Abstract
Background
Total knee arthroplasty (TKA) in patients with skeletal dysplasias is particularly challenging as a result of the anatomic variances and substantial bony deformities. Little has been written regarding technical considerations that should be made when performing TKA in skeletal dysplasia.
Questions/purposes
We describe special operative considerations that must be made when performing TKA on patients with skeletal dysplasia, including implant selection and ligamentous balancing.
Patients and Methods
We retrospectively reviewed 12 TKAs in eight patients with varying degrees of deformity (ranging from 30° of varus to 45° of valgus) secondary to three types of skeletal dysplasias: multiple hereditary exostosis, achondroplasia, and osteogenesis imperfecta. Clinical notes, operative records, and radiographic data were reviewed. Minimum followup was 1 year (average, 4 years; range, 1–10 years).
Results
We used customized implants in three of the 12 knees. Constrained tibial inserts were used in five knees. All 12 knees underwent releases (soft tissue or epicondylar osteotomy) to address gap balancing or patellar tracking. Average Knee Society scores improved from 35.9 preoperatively to 82.9 postoperatively and average function scores improved from 47.9 preoperatively to 96.7 postoperatively. Complications included two transient peroneal nerve palsies.
Conclusions
Special considerations must be made with regard to implant selection and ligamentous balancing as a result of the unusual anatomy and deformities that accompany skeletal dysplasia, but the short-term clinical results reveal consistent improvements in pain and function.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
As a result of the unusual anatomy and the severe deformities, special considerations must be made when performing TKA in patients with skeletal dysplasia. Three skeletal dysplastic conditions that may present with concomitant end-stage arthritic knees include multiple hereditary exostosis (MHE), achondroplasia, and osteogenesis imperfecta (OI). TKA in patients with skeletal dysplasia provides technical challenges with regard to implant selection and soft tissue balancing, but the short-term clinical results reveal consistent improvements in pain and function.
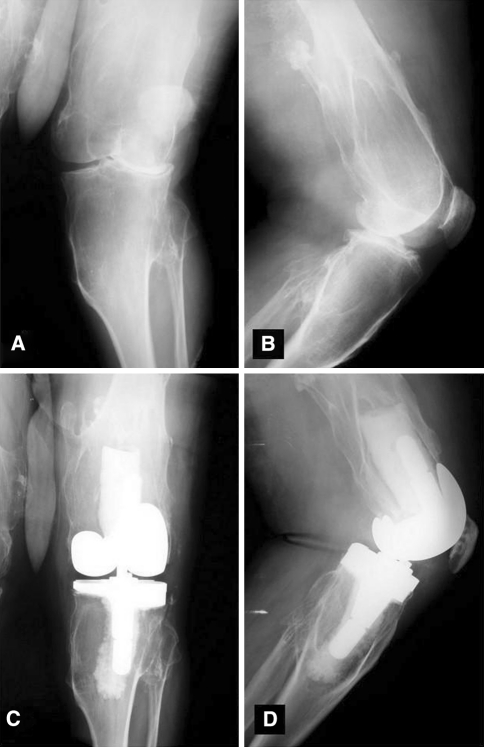
MHE is an autosomal-dominant condition secondary to a disorder in the EXT family of tumor suppressor genes [14, 18]. This genetic condition leads to multiple cartilage-capped bony excrescences arising from the metaphyseal ends of rapidly growing long bones. Patients commonly present with painless, slow-growing masses on both the upper and lower extremities. The most common location for these chondral masses are the metaphyses of long bones, particularly the distal femur [5, 12]. Deformities are also common in MHE, including valgus deformities of the knee [14] (Fig. 1).
Fig. 1A–D.
Preoperative (A) AP and (B) lateral radiographs of a patient with multiple hereditary exostosis demonstrate exostoses and valgus alignment. Postoperative (C) AP and (D) lateral radiographs demonstrate corrected deformity with use of a varus-valgus constraining implant and lateral tibial augment.
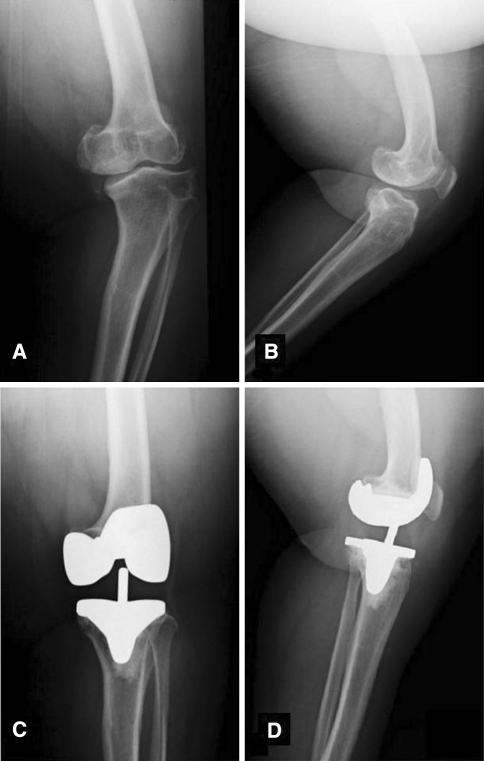
Achondroplasia is another skeletal dysplasia resulting from an autosomal-dominant mutation in the gene encoding fibroblast growth factor receptor-3 resulting in defects in enchondral bone formation [17]. Pertaining to knee anatomy, patients with achondroplasia have wide, flared metaphyses and metaphyseal angulation [1, 19]. They may also have lateral collateral ligament laxity and restriction in ROM with major flexion contractures [1, 19] (Fig. 2).
Fig. 2A–D.
Preoperative (A) AP and (B) lateral radiographs of a patient with achondroplasia demonstrate metaphyseal flaring and varus deformity. Postoperative (C) AP and (D) lateral radiographs demonstrating use of a mobile-bearing TKA in the setting of severe deformity.
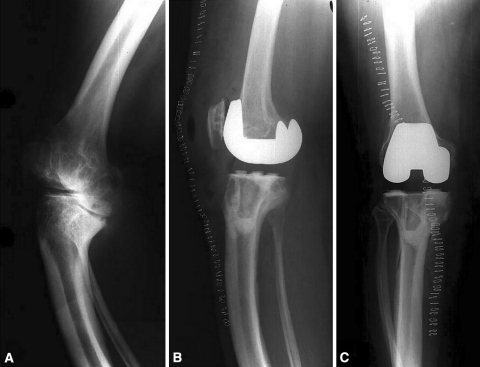
A third type of skeletal dysplasia is OI, which arises from an abnormality in Type 1 collagen secondary to mutations in the COL1A1 and COL1A2 genes [4]. As a result of the collogeneic abnormalities, both qualitative defects (manifested by abnormal collagen crosslinking), and quantitative defects (secondary to decreased production of collagen molecules) are present [3]. Patients present with bone fragility, multiple fractures, and limb deformities [3] (Fig. 3).
Fig. 3A–C.
(A) A preoperative radiograph (oblique view as a result of torsional deformity) of a patient with osteogenesis imperfecta demonstrates contorted tibial geometry. Postoperative (B) AP and (C) lateral radiographs demonstrate use of an all-polyethylene tibial component with modification of the tibial stem to accommodate the tibial deformity.
The purpose of this article is to present the technical considerations involved when performing TKA in patients with skeletal dysplasia.
Patients and Methods
We retrospectively reviewed eight patients with skeletal dysplasia who underwent 12 TKAs between October 1993 and July 2008. Clinical notes, radiographic images, and operative records were reviewed. Clinical data included length of followup, age, gender, height, weight, ROM, Knee Society scores and function scores [11], and any complications. Operative records were reviewed for exposure and special techniques used during the procedure (Table 1). One surgeon (RHK) evaluated all radiographs for preoperative alignment and deformity, postoperative alignment, and implant failure in accordance with the Knee Society radiographic evaluation system [7]. Operative records were reviewed to identify technical considerations used with regard to implant customization, surgical exposure, management of deformity, and soft tissue balancing (Table 1). No patients were recalled specifically for this study; all data were obtained from the medical records. We had prior Institutional Review Board approval.
Table 1.
Preoperative and postoperative clinical data for patients with skeletal dysplasia undergoing TKA
| Patient number | Gender | Height (inches) | Weight (pounds) | Diagnosis | Side | Age (years) | Preoperative alignment | Postoperative alignment | Preoperative ROM | Postoperative ROM | Preoperative Knee Society score/function score | Postoperative Knee Society score/function score | Followup (years) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 58 | 155 | OI | Right | 59 | 25° varus | 7° valgus | 5°–45° | 0°–100° | 16/45 | 90/100 | 3 | Soft tissue expanders; medial release; manual alteration of the all-polyethylene PS tibial component |
| 2 | Male | 62 | 155 | ACH | Right | 40 | 14° varus | 0° | 14°–78° | 5°–90° | 18/30 | 70/100 | 1 | Computer navigation; medial release; mobile-bearing PS |
| 3 | Female | 59 | 95 | MHE | Left | 80 | 11° valgus | 5° valgus | 5°–118° | 0°–100° | 60/60 | 90/100 | 3.5 | Lateral retinacular release; PS insert |
| 4 | Male | 63 | 193 | OI | Right | 24 | 17° valgus | 4° varus | 26°–90° | 10°–110° | 18/50 | 68/100 | 4 | Lateral retinacular release; TC3 insert |
| 5 | Male | 66 | 220 | MHE | Left | 71 | 45° valgus | 4° valgus | 0°–100° | 0°–110° | 40/50 | 89/100 | 4 | Lateral epicondylar osteotomy; tibial augment; lateral retinacular release; quadriceps snip; CCK; peroneal nerve palsy |
| Right | 72 | 45° valgus | 4° valgus | 0°–100° | 0°–110° | 40/50 | 87/100 | 3 | Lateral epicondylar osteotomy; tibial augment; CCK | |||||
| 6 | Male | 59 | 214 | MHE | Left | 60 | 15° valgus | 5° valgus | 0°–120° | 0°–100° | 49/45 | 90/80 | 5 | Lateral retinacular release; quadriceps snip; CCK |
| Right | 55 | 45° valgus | 5° valgus | 0°–120° | 0°–100° | 44/45 | 90/80 | 10 | Lateral retinacular release; proximal patellar realignment; CCK; lateral epicondylar osteotomy; peroneal nerve palsy | |||||
| 7 | Male | 54 | 113 | ACH | Left | 39 | 10° varus | 6° valgus | 0°–100° | 0°–80° | 40/60 | 86/100 | 4 | Medial release; PS insert |
| Right | 39 | 5° varus | 8° valgus | 0°–100° | 0°–90° | 40/60 | 88/100 | 4 | Medial release; hardware removal; PS insert | |||||
| 8 | Female | 42 | 95 | ACH | Left | 43 | 25° varus | 0° | 5°–130° | 0°–110° | 33/60 | 76/100 | 4 | Computer navigation; medial release; custom mobile-bearing RPF |
| Right | 43 | 30° varus | 5° varus | 5°–130° | 0°–105° | 33/20 | 71/100 | 3 | Medial release; lateral retinacular release; cement and screw technique; custom mobile-bearing RPF |
OI = osteogenesis imperfecta; ACH = achondroplasia; MHE = multiple hereditary exostosis; PS = posterior stabilized; CCK = constrained condylar knee; RPF = rotating-platform flexion.
Of the 12 TKAs, five were performed in patients with MHE, five in patients with achondroplasia, and two in patients with OI. The average age at the time of surgery was 52 years (range, 23–80 years). There were three women and five men. Three of the 12 knees had prior proximal tibial osteotomies. The mean height of the patients was 57.9 inches (range, 42–66 inches) and the mean weight was 155 pounds (range, 95–220 pounds). The average preoperative coronal malalignment was 30° of varus to 45° of valgus as measured with a goniometer on the AP radiographs. Three patients underwent staged bilateral TKA, whereas one patient underwent bilateral TKAs under the same anesthetic. Minimum followup was 1 year (average, 4 years; range, 1–10 years).
All surgeries were performed by two of the authors (GRS, DAD). We used an anterior midline incision and standard medial parapatellar arthrotomy in all patients, converting to a quadriceps snip [6, 9, 20] when necessary. A quadriceps snip was used in two knees when there was concern about adequate exposure and the attachment of the patellar tendon on the tibial tubercle. One knee was in a patient with achondroplasia with a preoperative valgus deformity of 15° and a preoperative ROM of 0° to 120°. The other knee was in a patient with MHE with a preoperative valgus deformity of 45° and preoperative ROM of 0° to 100°.
Preoperative placement of soft tissue expanders [13] was performed in one patient with OI with a severe preoperative varus deformity of 25° and a preoperative ROM of 5° to 45° to avoid potential postoperative wound complications.
Using standard instrumentation in 10 knees, attention was given to the angle of bone resection (based on preoperative radiographic templates), the depth of resection, and femoral and tibial rotational alignment. Computer navigation was used on two knees, one knee in a patient with achondroplasia with a 14° varus deformity and considerable varus femoral bow and in another patient with achondroplasia with a 25° varus deformity also with substantial bowing of the femur in patients with achondroplasia. Navigation was used in anticipation of difficulty with intramedullary instrumentation as a result of varus deformities and substantial femoral bowing [8].
For patients with large flexion contractures, additional distal femur was resected to achieve an acceptable extension gap. The femoral rotation was determined by a combination of techniques, including the epicondylar axis, AP axis, and gap balancing. Tibial rotation was based on anatomic landmarks.
Customized implants were used in three patients. Based on preoperative CT scans with three-dimensional reconstructions, custom tibial components with custom tibial inserts were manufactured for two knees as a result of the unusually small dimensions of the native tibia in a patient with achondroplasia. In one patient with OI, the all-polyethylene tibial component keel was customized intraoperatively by the surgeon to accommodate the deformed metaphyseal geometry.
One knee in a patient with achondroplasia with a previous proximal tibial osteotomy underwent removal of the proximal screw to allow the tibial component to seat properly.
To achieve proper ligamentous balancing, nine of the 12 knees in our series underwent soft tissue releases to achieve balanced gaps. Three knees with considerable preoperative valgus deformity required lateral epicondyle sliding osteotomies [2]. Two were in a patient with MHE with preoperative valgus deformities of 45° and another knee was in a patient also with MHE and a 45° preoperative valgus deformity. Six knees with preoperative varus deformities underwent medial releases. One knee was in a patient with OI with a preoperative varus deformity of 25°. Two knees were in a patient with achondroplasia, one knee with a preoperative varus deformity of 10° and the other knee with a 5° varus alignment. One patient with achondroplasia had knees with 30° and 25° varus deformity, which both required medial releases. Another knee was a 14° varus knee in a patient with achondroplasia. Varus-valgus constraining implants were used in five knees as a result of the inability to balance the flexion and extension gaps, whereas seven knees accepted posterior-stabilized tibial inserts.
To obtain central patellar tracking, lateral retinacular releases were performed in six knees. Two of these knees were in a patient with MHE who had a 45° preoperative valgus deformity on one knee and a 15° valgus alignment on the other knee. The knee with a 45° valgus deformity also underwent a concomitant proximal extensor mechanism realignment [10] to prevent lateral patellar subluxation. Other knees that underwent lateral release were in patients with MHE, one with a 45° preoperative valgus deformity and one with an 11° valgus deformity. A patient with OI with a preoperative valgus deformity of 17° also underwent a lateral release. One patient with achondroplasia with a 30° preoperative varus deformity also underwent a lateral release.
Two knees used tibial augments to accommodate bony defects in the lateral tibial plateau. Both of these knees were in a patient with MHE with 45° preoperative valgus deformities. The cement and screw technique [15, 16] was used in one knee to compensate for a posteromedial tibial defect in a patient with achondroplasia and a preoperative varus deformity of 30°.
Postoperatively, all patients began passive motion on a continuous passive motion machine. Physiotherapy was initiated on the day of operation or the first postoperative day and included walker or bilateral crutch ambulation (weightbearing as tolerated), knee ROM, muscle strengthening, and stair climbing.
The routine patient followup intervals used in this study were 2 weeks, 6 weeks, 3 months, 12 months, and every 2 years thereafter. Initial postoperative radiographs were obtained within the first 6 weeks after the surgical procedure. These included a standing AP view, a lateral radiograph, and a Merchant view. These same radiographs were obtained at sequential postoperative visits annually.
Results
Customized implants were used in three knees. Preoperatively manufactured custom tibial components were used in two knees, and one all-polyethylene tibial component was customized intraoperatively. Constrained tibial inserts were used in five of the 12 knees when soft tissue balancing was deemed inadequate. All 12 knees had releases of some form (medial release, lateral epicondylar osteotomy, and/or lateral retinacular release) to address gap balancing or patellar tracking (Table 1).
Average Knee Society scores improved from 35.9 (range, 16–60) preoperatively to 82.9 (range, 68–90) postoperatively and average function scores improved from 47.9 (range, 20–60) preoperatively to 96.7 (range, 80–100) postoperatively. All 12 knees were pain-free at their last followup. The preoperative coronal malalignment ranged from 30° of varus to 45° of valgus, whereas the postoperative coronal femoral-tibial alignment ranged from 5° of varus to 8° of valgus. Preoperative flexion averaged 103° (range, 45°–130°). Postoperative flexion averaged 99° (range, 85°–120°). Preoperative extension averaged 5° of a flexion contracture (range, 0°–26°) Postoperative extension averaged 1.3° of a flexion contracture (range, 0°–10°).
Complications included peroneal nerve palsies in two knees.
Discussion
Skeletal dysplastic conditions typically have concomitant anatomic variances with often substantial deformities that amplify the complexity of the typical TKA. The chondral metaphyseal masses and genu valgum deformity of MHE, the metaphyseal flaring with a narrow diaphysis in conjunction with genu varum and flexion contractures in achondroplasia, and the posttraumatic deformities with associated ligamentous laxity in OI require careful attention to preoperative planning and understanding the principles for dealing with severe deformity. We present the technical considerations involved when performing TKA in patients with skeletal dysplasia as well as the clinical knee scores, alignment, ROM, and complications.
This study has several limitations. First, as a result of the low prevalence of TKAs in patients with skeletal dysplasia, only a small cohort was available for review. Second, our followup is short-term and longer followup is needed to determine the long-term TKA outcomes in the setting of skeletal dysplasia and severe deformity. Third, TKAs were studied in three different types of skeletal dysplasias, each having their own individual pathologic presentations and deformities. Finally, full-length hip-knee-ankle radiographs were not available on the majority of patients, which impeded accurate alignment measurement and assessment of the deformities. Despite these shortcomings, the technical considerations noted in these cases still provide important points to consider when approaching patients undergoing TKA who have skeletal dysplasia.
Preoperative evaluation of the bony dimensions is critical, particularly in short-statured individuals such as in achondroplasia and OI to ensure appropriate fit of the keels and stems of the implants. Implant sizing in these cases of skeletal dysplasia needs special attention. If preoperative templating reveals particularly small bony anatomy, customized implants may need to be considered. The aspect ratio of the medial-lateral width and AP height of the femur should be determined to reestablish the femoral anatomic dimensions.
During correction of severe deformities in skeletal dysplasia, soft tissue imbalance must be anticipated. Soft tissue releases may be performed to achieve symmetric extension and flexion gaps once the deformities are corrected. When adequate balancing is unable to be obtained, increased constraint with the use of varus-valgus constraining tibial inserts should be available to achieve satisfactory stability. A hinge should also be made available in cases of severe ligamentous compromise. To obtain central patellar tracking, a lateral retinacular release may be used.
Clinically, short-term results demonstrated improvement in Knee Society scores and function scores as well as improved alignment. Complications included peroneal nerve palsies in two knees. These palsies both occurred in knees with MHE and both had preoperative valgus deformities of 45°. Both palsies were transient and had resolved by the 3-month postoperative visit.
Although surgically challenging, TKA in the context of skeletal dysplasia is a reasonable treatment option to provide pain relief and improved function. Technical considerations must be made with regard to implant selection and ligamentous balancing as a result of the unusual anatomy and deformities that accompany skeletal dysplasia.
Acknowledgments
We thank our Director of Clinical Research, Kendall Slutzky, for her efforts in organizing the manuscript.
Footnotes
One of the authors (DAD) has received funding from DePuy, a Johnson & Johnson Company. One of the authors (GRS) has received funding from Zimmer. The institution of the authors (DAD and RHK) has received funding from DePuy, a Johnson & Johnson Company.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Colorado Joint Replacement and Insall Scott Kelly Institute for Orthopaedics and Sports Medicine.
References
- 1.Bailey JA., 2nd Orthopaedic aspects of achondroplasia. J Bone Joint Surg Am. 1970;52:1285–1301. [PubMed] [Google Scholar]
- 2.Brilhault J, Lautman S, Favard L, Burdin P. Lateral femoral sliding osteotomy: lateral release in total knee arthroplasty for a fixed valgus deformity. J Bone Joint Surg Br. 2002;84:1131–1137. doi: 10.1302/0301-620X.84B8.12824. [DOI] [PubMed] [Google Scholar]
- 3.Burnei G, Vlad C, Georgescu I, Gavriliu TS, Dan D. Osteogenesis imperfecta: diagnosis and treatment. J Am Acad Orthop Surg. 2008;16:356–366. doi: 10.5435/00124635-200806000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Dagleish R. The human type I collagen mutation database. Nucleic Acids Res. 1997;25:181–187. doi: 10.1093/nar/25.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlin DC. Bone Tumors: General Aspects and an Analysis of 2, 276 Cases. Springfield, IL: Charles C. Thomas; 1957. [Google Scholar]
- 6.Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:59–68. doi: 10.1097/01.blo.0000214434.64774.d5. [DOI] [PubMed] [Google Scholar]
- 7.Ewald FC. The Knee Society total knee arthroplasty roentographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 8.Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132–136. doi: 10.1097/01.blo.0000229363.50361.25. [DOI] [PubMed] [Google Scholar]
- 9.Garvin KL, Scuderi G, Insall JN. Evolution of the quadriceps snip. Clin Orthop Relat Res. 1995;321:131–137. [PubMed] [Google Scholar]
- 10.Insall JN, Bullough PG, Burstein AH. Proximal ‘tube’ realignment of the patella for chondromalacia patellae. Clin Orthop Relat Res. 1979;144:63–69. [PubMed] [Google Scholar]
- 11.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 12.Jaffe HL. Tumors and Tumorous Conditions of the Bones and Joints. Philadelphia, PA: Lea & Febiger; 1958. [Google Scholar]
- 13.Manifold SG, Cushner FD, Craig-Scott S, Scott WN. Long-term results of total knee arthroplasty after the use of soft tissue expanders. Clin Orthop Relat Res. 2000;380:133–139. doi: 10.1097/00003086-200011000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Pierz KA, Stieber JR, Kusumi K, Dormans JP. Hereditary multiple exostoses: one center’s experience and review of etiology. Clin Orthop Relat Res. 2002;401:49–59. doi: 10.1097/00003086-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Ritter MA, Harty LD. Medial screws and cement: a possible mechanical augmentation in total knee arthroplasty. J Arthroplasty. 2004;19:587–589. doi: 10.1016/j.arth.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Ritter MA, Keating EM, Faris PM. Screw and cement fixation of large defects in total knee arthroplasty: a sequel. J Arthroplasty. 1993;8:63–65. doi: 10.1016/S0883-5403(06)80109-9. [DOI] [PubMed] [Google Scholar]
- 17.Rousseau F, Bonaventure J, Legeai-Mallet L. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- 18.Schmale GA, Conrad EU, Raskind WH. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am. 1994;76:986–992. doi: 10.2106/00004623-199407000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Shirley ED, Ain MC. Achondroplasia: manifestations and treatment. J Am Acad Orthop Surg. 2009;17:231–241. doi: 10.5435/00124635-200904000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Younger AS, Duncan CP, Masri BA. Surgical exposures in revision total knee arthroplasty. J Am Acad Orthop Surg. 1998;6:55–64. doi: 10.5435/00124635-199801000-00006. [DOI] [PubMed] [Google Scholar]