Abstract
Background
Resistant organisms are difficult to eradicate in infected total knee arthroplasty. While most surgeons use antibiotic-impregnated cement in these revisions, the delivery of the drug in adequate doses is limited in penetration and duration. Direct infusion is an alternate technique.
Questions/purposes
We asked whether single-stage revision and direct antibiotic infusion for infected TKA would control infection in patients with methicillin-resistant Staphylococcus aureus (MRSA) infections.
Methods
We retrospectively reviewed 18 patients (18 knees) with MRSA with one-stage revision protocol that included débridement, uncemented revision of total knee components, and intraarticular infusion of 500 mg vancomycin via Hickman catheter once or twice daily for 6 weeks; we used no intravenous antibiotics after the first 24 hours. We monitored serum vancomycin levels to maintain levels between 3 and 10 μg/mL. Minimum followup was 27 months (range, 27–75 months). Mean followup was 62 months, (range, 27–96 months).
Results
Infection was controlled at last followup in all but one patient with a recurrence of the MRSA. The patient was reoperated at 5 months; a necrotic bone fragment was removed, the knee was débrided and revised, and the antibiotic infusion protocol readministered. The patient remained free of infection 42 months postoperatively. At 2-year followup, the mean Knee Society score was 83. We observed no radiographic evidence of implant migration.
Conclusions
One-stage revision and 6 weeks of intraarticular vancomycin administration controlled infection in MRSA infected TKA with no apparent complications.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Revision for infected TKA generally is performed in two stages with antibiotics administered intravenously for 6 weeks after the first stage [14, 35, 52]. This first stage consists of thorough débridement and closure over a spacer made with antibiotic-impregnated cement, and the second stage is performed after sufficient healing has occurred, usually within 3 months, and consists of fixation of the final implants with antibiotic-impregnated cement [14, 35]. The purpose of the cement spacer is to deliver antibiotics at high concentration to a local area and to hold the joint space in preparation for the final implantation [4, 5]. Although this technique controls the infection in many patients, reinfection is common, ranging from 24–82% in cases involving resistant bacteria [15, 23, 34, 45], and the consequences of these reinfections are serious. Control of infection has been reported for the second revision after revision for infection protocol failed [3], but most authors report high complication rates including reinfection [11, 41, 46]. In one study failure of fusion occurred in 75% of cases initially treated for infection with long, cemented stems and amputation was performed in four of 24 knees treated for reinfection [11]. Five of the 24 knees continued to have chronic infection. Repeat rerevision for loosening, pain, and infection was necessary in 52% of one series [41], and chronic pain was reported in 50% of revisions for infection in the knees that achieved control of the infection [46]. These can be difficult and disappointing procedures. Designing the operation to avoid catastrophic rerevision should include efforts to ensure mechanical fixation without destroying bone stock and to avoid development of resistant organisms.
Delivery of antibiotics from antibiotic-impregnated cement is limited. Although concentrations can be high during the first 2 days, they fall to very low levels after 72 hours [2, 35]. In many cases, no detectable antibiotics are present in the synovial fluid after the first week [2, 35, 44]. Furthermore, the low concentration of antibiotics at the surface of these spacers promotes the growth of antibiotic-resistant bacteria [27, 29]. The other common mode of delivery of antibiotics to infected joints, intravenous administration, generally achieves low synovial fluid concentration [18, 22, 31, 32, 35, 47], and it is especially low for drugs that have poor soft tissue penetration such as gentamicin [35].
In veterinary practice, direct intraarticular injection of antibiotics has been used for treatment of pyarthrosis for decades [1, 18, 36, 47], and the reported intraarticular concentration is many orders of magnitude higher than that achieved by intravenous administration of a much higher dose [20]. Direct infusion of antibiotics also has been used successfully in humans to salvage acutely and chronically infected TKA [7, 31, 40]. In one study 17 of 20 cases of débridement and direct antibiotic infusion were free of infection greater than 30 months postoperative [7]. In another study 10 of 12 chronically infected arthroplasties had successful suppression with débridement and direct antibiotic infusion [31]. Intraarticular infusion with antibiotics in humans with indwelling catheters can achieve extremely high levels, and they can be maintained for as long as the catheters are left in place [7, 31, 32, 40]. Antibiotic levels are reportedly hundreds or even thousands of times higher with direct infusion of antibiotics than can be achieved with intravenous administration [32, 40]. Although this technique is not commonly used, direct infusion of antibiotics using infusion pumps after débridement reportedly allowed a high percentage of patients with infected TKA to maintain their prostheses, likely because of the extremely high concentration of antibiotics (> 500 μg/mL gentamicin) achieved with intraarticular infusion [32]. Using indwelling infusion pumps is expensive and technically difficult, but Hickman catheters are less costly and are relatively easy to use and maintain over a 6-week period [25, 40].
Resistant organisms are especially difficult to treat in TKA. The common method of treatment—débridement, insertion of antibiotic-laden polymethylmethacrylate spacer, 6-week course of intravenous antibiotics, and revision arthroplasty—has a high failure rate, ranging from 24–82%, despite the use of aggressive antibiotic treatment [12, 16, 23, 30, 34].
We therefore describe a technique to treat infected total knee arthroplasty by immediate exchange arthroplasty using cementless implants and administration of antibiotics by infusion directly into the knee. We then (1) determined the ability of the technique to control methicillin-resistant Staphylococcus aureus (MRSA), (2) evaluated clinical and radiographic signs of loosening, and (3) describe the pharmacokinetics of this method of antibiotic delivery to the knee.
Patients and Methods
We prospectively followed all 18 patients (18 knees) with TKA infected with MRSA bacteria referred to the senior author (LAW) for treatment between January 2001 and January 2007. All patients had established chronic infections of greater than 3 months’ duration. Eleven patients were women and seven were men. Mean age was 69 ± 6 years (range, 58–84 years). All patients had important comorbidities: nine patients had Type II diabetes, 12 had chronic dependency edema and stasis dermatitis, nine had morbid obesity, and 15 had malnutrition and hypoalbuminemia. Seventeen of the 18 patients had two or more comorbidities. Four of these patients had previous two-stage revisions for infection with antibiotic-laden cement spacers and antibiotic cement with revision implants. Seven patients had primary total knee components and a previous infection treated by incision and drainage followed by 2 to 6 weeks of intravenous antibiotics. Four patients had previous operations after their primary TKA for patellar tendon avulsion or patellar subluxation. All patients were entered into a treatment protocol that included débridement, revision of TKA with uncemented components, and direct antibiotic infusion. Two additional patients (two knees) were treated in 2009 and have been included in this study to evaluate and compare intraarticular and serum antibiotic concentration achieved with intraarticular antibiotics. Eleven patients had cemented components that included cemented diaphyseal engaging stems in the femur and tibia (Figs. 1, 2). The minimum followup was 27 months (mean, 62 months, range, 27 to 96 months). No patients were lost to followup.
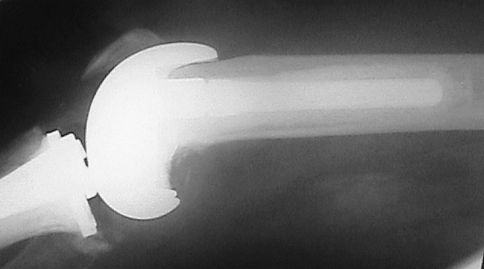
Fig. 1.
A preoperative lateral radiograph shows an infected TKA with evidence of deep cement penetration and massive bone loss in the femur. Reprinted with permission and © 2008 Elsevier from Whiteside LA. Two-stage exchange for infected TKA—opposes. Semin Arthroplasty. 2008;19:121–125 [51].
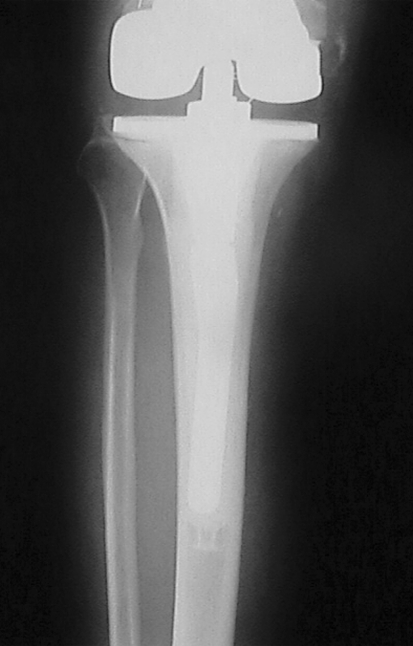
Fig. 2.
A preoperative anteroposterior radiograph of the tibia in the same knee as Fig. 1 also shows bone loss and cement penetration along the tibial stem. Reprinted with permission and © 2008 Elsevier from Whiteside LA. Two-stage exchange for infected TKA—opposes. Semin Arthroplasty. 2008;19:121–125 [51].
Surgical treatment included thorough removal of nonabsorbable sutures; complete synovectomy; vascularized osteoperiosteal flap osteotomy to expose diaphyseal cement mantles if necessary; and meticulous cement removal using a three-phase débridement starting with rongeurs, followed by curettes, and finishing with high-torque reamer to burr away all surfaces exposed to cement. During débridement, hand-pump irrigation with saline solution of vancomycin (1 g/L), polymyxin (30,000 units/L), and bacitracin (50,000 units/L) was performed repeatedly. After the débridement was finished, the area was cleaned and redraped, surgical gowns and gloves were changed, and instruments were washed and soaked in the same type of antibiotic solution used for irrigation. Revision total knee implants were inserted using smooth, fluted, press-fit diaphyseal-engaging titanium alloy stems and porous-coated surfaces applied directly to available bone. No cement was used to fix the implants to bone and no bone graft was used to fill defects. Intraoperatively the patients received 1 g vancomycin and the same dose was given twice postoperatively at 12-hour intervals. After 24 hours, no further intravenous antibiotics were given. A Constavac (Stryker, Kalamazoo, MI) drain was used for 24–48 hours postoperatively, but the blood was not reinfused.
To improve the chances of maintaining intraarticular access for 6 weeks, two Hickman catheters (CR Bard Inc., Salt Lake City, UT) were inserted (Fig. 3). These catheters are Teflon® tubes with a fibrous cuff that allows fibrous tissue ingrowth to seal the entry point and prevent ingress and egress of fluid around the catheter. The catheters were inserted through the lateral thigh, penetrating the vastus lateralis muscle and entering the suprapatellar area of the knee. The fibrous cuff was placed approximately 5 mm deep to the dermis. The catheters were sutured to the skin surface with silk sutures on two sides and the injection portals were taped to the surface of the skin. The external portals were each fitted with a Luer lock module and cap to allow injection with a syringe. The junctions were sealed with Betadine® ointment. Seven of the 18 knees lost one of the catheters during the six-week infusion interval, but none lost both catheters.
Fig. 3A–D.
This drawing illustrates the injection portals (A) that are outside the skin, the fibrous cuffs that are approximately 5 mm deep to the dermis (B), the catheters inside the synovial cavity of the knee (C), and outflow of the antibiotic through the synovial membrane and into the regional veins (D). The fibrous cuffs seal the catheters so that contaminants do not enter the knee and joint fluid does not leak out.
Intraarticular injection of antibiotics began in the evening of the first day after surgery. Vancomycin 500 mg in 10 mL saline was administered every 12 to 24 hours, and the injections were alternated between the two catheters to maintain open access. The catheters were not flushed but were capped and clamped to maintain a reliable seal. Vancomycin is unstable in solution and must not be injected in the knee in concentrations of greater than 100 mg/mL. To avoid precipitation, the concentration used in the knees was limited to 50 mg/mL. Ten milliliters of this solution (500 mg vancomycin) was injected once or twice daily for 6 weeks, and serum peak and trough levels were measured twice weekly. The frequency and dose were modified to maintain the serum trough levels between 3 and 10 μg/mL. These serum concentrations were achieved in all patients. Intraarticular vancomycin levels were measured just before one of the daily injections to determine trough concentration and one hour after injection to determine peak concentration. Peak and trough serum vancomycin levels were measured using fluorescent polarization immunoarray (FPIA) [6, 24] after the third dose and twice weekly until the catheters were removed. Intraarticular concentrations of vancomycin were measured after the second week of treatment in two knees (two patients) not included in the MRSA series. The samples were removed by arthrocentesis immediately before and after injection of the intraarticular antibiotic dose. These samples were diluted by a factor of 10, 100, 1000, and 5000 to achieve a concentration that could be measured by the FPIA method.
Patients were mobilized the first day postoperatively and allowed full weight bearing. We used splinting in extension in six knees to protect a repaired quadriceps mechanism or gastrocnemius flap. The remaining knees were started on active and passive range of motion exercises with the supervision of a physiotherapist.
After 6 weeks of treatment, the Hickman catheters were removed surgically and the joint fluid was cultured. The patients were seen 2 weeks later for suture removal, at 3 months for evaluation, and then at yearly intervals for evaluation.
All knees (except the one reoperated for osteomyelitis) were evaluated for tenderness, erythema, and induration by three months postoperative. Serum C-reactive protein concentration and sedimentation rate were evaluated at three months. C-reactive protein level less than 25% above normal and sedimentation rate less than 50% elevated were considered signs of resolved infection. After three months, no additional laboratory tests were obtained. Knee scores were determined using the Knee Society Clinical Rating System [13].
We routinely obtained anteroposterior and lateral radiographs at each office visit. All knees had radiographs taken at 1 month and 3 months, and then at yearly intervals after surgery. One of us (LAW) identified and measured radiolucent lines with a ruler accurate to 0.5 mm. We chose the tibial tubercle and fibular head as landmarks to measure tibial component migration, and the distances from the undersurface of the tibial baseplate to the top of the fibular head and to the tibial tubercle were measured on each radiographic examination. The medial and lateral epicondyles were chosen as the femoral bone landmarks for femoral component migration. Femoral component position was measured relative to the femoral bone landmarks by drawing a line that joined the distal surface of the implant and measuring the distance between this line and the medial and lateral epicondyles. Radiographic signs of migration were defined as a radiolucent line that increased by more than 1 mm on one side of a diaphyseal stem or greater than 1-mm change in distance relative to one of the bone landmarks on two successive radiographic examinations.
Results
Seventeen of the 18 patients had no clinical sign of infection at last followup. All patients except one had laboratory evidence of resolved infection by 3 months postoperative. None of the synovial fluid cultures taken at the time of catheter removal was positive for bacteria. One patient had elevated sedimentation rate and C-reactive protein concentration at 3 months postoperative and redeveloped clinically apparent infection with MRSA 5 months after initial revision and débridement. The knee was reoperated and a fragment of necrotic bone measuring 2 to 3 cm on the anterior surface of the femur was found. This area was under the suprapatellar pouch and had been overlooked at the original débridement. This necrotic bone was excised and the knee was débrided. The implants were loosely adherent to bone. Purulent material and granulation tissue were present in all interfaces, suggesting that the infection had not been eradicated by the initial débridement. Complete redébridement was done, Hickman catheters were inserted, and the knee was treated for 6 weeks with intraarticular vancomycin. At 42 months postoperatively, this knee had no clinical signs of infection. Other than the case with persistent infection, none of the implants has been revised for loosening.
All patients achieved full weightbearing by 3 months after surgery. Mean Knee Society Score was 78 ± 8 at 1 year, 83 ± 9 at 2 years, 84 ± 8 at 5 years, 85 ± 10 at 6 years, and 84 at 8 years. We detected no sign of radiographic migration of the implants in any of the knees (Figs. 4, 5). One knee had a complete radiolucent line around the tibial component, but none of the lines measured greater than 1 mm in diameter, and none widened. Sixteen of the 18 patients (88%) had at least one radiographic view with a radiolucent line under the tibial porous surface at 2 years postoperatively, and 13 knees (72%) had a radiolucent line under the femoral component (all under the anterior femoral flange, which was not porous-coated). None of these lines widened, and all measured 1 mm or less. Four knees had anterior gaps of 2 to 4 mm dating from the time of surgery. These gaps have remained radiographically stable throughout the followup period.
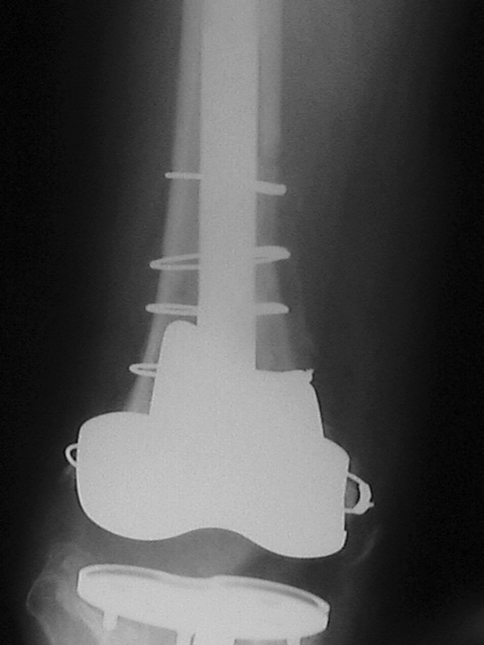
Fig. 4.
An anteroposterior radiograph shows the femur for the same knee as in Fig. 1 at 1 year postoperatively. Bone healing has occurred and the cementless implant is stable. Reprinted with permission and © 2008 Elsevier from Whiteside LA. Two-stage exchange for infected TKA—opposes. Semin Arthroplasty. 2008;19:121–125 [51].
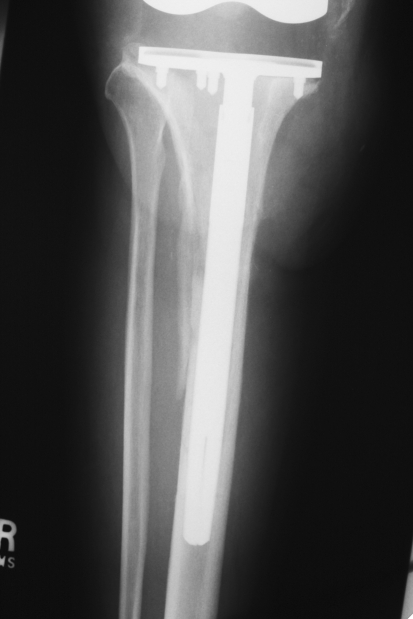
Fig. 5.
An anteroposterior radiograph shows the tibia of the same knee as in Fig. 1 at 1 year postoperatively. The long-stem tibial component is stable and evidence of bone healing is apparent. Reprinted with permission and © 2008 Elsevier from Whiteside LA. Two-stage exchange for infected TKA—opposes. Semin Arthroplasty. 2008;19:121–125 [51].
Mean serum vancomycin peak concentration was 6.1 ± 4.1 μg/mL and mean serum vancomycin trough concentration level was 3.2 ± 1.0 μg/mL at 2 weeks postoperatively. In the two knees (two patients) in which synovial fluid antibiotic concentration was measured, intraarticular vancomycin peak levels were 10,233 μg/mL and 20,167 μg/mL. Intraarticular vancomycin trough levels were 724 μg/mL and 543 μg/mL. All patients maintained trough levels in the desired range. Five patients required decreasing the dosage to 500 mg given once daily, and one required stopping the antibiotics for 4 days. Three required discontinuation of the antibiotic infusion for 2–3 days because of local inflammatory response to precipitated vancomycin. Six patients (six knees) (33%) had elevated blood urea nitrogen and creatinine levels during the 6 weeks of antibiotic infusion and required temporary discontinuation of intraarticular vancomycin for 2 days. Intraarticular infusion was then resumed at a lower dose. None required complete discontinuation of vancomycin infusion for more than four days.
Discussion
Direct antibiotic infusion and single-stage revision using porous-surface implants was developed to eradicate the infection and to provide a well-fixed implant without the morbidity and inconvenience of an antibiotic spacer and second surgical procedure. MRSA infection is especially challenging [12, 15, 23, 45], so we reviewed our results of this procedure in a consecutive series of patients with this bacteriological diagnosis. We sought to determine the effectiveness of this technique to eradicate the infection and to achieve stable fixation of the implants to bone. We also tested peak and trough antibiotic levels in the serum and joint to establish the basic pharmacokinetics of vancomycin injected into the knee.
Validity of the clinical results of this study is limited by the small number of cases, the retrospective nature of the study, the lack of controls and comparative groups, and the relatively short followup time. However, these limitations are overcome to a certain extent by the prospective collection of the data, the completeness of followup, and the fact that all patients were included. Collecting a very large series of infections with resistant organisms would not be possible, so this type of study requires that we accept certain limitations. These limitations do not affect the pharmacokinetic data. Control of the infection was successful in 17 of 18 patients with the first procedure, and also was successful in the failed procedure after débridement and repeat revision. Two-stage revision, using intravenous antibiotics and a bone-cement spacer loaded with antibiotics to deliver antibiotics into the joint is considered the conservative surgical approach to this condition [9, 11, 14, 35], but its clinical results are disappointing. Reinfection rates varying from 11% to 24% have been reported in centers that are highly experienced in care of these difficult cases using two-stage débridement and reimplantation [12, 15, 23, 45]. Our success rate compares favorably to these published results and suggests that direct exchange with antibiotic infusion may offer advantages over the current, more popular treatment methods (Table 1).
Table 1.
Failure rate of total knee arthroplasty for infection with resistant organisms
Fixation of the implants with one-stage revision and cementless implants was successful in this series. Fixation of the implants to bone consistently has been a problem in revision TKA, especially in series with MRSA infection. Mechanical failure rates of 20% to 40% are reported in centers with high surgical volume and recognized expertise in this field [39, 41, 46]. Fixation of implants with cement consistently is less successful in revision cases than in primary cases [11, 39, 48–51], and is likely to be even more difficult with persistent infection with indolent bacteria. Cementless fixation with porous devices in revision arthroplasty has had a high rate of successful fixation in the hip and knee and has become the dominant mode of fixation in revision THA [8, 10, 30]. Our results using cementless fixation technique for revision of infected TKA resemble those of cementless revision THA, and appear to offer an advantage over cemented fixation.
Antibiotics in synovial joints are absorbed in a manner similar to that of antibiotics injected intramuscularly and result in similar serum concentrations [18, 38], so some peripheral therapeutic effect of the antibiotics would be expected. There is also evidence that antibiotics injected into the joint produce concentrations in the adjacent bone that far exceed those achieved by intravenous administration [47], suggesting local therapeutic effects beyond the synovial cavity would be more effective with intraarticular than with intravenous administration of antibiotics.
Our data show that intraarticular infusion of antibiotics achieves concentrations in the synovial fluid that are hundreds of times higher than can be achieved with antibiotic-loaded spacers and intravenous antibiotics. This is similar to the levels reported in earlier reports of direct infusion in humans [26, 32] and in veterinary practice [18]. Intravenous antibiotics, generally used for six weeks after surgery [14], can produce synovial fluid concentrations as high as 50% serum levels when cephalosporins are used [37], but less than 20% of serum concentration when gentamicin is used [18]. The synovial fluid concentration of aminoglycosides exceeds the minimal inhibitory concentration for common organisms only transiently when intravenous antibiotics are used [18]. In cases of resistant bacteria or gram-negative organisms, the levels are too low to be effective [26]. Intraarticular injection of third-generation cephalosporins in horses results in concentrations in synovial fluid in the range of 5,000 to 10,000 μg/mL, while intravenous administration resulted in concentrations of 7–10 μg/mL [22]. Antibiotic concentrations achieved with antibiotic-loaded cement spacers can be high early, but decrease rapidly after the first 24 hours [2, 21, 35, 43]. Depot methods that release antibiotics more rapidly must be used with caution to avoid dangerously high dosage [20]. Although higher initial concentration can be achieved by using larger amounts of antibiotics in the cement [2, 42], only the entrapped antibiotic powder at the surface dissolves into the joint fluid. The total percentage released is reportedly from 1.3% to 14.8% [28]. After 5 days antibiotic release is minimal [17], but low levels may be detected for as long as 340 days postoperative [19]. In cases of resistant bacteria or gram-negative organisms, the levels may be too low to be effective [26]. The low concentration of antibiotics in the synovial fluid and on the surface of the spacer produces conditions that foster development of resistant bacteria [26]. These small colony variants require higher concentrations and longer exposure to high antibiotic levels than can be obtained with intravenous antibiotics and antibiotic-loaded cement spacers [26, 33].
Delivery of antibiotics in concentrations that are achievable with direct infusion requires rethinking the concept of antibiotic resistance. Antibiotic concentration many times higher than minimal bactericidal concentrations can be achieved readily with this technique and can be maintained for weeks even in cases with highly resistant organisms. Currently the single-stage procedure—including débridement and insertion of Hickman catheters followed 6 weeks later by removal of the catheters—is the practice in our institution. Even in cases with highly resistant organisms, we anticipate success using this technique.
Acknowledgments
We thank William Andrea, CMI, for preparation of the illustration, and Diane Morton, MS, for editorial assistance with the manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the research protocol, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
An Institutional Review Board and hospital committee reviewed and approved the protocol for this study when it was undertaken.
This work was performed at Missouri Bone and Joint Research Foundation.
References
- 1.Adams SB, Lescun TB. How to treat septic joints with constant intra-articular infusion of gentamicin or amikacin. AAEP Proc. 2000;46:188–192. [Google Scholar]
- 2.Anguita-Alonso P, Rouse MS, Piper KE, Jacofsky DJ, Osmon DR, Patel R. Comparative study of antimicrobial release kinetics from polymethylmethacrylate. Clin Orthop Relat Res. 2006;445:239–244. doi: 10.1097/01.blo.0000201167.90313.40. [DOI] [PubMed] [Google Scholar]
- 3.Backe HA, Jr, Wolff DA, Windsor RE. Total knee replacement infection after 2-stage reimplantation: results of subsequent 2-stage reimplantation. Clin Orthop Relat Res. 1996;331:125–131. doi: 10.1097/00003086-199610000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz HW, Elson RA, Engelbrecht E, Lodenkamper H, Rottger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63:342–353. doi: 10.1302/0301-620X.63B3.7021561. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz HW, Elson RA, Heinert K. Antibiotic-loaded acrylic cement: current concepts. Clin Orthop Relat Res. 1984;190:96–108. [PubMed] [Google Scholar]
- 6.Dandliker WB, Feigen GA. Quantification of the antigen-antibody reaction by the polarization of fluorescence. Biochem Biophys Res Commun. 1961;5:299. doi: 10.1016/0006-291X(61)90167-X. [DOI] [PubMed] [Google Scholar]
- 7.Davenport K, Traina S, Perry C. Treatment of acutely infected arthroplasty with local antibiotics. J Arthroplasty. 1991;6:179–183. doi: 10.1016/s0883-5403(11)80014-8. [DOI] [PubMed] [Google Scholar]
- 8.Garazzino S, Aprato A, Baietto L, D’Avolio A, Maiello A, DeRosa FG, Aloj D, Siccardi M, Biasibetti A, Masse A, DiPerri G. Glycopeptide bone penetration in patients with septic pseudoarthrosis of the tibia. Clin Pharmacokinet. 2008;47:793–805. doi: 10.2165/0003088-200847120-00004. [DOI] [PubMed] [Google Scholar]
- 9.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton WG, Cashen DV, Ho H, Hopper RH, Jr, Engh CA. Extensively porous-coated stems for femoral revision: a choice for all seasons. J Arthroplasty. 2007;22(4 suppl 1):106–110. doi: 10.1016/j.arth.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Hanssen AD, Trousdale RT, Osmon DR. Patient outcome with reinfection following reimplantation for the infected total knee arthroplasty. Clin Orthop Relat Res. 1995;321:55–67. [PubMed] [Google Scholar]
- 12.Hirakawa K, Stulberg BN, Wilde AH, Bauer TW, Secic M. Results of two-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–28. doi: 10.1016/S0883-5403(98)90071-7. [DOI] [PubMed] [Google Scholar]
- 13.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 14.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087–1098. [PubMed] [Google Scholar]
- 15.Kilgus DJ, Howe DJ, Strang A. Results of periprosthetic hip and knee infections caused by resistant bacteria. Clin Orthop Relat Res. 2002;404:116–124. doi: 10.1097/00003086-200211000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Kordelle J, Frommelt L, Kluber D, Seemann K. Results of one-stage endoprosthesis revision in periprosthetic infection caused by methicillin-resistant Staphylococcus aureus [in German] Z Orthop Ihre Grenzgeb. 2000;138:240–244. doi: 10.1055/s-2000-10143. [DOI] [PubMed] [Google Scholar]
- 17.Kuechle DK, Landon GC, Musher DM, Noble PC. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin Orthop Relat Res. 1991;264:302–308. [PubMed] [Google Scholar]
- 18.Lloyd KC, Stover SM, Pascoe JR, Baggot JD, Kurpershoek C, Hietala S. Plasma and synovial fluid concentrations of gentamicin in horses after intra-articular administration of buffered and unbuffered gentamicin. Am J Vet Res. 1988;49:644–649. [PubMed] [Google Scholar]
- 19.Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty. 1998;13:331–338. doi: 10.1016/S0883-5403(98)90179-6. [DOI] [PubMed] [Google Scholar]
- 20.McLaren AC. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin Orthop Relat Res. 2004;427:101–106. doi: 10.1097/01.blo.0000143554.56897.26. [DOI] [PubMed] [Google Scholar]
- 21.McLaren RL, McLaren AC, Vernon BL. Generic tobramycin elutes from bone cement faster than proprietary tobramycin. Clin Orthop Relat Res. 2008;466:1372–1376. doi: 10.1007/s11999-008-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills ML, Rush BR, St Jean G, Guaghan EM, Mosier D, Gibson E, Freeman L. Determination of synovial fluid and serum concentrations, and morphologic effects of intraarticular ceftiofur sodium in horses. Vet Surg. 2000;29:398–406. doi: 10.1053/jvet.2000.9141. [DOI] [PubMed] [Google Scholar]
- 23.Mittal Y, Fehring TK, Hanssen A, Marculescu C, Odum S, Osmon D. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J Bone Joint Surg Am. 2007;89:1227–1231. doi: 10.2106/JBJS.E.01192. [DOI] [PubMed] [Google Scholar]
- 24.Interference Testing in Clinical Chemistry; Tentative Guideline. NCCLS Publication EP7-T. Villanova, PA: National Committee for Clinical Laboratory Standards; 1986. [Google Scholar]
- 25.Nayfeh T, Whiteside LA, Hirsch M. Direct exchange treatment of septic total joint arthroplasty with intra-articular infusion of antibiotics: technique and early results. Orthopedics. 2004;27:987–988. doi: 10.3928/0147-7447-20040901-40. [DOI] [PubMed] [Google Scholar]
- 26.Neut D, Hendriks JG, Horn JR, Mei HC, Busscher HJ. Pseudomonas aeruginosa biofilm formation and slime excretion on antibiotic-loaded bone cement. Acta Orthop. 2005;76:109–114. doi: 10.1080/00016470510030427. [DOI] [PubMed] [Google Scholar]
- 27.Neut D, Belt H, Stokroos I, Horn JR, Mei HC, Busscher HJ. Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J Antimicrob Chemother. 2001;47:885–891. doi: 10.1093/jac/47.6.885. [DOI] [PubMed] [Google Scholar]
- 28.Neut D, Belt H, Horn JR, Mei HC, Busscher HJ. The effect of mixing on gentamicin release from polymethylmethacrylate bone cements. Acta Orthop Scand. 2003;74:670–676. doi: 10.1080/00016470310018180. [DOI] [PubMed] [Google Scholar]
- 29.Neut D, Horn JR, Kooten TG, Mei HC, Busscher HJ. Detection of biomaterial-associated infections in orthopaedic joint implants. Clin Orthop Relat Res. 2003;413:261–268. doi: 10.1097/01.blo.0000073345.50837.84. [DOI] [PubMed] [Google Scholar]
- 30.Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res. 2009;467:1732–1739. doi: 10.1007/s11999-009-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry CR, Hulsey RE, Mann FA, Miller GA, Pearson RL. Treatment of acutely infected arthroplasties with incision, drainage, and local antibiotics delivered via an implantable pump. Clin Orthop Relat Res. 1992;281:216–223. [PubMed] [Google Scholar]
- 32.Perry CR, Pearson RL. Local antibiotic delivery in the treatment of bone and joint infections. Clin Orthop Relat Res. 1991;263:215–226. [PubMed] [Google Scholar]
- 33.Rusthoven JJ, Davies TA, Lerner SA. Clinical isolation and characterization of aminoglycoside-resistant small colony variants of Enterobacter aerogenes. Am J Med. 1979;67:702–706. doi: 10.1016/0002-9343(79)90269-9. [DOI] [PubMed] [Google Scholar]
- 34.Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53. doi: 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed] [Google Scholar]
- 35.Salvati EA, Callaghan JJ, Brause BD, Klein RF, Small RD. Reimplantation in infection: elution of gentamicin from cement and beads. Clin Orthop Relat Res. 1986;207:83–93. [PubMed] [Google Scholar]
- 36.Schneider RK. Treatment of posttraumatic septic arthritis. AAEP Proc. 1988;44:167–171. [Google Scholar]
- 37.Schurman DJ, Burton DS, Kajiyama G. Cefoxitin antibiotic concentration in bone and synovial fluid. Clin Orthop Relat Res. 1982;168:64–68. [PubMed] [Google Scholar]
- 38.Schurman DJ, Hirshman HP, Kajiyama G, Moser K, Burton DS. Cefazolin concentrations in bone and synovial fluid. J Bone Joint Surg Am. 1978;60:359–362. [PubMed] [Google Scholar]
- 39.Shannon BD, Klassen JF, Rand JA, Berry DJ, Trousdale RT. Revision total knee arthroplasty with cemented components and uncemented intramedullary stems. J Arthroplasty. 2003;18(suppl 1):27–32. doi: 10.1016/S0883-5403(03)00301-2. [DOI] [PubMed] [Google Scholar]
- 40.Shaw JA. The use of long-term indwelling catheters for local antibiotic administration into infected joints: a concept report. J Orthop Tech. 1995;3:181–184. [Google Scholar]
- 41.Stuart MJ, Larson JE, Morrey BF. Reoperation after condylar revision total knee arthroplasty. Clin Orthop Relat Res. 1993;286:168–173. [PubMed] [Google Scholar]
- 42.Thabe H, Schill S. Two-stage reimplantation with an application spacer and combined with delivery of antibiotics in the management of prosthetic joint infection. Oper Orthop Traumatol. 2007;19:78–100. doi: 10.1007/s00064-007-1196-4. [DOI] [PubMed] [Google Scholar]
- 43.Belt H, Neut D, Schenk W, Horn JR, Mei HC, Busscher JH. Gentamicin release from polymethylmethacrylate bone cements and Staphylococcus aureus biofilm formation. Acta Orthop Scand. 2000;71:269–625. doi: 10.1080/000164700317362280. [DOI] [PubMed] [Google Scholar]
- 44.Belt H, Neut D, Uges DR, Schenk W, Horn JR, Mel HC, Busscher HJ. Surface roughness, porosity, wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials. 2000;21:1981–1987. doi: 10.1016/S0142-9612(00)00082-X. [DOI] [PubMed] [Google Scholar]
- 45.Volin SJ, Hinrichs SH, Garvin KL. Two-stage reimplantation of total joint infections: a comparison of resistant and non-resistant organisms. Clin Orthop Relat Res. 2004;427:94–100. doi: 10.1097/01.blo.0000143559.34143.3d. [DOI] [PubMed] [Google Scholar]
- 46.Wang CJ, Huang TW, Wang JW, Chen HS. The often poor clinical outcome of infected total knee arthroplasty. J Arthroplasty. 2002;17:608–614. doi: 10.1054/arth.2002.32700. [DOI] [PubMed] [Google Scholar]
- 47.Werner LA, Hardy J, Bertone AL. Bone gentamicin concentration after intra-articular injection or regional intravenous perfusion in the horse. Vet Surg. 2003;32:559–565. doi: 10.1111/j.1532-950X.2003.00559.x. [DOI] [PubMed] [Google Scholar]
- 48.Whiteside LA. Treatment of infected total knee arthroplasty. Clin Orthop Relat Res. 1994;299:169–172. [PubMed] [Google Scholar]
- 49.Whiteside LA. Cementless fixation issues in revision total knee arthroplasty. Instr Course Lect. 1999;48:177–182. [PubMed] [Google Scholar]
- 50.Whiteside LA. Two-stage exchange for infected TKA—opposes. Semin Arthroplasty. 2008;19:121–125. doi: 10.1053/j.sart.2007.12.030. [DOI] [Google Scholar]
- 51.Whiteside LA, Bicalho PS. Radiologic and histologic analysis of morselized allograft in revision total knee replacement. Clin Orthop Relat Res. 1998;357:149–156. doi: 10.1097/00003086-199812000-00020. [DOI] [PubMed] [Google Scholar]
- 52.Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Two-stage reimplantation for the salvage of total knee arthroplasty complicated by infection: further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72:272–278. [PubMed] [Google Scholar]