral component flexion compared with knees that did not impinge. We found similar lateral radiographic alignment among the three groups and no relationship between component position and anterior tibial post wear damage.
Currently, considerable interest exists in highly crosslinked polyethylene for TKA. Elevated crosslinked polyethylenes reduce wear rates in TKA simulator studies [8, 19, 21], but this benefit must be balanced with the mechanical changes that accompany the increased crosslinking, specifically reduced fracture toughness and reduced resistance to fatigue crack propagation [3, 6, 10]. These adverse changes in the mechanical properties of highly crosslinked polyethylene are particularly concerning when viewed in the context of anterior tibial post impingement. Ideally, the use of highly crosslinked polyethylene in PS TKAs should be accompanied by a design with limited anterior tibial post wear damage and impingement to offset the mechanical limitations and potential for post fracture.
We found differences in both the total wear damage scores and location of wear damage among the tibial posts of the three implants and, through a multivariate linear regression model, demonstrated design is indeed the primary factor influencing total post wear damage, and in particular anterior post wear damage, in PS TKAs. Although PS knee designs have good long-term survival and functional results, wear damage of the tibial post remains a concern both for the added source of particulate debris created by post damage and for the evidence that it provides of the kinematic constraints provided by the PS post-cam mechanism in vivo. Unintended constraint such as anterior impingement should be addressed through design modifications for future PS knee arthroplasties.
Acknowledgments
We thank Joseph Lipman, MS, for his assistance with the 3D analysis and measurements of the tibial inserts; Sean LeFloch, BS, for his help in data collection; and Reed LaSala for his work grading the implant retrievals.
Footnotes
SBH receives royalties from Smith and Nephew, Inc (Memphis, TN), is a paid consultant for Smith and Nephew, and receives research or institutional support from Smith and Nephew. TMW receives royalties from Mathys AG (Bettlach, Switzerland); holds stock or stock options in Exactech; and receives research or institutional support from Zimmer, Synthes Spine (West Chester, PA), and Stryker (Mahwah, NJ). One or more of the authors received funding from the Clark and Kirby Foundations (TMW, NHK) and the Hospital for Special Surgery Knee Service Research Fund (TMW, SBH).
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Bal BS, Greenberg D, Li S, Mauerhan D R, Schultz L, Cherry K. Tibial post failures in a condylar posterior cruciate substituting total knee arthroplasty. J Arthroplasty. 2008;5:650–655. doi: 10.1016/j.arth.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Banks SA, Harman MK, Hodge WA. Mechanism of anterior impingement damage in total knee arthroplasty. J Bone Joint Surg Am. 2002;84(Suppl 2):37–42. doi: 10.2106/00004623-200200002-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bradford L, Baker D, Ries MD, Pruitt LA. Fatigue crack propagation resistance of highly crosslinked polyethylene. Clin Orthop Relat Res. 2004;429:68–72. doi: 10.1097/01.blo.0000150124.34906.34. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan JJ, O’Rourke MR, Goetz DD, Schmalzried TP, Campbell PA, Johnston RC. Tibial post impingement in posterior-stabilized total knee arthroplasty. Clin Orthop Relat Res. 2002;404:83–88. doi: 10.1097/00003086-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Clarke HD, Math KR, Scuderi GR. Polyethylene post failure in posterior stabilized total knee arthroplasty. J Arthroplasty. 2004;5:652–657. doi: 10.1016/j.arth.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Cole JC, Lemons JE, Eberhardt AW. Gamma irradiation alters fatigue-crack behavior and fracture toughness in 1900H and GUR 1050 UHMWPE. J Biomed Mater Res. 2002;5:559–566. doi: 10.1002/jbm.10335. [DOI] [PubMed] [Google Scholar]
- 7.Ewald FC. The knee society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 8.Fisher J, McEwen HM, Tipper JL, Galvin AL, Ingram J, Kamali A, Stone MH, Ingham E. Wear, debris, and biologic activity of cross-linked polyethylene in the knee: benefits and potential concerns. Clin Orthop Relat Res. 2004;428:114–119. doi: 10.1097/01.blo.0000148783.20469.4c. [DOI] [PubMed] [Google Scholar]
- 9.Furman BD, Lipman J, Kligman M, Wright TM, Haas SB. Tibial post wear in posterior-stabilized knee replacements is design-dependent. Clin Orthop Relat Res. 2008;11:2650–2655. doi: 10.1007/s11999-008-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomoll A, Wanich T, Bellare A. J-integral fracture toughness and tearing modulus measurement of radiation cross-linked UHMWPE. J Orthop Res. 2002;6:1152–1156. doi: 10.1016/S0736-0266(02)00073-6. [DOI] [PubMed] [Google Scholar]
- 11.Hamai S, Miura H, Higaki H, Shimoto T, Matsuda S, Iwamoto Y. Evaluation of impingement of the anterior tibial post during gait in a posteriorly-stabilised total knee replacement. J Bone Joint Surg Br. 2008;90:1180–1185. doi: 10.1302/0301-620X.90B9.20298. [DOI] [PubMed] [Google Scholar]
- 12.Hanson GR, Suggs JF, Kwon YM, Freiberg AA, Li G. In vivo anterior tibial post contact after posterior stabilizing total knee arthroplasty. J Orthop Res. 2007;11:1447–1453. doi: 10.1002/jor.20417. [DOI] [PubMed] [Google Scholar]
- 13.Hendel D, Garti A, Weisbort M. Fracture of the central polyethylene tibial spine in posterior stabilized total knee arthroplasty. J Arthroplasty. 2003;5:672–674. doi: 10.1016/S0883-5403(03)00192-X. [DOI] [PubMed] [Google Scholar]
- 14.Hood RW, Wright TM, Burstein AH. Retrieval analysis of total knee prostheses: a method and its application to 48 total condylar prostheses. J Biomed Mater Res. 1983;5:829–842. doi: 10.1002/jbm.820170510. [DOI] [PubMed] [Google Scholar]
- 15.Huang CH, Liau JJ, Huang CH, Cheng CK. Influence of post-cam design on stresses on posterior-stabilized tibial posts. Clin Orthop Relat Res. 2006;450:150–156. doi: 10.1097/01.blo.0000218739.76871.28. [DOI] [PubMed] [Google Scholar]
- 16.Huang CH, Liau JJ, Huang CH, Cheng CK. Stress analysis of the anterior tibial post in posterior stabilized knee prostheses. J Orthop Res. 2007;4:442–449. doi: 10.1002/jor.20336. [DOI] [PubMed] [Google Scholar]
- 17.Insall JN, Lachiewicz PF, Burstein AH. The posterior stabilized condylar prosthesis: a modification of the total condylar design. Two to four-year clinical experience. J Bone Joint Surg Am. 1982;64:1317–1323. [PubMed] [Google Scholar]
- 18.Li G, Papannagari R, Most E, Park SE, Johnson T, Tanamal L, Rubash HE. Anterior tibial post impingement in a posterior stabilized total knee arthroplasty. J Orthop Res. 2005;3:536–541. doi: 10.1016/j.orthres.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 19.McEwen HM, Barnett PI, Bell CJ, Farrar R, Auger DD, Stone MH, Fisher J. The influence of design, materials and kinematics on the in vitro wear of total knee replacements. J Biomech. 2005;2:357–365. doi: 10.1016/j.jbiomech.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Mikulak SA, Mahoney OM, dela Rosa MA, Schmalzried TP. Loosening and osteolysis with the press-fit condylar posterior-cruciate-substituting total knee replacement. J Bone Joint Surg Am. 2001;83:398–403. doi: 10.2106/00004623-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Muratoglu OK, Bragdon CR, Jasty M, O’Connor DO, Knoch RS, Harris WH. Knee-simulator testing of conventional and cross-linked polyethylene tibial inserts. J Arthroplasty. 2004;7:887–897. doi: 10.1016/j.arth.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Puloski SK, McCalden RW, MacDonald SJ, Rorabeck CH, Bourne RB. Tibial post wear in posterior stabilized total knee arthroplasty: an unrecognized source of polyethylene debris. J Bone Joint Surg Am. 2001;83:390–397. doi: 10.2106/00004623-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Stiehl JB, Komistek RD, Dennis DA, Paxson RD, Hoff WA. Fluoroscopic analysis of kinematics after posterior-cruciate-retaining knee arthroplasty. J Bone Joint Surg Br. 1995;77:884–889. [PubMed] [Google Scholar]