Abstract
In this study we investigated the effects of intragastric infusion of palatable basic taste substances (umami, sweet, and salty) on the activity of the vagal gastric afferent nerve (VGA), the vagal celiac efferent nerve (VCE), and the splanchnic adrenal efferent nerve (SAE) in anesthetized rats. To test the three selected taste groups, rats were infused with inosine monophosphate (IMP) and l-glutamate (GLU) for umami, with glucose and sucrose for sweet, and with sodium chloride (NaCl) for salty. Infusions of IMP and GLU solutions significantly increased VGA activity and induced the autonomic reflex, which activated VCE and SAE; these reflexes were abolished after sectioning of the VGA. Infusions of glucose, sucrose and NaCl solutions, conversely, had no significant effects on VGA activity. These results suggest that umami substances in the stomach send information through the VGA to the brain and play a role in the reflex regulation of visceral functions.
Keywords: Umami substance, Vagal gastric afferent, Vagal celiac efferent, Splanchnic adrenal efferent, Autonomic nerve reflex
Introduction
Taste and smell stimulation of the oropharyngeal cavity elicits several digestive, endocrinological, thermogenic, cardiovascular, and renal responses, which are collectively termed cephalic phase responses [1–3]. The gastric phase response, however, involves fewer reflexive responses than the cephalic phase and has not yet been well described.
Umami, one of the five basic tastes, is induced by free l-glutamate (GLU) and 5′-mononucleotides, for example inosine 5′-monophosphate (IMP) and guanine 5′-monophosphate (GMP). Umami taste stimulation of the oral cavity with monosodium glutamate (MSG) solution activates the efferent activity of the gastric, pancreatic, and hepatic vagus nerves [4, 5] and is associated with an increase in insulin secretion in rats [6]. In the stomach (gastric phase), the vagal gastric afferent nerve (VGA) can detect mechanical and chemical (acid and alkali) stimulation in both feline [7, 8] and rodent models [9]. Mathis et al. [10] reported that the afferent nerve detects gastric distention in the rat but fails to detect the luminal nutrient information, for example carbohydrate and protein content. However, we previously found that the VGA in the rat responded to an intragastric infusion of MSG solution [11] and that this response was specific to GLU and did not generalize to other amino acids [12]. Moreover, intragastric infusion of the MSG solution caused a vago-vagal reflex that increased the efferent activity of the gastric and pancreatic vagus nerves in the rat [11]. It is still unclear whether IMP also affects VGA activity. In addition, there has been no report that IMP and MSG could affect the efferent activity of the celiac vagus nerve (VCE), which regulates intestinal function, and the adrenal splanchnic nerve (SAE), which affects the release of catecholamine. In this study, we examined the possibility that intragastric infusion of umami substances can cause changes in VGA activity and affect both VCE and SAE activity in anesthetized rats.
Materials and methods
Animals and diets
Male Wistar (Slc: Wistar) rats weighing 250–300 g were obtained from Japan SLC (Hamamatsu, Japan). Food (MF; Oriental Yeast, Tokyo, Japan) and water were freely available until the day of the experiment; 12 h before the experiment, food, but not water, was removed. All of the experimental procedures were approved by the Institutional Animal Care and Use Committee and conformed to the standards for the use of laboratory animals published by the Institute of Laboratory Animal Resources (National Academy of Sciences, USA).
Experiments
The surgical techniques and other experimental methods have been extensively documented elsewhere [5]. Briefly, the animals were anesthetized with an intraperitoneal injection of 1–1.2 g/kg urethane (Wako Pure Chemicals, Osaka, Japan). The taste substance solutions for intragastric infusion were applied through a catheter inserted either into the forestomach or into the stomach through a small incision in the duodenum. The output line was captured by another catheter placed through the pylorus. To eliminate the mechanical response of intragastric infusion, the output catheter was not shut. The pylorus of the stomach was ligated with a silk suture. Using a dissecting microscope, nerve filaments were isolated from the severed peripheral end of the gastric branch of the ventral vagus nerve, and afferent nerve activity was recorded as multi-units with a pair of silver wire electrodes. The hepatic and accessory celiac branches of the vagus nerve were intact. Efferent nerve activity was similarly recorded from a nerve filament dissected from the severed central end of the celiac branch of the vagus nerve and the adrenal branch of the splanchnic nerve. Nerve activity was amplified (DPA-100E; Dia Medical, Tokyo, Japan), displayed on an oscilloscope, and stored on magnetic tape. A rate meter (DSE-325P; Dia Medical) with a rest time of 5 s was used to observe the time course of the nerve activity that was recorded with a pen recorder (Recti-Horiz-8K; Sanei, Tokyo, Japan). The discharge rate is expressed as the mean ± standard error of the mean (SEM). Solutions of IMP (1, 3, 10, and 30 mM) or MSG (150 mM) were used as umami substance. Sweet and salty substances were a 277.5 mM solution of glucose, a 292.1 mM solution of sucrose, and a 154 mM solution of NaCl (physiological saline). MSG, glucose, sucrose, and NaCl solutions were isotonic solutions [4, 12]. Thirty millimolar mannose was used as a hypotensive solution. IMP, MSG, glucose, sucrose, NaCl, and mannose were each dissolved in water. These solutions were infused into the stomach in 2 ml volumes at a flow rate of 1 ml/min with a catheter. After verifying that the solution had an effect on vagal gastric afferent nerve activity, we examined the effects of the solution on the efferent activity of the celiac vagus and the adrenal splanchnic nerves. For vagotomized experiments, the gastric branches of both the ventral and dorsal vagus nerve were vagotomized, and the efferent activity was recorded 30 min after the baseline recording, for signal stabilization. We then examined the effects of the solution on the vital functions, including heart rate, systolic blood pressure, diastolic blood pressure, mean blood pressure, respiration rate, and body temperature of the rats that were not used for the recording of nerve activity. These vital signals were measured with a transducer (MLT0699, MLT1010, ADInstruments, Castle Hill, Australia) and a thermometer (BAT-12, Physitemp Instruments, Clifton, NJ, USA) and were acquired via a PowerLab interface (PowerLab, ADInstruments), and viewed online using LabCart 7 software for Windows (ADInstruments). After the experiment was finished, the rat was euthanized with an overdose of urethane. Individual experimental data were acquired from 91 rats. Inosine 5′-monophosphate disodium salt (IMP) and MSG were obtained from Ajinomoto (Tokyo, Japan). Glucose, sucrose, NaCl, and mannose were obtained from Wako Pure Chemicals (Osaka, Japan).
Data analysis
The effects of intragastric infusion on nerve activity were determined by comparing the mean number of spikes per 5 s over 50 s (i.e., the mean value of 10 successive measured samples) before the infusion of taste substances and 30 min after. The treatment effects were statistically evaluated using Student’s paired t test or a one-way repeated ANOVA with a Dunnett’s multiple comparison post-hoc test. A probability of p < 0.05 was considered statistically significant.
Results
To examine the effects of IMP on VGA activity, we used intragastric infusions of the IMP solution and recorded VGA activity. After infusion with the 30 mM IMP solution, a long-lasting increase in afferent activity was observed (Fig. 1a). The time course response to IMP on VGA activity is summarized in Fig. 1b. The discharge rate before and 10, 20, and 30 min after infusion was 64.5 ± 1.3, 77.5 ± 2.6*, 76.8 ± 4.5*, and 86.5 ± 3.9** impulses/5 s, respectively (n = 5, *p < 0.05, **p < 0.01 compared with the value of the pre-IMP infusion, one-way repeated ANOVA with post-hoc Dunnett’s method). These data show that the effect of 30 mM IMP on VGA activity is highest 30 min after intragastric infusion. Thus, we determined that nerve activity was evaluated 30 min after administration of the taste substances. The concentration-dependent response to IMP on VGA activity is summarized in Fig. 1c. The change in discharge rate for the 1, 3, 10, and 30 mM IMP infusions was 0.0 ± 2.7, 14.3 ± 3.4*, 18.0 ± 5.3*, and 22.0 ± 3.8** impulses/5 s, respectively (n = 5, *p < 0.05, **p < 0.01 compared with the value of the pre-IMP infusion, paired t test). This shows that afferent discharge rates above baseline were dose-dependently increased 30 min after each dose of the IMP application (3, 10, and 30 mM); however, intragastric infusion of 1 mM IMP had no significant effect on the discharge rates. Therefore, the threshold concentration for IMP had to be below 3 mM.
Fig. 1.
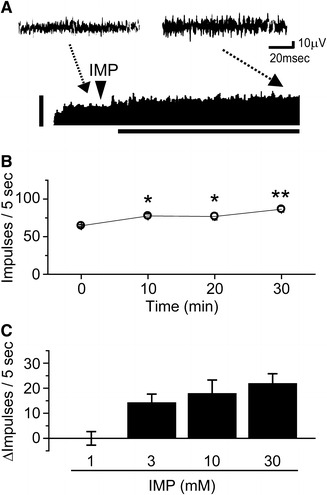
Effects of intragastric infusion of IMP on the afferent discharge rate of a vagal gastric nerve (VGA) filament. a Example of intragastric infusion of 30 mM IMP on VGA activity. Actual wave recording at each point was displayed before (left) and after 30 min of (right) intragastric infusion of IMP (Top). Representative recording of gastric afferent discharge displayed as a sequential rate histogram after intragastric infusion of IMP (Bottom). Arrowheads indicate the points at which the IMP solution was infused. The vertical bar indicates 100 impulses/5 s. The horizontal bar indicates 30 min. b The time course of changes in VGA activity from 5 different rats in which 30 mM IMP was administrated. *p < 0.05, **p < 0.01, one-way repeated ANOVA with the post-hoc Dunnett’s method. Vertical bars indicate ±SEM. c Dose-dependent effects of luminal IMP on the afferent discharge rate of VGA activity. Each IMP solution (1, 3, 10, and 30 mM) was introduced into the rat stomach, and the mean change in discharge rate above baseline at 30 min was plotted. Columns and vertical bars represent mean ± SEM from 5 different rats
Next, to examine the effects of other palatable taste substances on VGA activity, we used intragastric infusion of MSG, glucose, sucrose, and NaCl solutions and recorded VGA activity. After infusion with the MSG solution, an increase in afferent activity was observed (n = 6, p < 0.01 compared with the value of the pre-MSG infusion, paired t test; Fig. 2; Table 1). However, intragastric infusion of glucose (n = 5), sucrose (n = 5), and NaCl (n = 5) solutions resulted in no significant changes in the discharge rate, as summarized in Fig. 2 and Table 1. With the exception of IMP, all of the infusion solutions were isotonic. We then addressed the possibility that hypotonic solutions could have an effect on VGA activity. Intragastric infusion of 30 mM mannose, which is more hypotonic than 30 mM IMP, had no significant effect on discharge rates after 30 min (n = 5; Table 1).
Fig. 2.
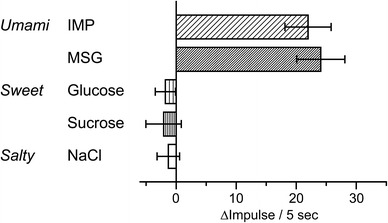
Effects of intragastric infusion of taste substances on the afferent discharge rate of a VGA filament. Each solution (30 mM IMP, 150 mM MSG, 277.5 mM glucose, 292.1 mM sucrose, and 154 mM NaCl) was introduced into the rat stomach, and the mean discharge rate above baseline at 30 min was plotted. Columns and horizontal bars represent mean ± SEM from 5 rats
Table 1.
Effects of intragastric infusion of taste substances on gastric vagal afferent activity
| Taste substances | Gastric afferent discharge rate (impulses/5 s ± SEM) | ||
|---|---|---|---|
| Before | After 30 min | ΔDischarge rate | |
| Umami | |||
| 30 mM IMP | 64.5 ± 1.3 | 86.5 ± 3.9** | 22.0 ± 3.8 |
| 150 mM MSG | 65.6 ± 4.7 | 89.7 ± 7.4** | 24.1 ± 4.0 |
| Sweet | |||
| 277.5 mM Glucose | 57.2 ± 4.5 | 55.4 ± 3.6 | −1.8 ± 1.7 |
| 292.1 mM Sucrose | 57.7 ± 3.2 | 55.6 ± 2.1 | −2.1 ± 2.9 |
| Salty | |||
| 154 mM NaCl | 61.3 ± 2.9 | 60.0 ± 1.8 | −1.3 ± 1.9 |
| Hypotonic solution | |||
| 30 mM Mannose | 55.8 ± 3.2 | 54.5 ± 2.9 | −1.4 ± 2.6 |
** p < 0.01, paired t test
To investigate the autonomic reflex, we next examined the effects of intragastric infusion of IMP and MSG solutions on VCE activity. After intragastric infusion of IMP solution a significant increase was seen in VCE activity (n = 4, p < 0.05, paired t test; Fig. 3a, c; Table 2). A long-lasting increase in efferent activity was also observed after intragastric infusion of the MSG solution (n = 5, p < 0.01, paired t test; Fig. 3b, c; Table 2). We then addressed the hypothesis that these gastro-intestinal reflex effects were mediated via the VGA pathway. When the VGA was sectioned, intragastric infusion of IMP and MSG solutions had no effect on VCE activity (IMP, n = 4; MSG, n = 4; Table 2).
Fig. 3.
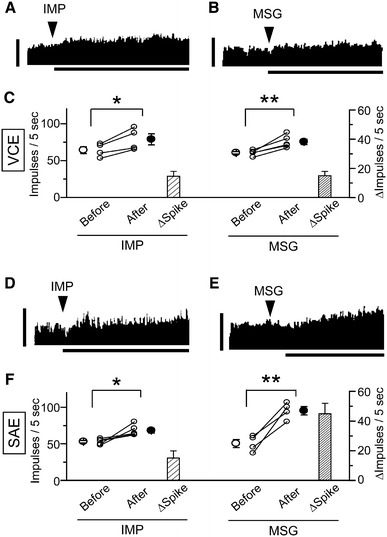
Effects of intragastric infusion of umami substances on the efferent discharge rate of a vagal celiac nerve (VCE) and a sympathetic adrenal nerve (SAE) filament. a, b Examples of intragastric infusion of a 30 mM IMP and b 150 mM MSG on VCE activity. Arrowheads indicate points at which the umami substance solution was infused. The vertical bar indicates 100 impulses/5 s. The horizontal bar indicates 30 min. c Summary plot and summary of changes in discharge rate showing the impulse values of IMP and MSG on VCE activity measured before and after administration. Data from the same rat are connected by a line. Open and closed circles with bars represent the mean ± SEM. Columns and vertical bars represent mean changes in spike ±SEM. d, e Examples of intragastric infusion of d 30 mM IMP and e 150 mM MSG on SAE activity. Arrowheads indicate points at which the umami substance solution was infused. The vertical bar indicates 100 impulses/5 s. The horizontal bar indicates 30 min. f Summary plot and summary of changes in discharge rate showing the impulse values of IMP and MSG on SAE activity measured before and after administration. Data from the same rat are connected by a line. Open and closed circles with bars represent the mean ± SEM. Columns and vertical bars represent mean changes in spike ±SEM. *p < 0.05, **p < 0.01, paired t test
Table 2.
Effects of intragastric infusion of umami substances on vagal celiac and sympathetic adrenal efferent activity
| Nerve discharge rate (impulses/5 s ± SEM) | ||
|---|---|---|
| 30 mM IMP | 150 mM MSG | |
| Vagal celiac efferent | ||
| Normal gastric afferent | ||
| Before | 64.2 ± 4.6 | 60.5 ± 1.7 |
| After 30 min | 78.8 ± 7.5* | 75.5 ± 3.8** |
| ΔDischarge rate | 14.6 ± 3.4 | 15.1 ± 4.8 |
| Vagotomized | ||
| Before | 59.6 ± 2.1 | 73.1 ± 2.2 |
| After 30 min | 60.4 ± 1.8 | 73.9 ± 1.3 |
| ΔDischarge rate | 0.8 ± 0.8 | 0.2 ± 1.9 |
| Sympathetic adrenal efferent | ||
| Normal gastric afferent | ||
| Before | 53.0 ± 1.7 | 50.4 ± 5.5 |
| After 30 min | 68.1 ± 3.4* | 95.2 ± 5.7** |
| ΔDischarge rate | 15.0 ± 3.0 | 44.9 ± 7.1 |
| Vagotomized | ||
| Before | 68.1 ± 1.4 | 66.9 ± 4.3 |
| After 30 min | 68.3 ± 3.3 | 70.5 ± 4.1 |
| ΔDischarge rate | 0.8 ± 1.8 | 3.6 ± 1.7 |
* p < 0.05, paired t test, ** p < 0.01, paired t test
We also examined the effects of intragastric infusion of IMP and MSG solutions on SAE activity. Intragastric infusion of IMP solution significantly increased SAE activity (n = 5, p < 0.05, paired t test; Fig. 3d, f; Table 2). Intragastric infusion of MSG solution also led to a gradual and clear increase in the discharge rate (n = 4, p < 0.01, paired t test; Fig. 3e, f; Table 2). We then addressed whether these gastro-adrenal reflex effects were mediated via the VGA pathway. When the VGA was sectioned, intragastric infusion of IMP and MSG solutions had no effect on SAE activity (IMP, n = 4; MSG, n = 5; Table 2). Because of the increase in SAE activity on intragastric infusion of IMP and MSG, we investigated the effect of intragastric infusion of IMP and MSG solutions on vital functions (heart rate, systolic blood pressure, diastolic blood pressure, mean blood pressure, respiration rate, body temperature). However, intragastric infusion of IMP (n = 5) and MSG (n = 5) solutions resulted in no significant changes in the vital functions, as summarized in Table 3. We did not investigate the effect of glucose, sucrose, or NaCl solutions on VCE or SAE activity because these solutions did not cause a significant change in VGA activity.
Table 3.
Effects of intragastric infusion of umami substances on the vital functions
| Umami substances | Before | 10 min | 20 min | 30 min |
|---|---|---|---|---|
| 30 mM IMP | ||||
| Heart rate (BPM) | 394.5 ± 22.5 | 397.4 ± 21.7 | 400.5 ± 26.0 | 402.5 ± 24.0 |
| Systolic blood pressure (mmHg) | 96.7 ± 6.7 | 100.3 ± 6.1 | 95.3 ± 7.2 | 93.0 ± 5.3 |
| Diastolic blood pressure (mmHg) | 53.7 ± 5.3 | 55.6 ± 5.2 | 53.0 ± 4.6 | 51.6 ± 3.4 |
| Mean blood pressure (mmHg) | 66.8 ± 5.2 | 69.0 ± 3.3 | 65.9 ± 4.9 | 64.3 ± 3.5 |
| Respiration rate (BPM) | 101.2 ± 7.9 | 100.8 ± 8.9 | 103.2 ± 7.2 | 103.2 ± 8.0 |
| Body temperature (°C) | 37.3 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.2 ± 0.1 |
| 150 mM MSG | ||||
| Heart rate (BPM) | 389.6 ± 9.6 | 387.3 ± 8.5 | 392.4 ± 9.8 | 396.0 ± 11.9 |
| Systolic blood pressure (mmHg) | 79.3 ± 6.2 | 78.6 ± 6.0 | 78.3 ± 5.2 | 75.7 ± 5.6 |
| Diastolic blood pressure (mmHg) | 44.7 ± 4.2 | 44.3 ± 3.8 | 44.7 ± 3.5 | 43.8 ± 3.8 |
| Mean blood pressure (mmHg) | 56.3 ± 5.0 | 55.7 ± 4.6 | 56.0 ± 4.3 | 54.4 ± 4.6 |
| Respiration rate (BPM) | 116.5 ± 14.1 | 113.4 ± 12.6 | 113.7 ± 13.6 | 111.9 ± 11.7 |
| Body temperature (°C) | 37.2 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.1 |
BPM beat per minute
Discussion
In this work, we illustrate that intragastric infusion of IMP causes changes in VGA activity and has an effect on autonomic reflexes, whereas administration of glucose, sucrose, and NaCl has no effect. Previously, we reported that intragastric infusion of MSG increased afferent activity of the vagal gastric nerve in the rat [11, 12]. In this study, intragastric infusion of IMP gradually and dose-dependently increased the afferent activity of the vagal gastric nerve (Fig. 1; Table 1). This finding was not because of hypo-osmolality because the 30 mM mannose, which is more hypotonic than the 30 mM IMP (Table 1), had no effect. Moreover, the enhancement of VGA activity by IMP and MSG caused vago-vagal and vago-sympathetic reflexes. Intragastric infusion of IMP affected both VCE and SAE activity in the same manner as MSG (Fig. 3; Table 2), and sectioning of the VGA blocked the effects of both IMP and MSG (Table 2). Recently, taste receptor (T1R1/T1R3) and metabotropic glutamate receptor 1, which are expressed on the tongue and are possible candidates for luminal amino acid sensors, were found in the gastrointestinal tract of the mouse and rat [13–15]. In addition, it has been suggested that T1R1/T1R3 could be activated by both glutamate and IMP [16, 17]. These data suggest that different umami substances might activate the same sensor on the gastric lumen rather than directly stimulating the autonomic centers within the brain.
The increase in VCE activity triggered by IMP and MSG infusions may contribute to motility and secretion in the small intestine. Vagal stimulation causes either excitation followed by inhibition or inhibition followed by excitation of the motility in the isolated small intestine in dogs [18, 19] and rabbits [20], which indicates that the vagal efferent nerve contains both excitatory and inhibitory pathways. Thus, it is unclear whether the increase in VCE activity observed in our study would increase or reduce the motility of the small intestine; however, some effect on the motility of the small intestine is predicted. Moreover, vagal stimulation increases the intestinal secretion of mucosal bicarbonate in cats [21, 22] and rats [22]. These data indicate that IMP and MSG can control small intestine function in the gastric phase.
The increase in SAE activity in response to 30 mM IMP and 150 mM MSG infusions may cause an increase in the release of catecholamines from the adrenal medulla. In Wistar (Slc: Wistar) rats, 120 mM MSG is weakly aversive for intake, but MSG concentrations higher than 240 mM are significantly aversive for intake [23]. In our study, therefore, it is likely that the increase in SAE activity was a response to a rather stressful stimulation even though the vital functions did not change (Table 3). Others have hypothesized that the cephalic phase of thermogenesis may be related to levels of catecholamines [24, 25]. Recently, Kondoh and Torii [26] reported that MSG intake suppressed weight gain in the rat, which may be partially related to the release of catecholamines in the cephalic and gastric phases.
This study has shown that intragastric infusion of glucose, sucrose, and NaCl has no effect on VGA activity (Fig. 2). This result is consistent with previous studies showing that application of 2 M glucose and 154 mM NaCl to the gastric mucosa had no effect on VGA activity in the rat [9, 12]. Our results support this finding and suggest that the isotonic NaCl solution is unable to activate the sodium sensor in the stomach. However, the report that the glucosensitive fibers are present in the antral portion of the stomach in the cat [27] is not consistent with our result. This disagreement may result from different recording methods or species. We used a multi-units recording of VGA, whereas the previous method used extracellular recordings from nodose ganglion neurons. Clarke and Davison used single-unit recording in their study [9] and found that application of glucose to the gastric mucosa had no significant effect on VGA activity in the rat. Evidently, then, the disagreement is not a result of the recording method. As for the difference between species, it is known that cats cannot taste sweet substances, but rats can [28]. This difference may be attributed to differences in the species’ stomachs. Our data support Clarke and Davison’s report [9] and suggest that a sensor for sweet substances, for example glucose and sucrose, may not exist in the stomach of the rat.
In this study, among the preferred substances ingested for energy (sweet), minerals (salty), or protein (umami), only umami was sensed by the luminal mucosa in the stomach. This substance sent information through the VGA to the brain, where the information played a role in the reflex regulation of visceral functions. These effects seem to be important for the process of nutritional ingestion and metabolism, as well as for processes in the cephalic phase.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Giduck SA, Threatte RM, Kare MR. Cephalic reflexes: their role in digestion and possible roles in absorption and metabolism. J Nutr. 1987;117:1191–1196. doi: 10.1093/jn/117.7.1191. [DOI] [PubMed] [Google Scholar]
- 2.Mattes RD. Physiological responses to sensory stimulation by food: nutritional implications. J Am Diet Assoc. 1997;97:406–410. doi: 10.1016/S0002-8223(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 3.Zafra MA, Molina F, Puerto A. The neural/cephalic phase reflexes in the physiology of nutrition. Neurosci Biobehav Rev. 2006;30:1032–1044. doi: 10.1016/j.neubiorev.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Niijima A. Effect of umami taste stimulations on vagal efferent activity in the rat. Brain Res Bull. 1991;27:393–396. doi: 10.1016/0361-9230(91)90131-3. [DOI] [PubMed] [Google Scholar]
- 5.Niijima A. Effects of oral and intestinal stimulation with umami substance on gastric vagus activity. Physiol Behav. 1991;49:1025–1028. doi: 10.1016/0031-9384(91)90218-D. [DOI] [PubMed] [Google Scholar]
- 6.Niijima A, Togiyama T, Adachi A. Cephalic-phase insulin release induced by taste stimulus of monosodium glutamate (umami taste) Physiol Behav. 1990;48:905–908. doi: 10.1016/0031-9384(90)90247-2. [DOI] [PubMed] [Google Scholar]
- 7.Iggo A. Gastric mucosal chemoreceptors with vagal afferent fibres in the cat. Q J Exp Physiol Cogn Med Sci. 1957;42:398–409. doi: 10.1113/expphysiol.1957.sp001284. [DOI] [PubMed] [Google Scholar]
- 8.Davison JS. Response of single vagal afferent fibres to mechanical and chemical stimulation of the gastric and duodenal mucosa in cats. Q J Exp Physiol Cogn Med Sci. 1972;57:405–416. doi: 10.1113/expphysiol.1972.sp002176. [DOI] [PubMed] [Google Scholar]
- 9.Clarke GD, Davison JS. Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. J Physiol. 1978;284:55–67. doi: 10.1113/jphysiol.1978.sp012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathis C, Moran TH, Schwartz GJ. Load-sensitive rat gastric vagal afferents encode volume but not gastric nutrients. Am J Physiol Regul Integr Comp Physiol. 1988;274:R280–R286. doi: 10.1152/ajpregu.1998.274.2.R280. [DOI] [PubMed] [Google Scholar]
- 11.Niijima A. Reflex effects of oral, gastrointestinal and hepatoportal glutamate sensors on vagal nerve activity. J Nutr. 2000;130:971S–973S. doi: 10.1093/jn/130.4.971S. [DOI] [PubMed] [Google Scholar]
- 12.Uneyama H, Niijima A, San Gabriel A, Torii K. Luminal amino acid sensing in the rat gastric mucosa. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1163–G1170. doi: 10.1152/ajpgi.00587.2005. [DOI] [PubMed] [Google Scholar]
- 13.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 14.Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 15.San Gabriel AM, Maekawa T, Uneyama H, Yoshie S, Torii K. mGluR1 in the fundic glands of rat stomach. FEBS Lett. 2007;581:1119–1123. doi: 10.1016/j.febslet.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 18.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama S. Effects of stimulation of the vagus nerve on the movements of the small intestine. Jpn J Physiol. 1965;15:243–252. doi: 10.2170/jjphysiol.18.373. [DOI] [PubMed] [Google Scholar]
- 20.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1901;26:125–138. doi: 10.1113/jphysiol.1901.sp000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nylander O, Flemström G, Delbro D, Fändriks L. Vagal influence on gastroduodenal HCO3− secretion in the cat in vivo. Am J Physiol Gastrointest Liver Physiol. 1987;252:G522–G528. doi: 10.1152/ajpgi.1987.252.4.G522. [DOI] [PubMed] [Google Scholar]
- 22.Fändriks L, Jönson C. Vagal and sympathetic control of gastric and duodenal bicarbonate secretion. J Intern Med Suppl. 1990;732:103–107. doi: 10.1111/j.1365-2796.1990.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 23.Kondoh T, Mori M, Ono T, Torii K. Mechanisms of umami taste preference and aversion in rats. J Nutr. 2000;130:966S–970S. doi: 10.1093/jn/130.4.966S. [DOI] [PubMed] [Google Scholar]
- 24.LeBlanc J, Cabanac M, Samson P. Reduced postprandial heat production with gavage as compared with meal feeding in human subjects. Am J Physiol. 1984;246:E95–E101. doi: 10.1152/ajpendo.1984.246.1.E95. [DOI] [PubMed] [Google Scholar]
- 25.Diamond P, Brondel L, LeBlanc L. Palatability and postprandial thermogenesis in dogs. Am J Physiol. 1985;248:E75–E79. doi: 10.1152/ajpendo.1985.248.1.E75. [DOI] [PubMed] [Google Scholar]
- 26.Kondoh T, Torii K. MSG intake suppresses weight gain, fat deposition, and plasma leptin levels in male Sprague–Dawley rats. Physiol Behav. 2008;95:135–144. doi: 10.1016/j.physbeh.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 27.El Ouazzani T, Mei N. Acido- et glucorecepteurs vagaux de la région gastro-duodénale. Exp Brain Res. 1981;42:442–452. doi: 10.1007/BF00237509. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Li W, Wang H, Bayley DL, Cao J, Reed DR, Bachmanov AA, Huang L, Legrand-Defretin V, Beauchamp GK, Brand JG. Cats lack a sweet taste receptor. J Nutr. 2006;136:1932S–1934S. doi: 10.1093/jn/136.7.1932S. [DOI] [PMC free article] [PubMed] [Google Scholar]


