Abstract
Nonylphenol (NP) is an endocrine disruptor present as a pollutant in river sediment. Biodegradation of NP can reduce its toxicological risk. As sediments are mainly anaerobic, degradation of linear (4-n-NP) and branched nonylphenol (tNP) was studied under methanogenic, sulphate reducing and denitrifying conditions in NP polluted river sediment. Anaerobic bioconversion was observed only for linear NP under denitrifying conditions. The microbial population involved herein was further studied by enrichment and molecular characterization. The largest change in diversity was observed between the enrichments of the third and fourth generation, and further enrichment did not affect the diversity. This implies that different microorganisms are involved in the degradation of 4-n-NP in the sediment. The major degrading bacteria were most closely related to denitrifying hexadecane degraders and linear alkyl benzene sulphonate (LAS) degraders. The molecular structures of alkanes and LAS are similar to the linear chain of 4-n-NP, this might indicate that the biodegradation of linear NP under denitrifying conditions starts at the nonyl chain. Initiation of anaerobic NP degradation was further tested using phenol as a structure analogue. Phenol was chosen instead of an aliphatic analogue, because phenol is the common structure present in all NP isomers while the structure of the aliphatic chain differs per isomer. Phenol was degraded in all cases, but did not affect the linear NP degradation under denitrifying conditions and did not initiate the degradation of tNP and linear NP under the other tested conditions.
Keywords: 4-n-nonylphenol, Biodegradation, Denitrifying conditions, Alkane, LAS
Introduction
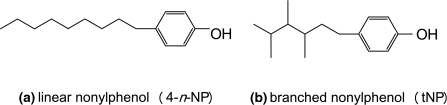
Nonylphenol (NP) is a hydrophobic compound which is widely used as intermediate in the production of nonylphenol ethoxylates (Giger et al. 1984; Liber et al. 1999). These nonylphenol ethoxylates are used in household and cleaning products as a surfactant. They are transformed in anaerobic waste water treatment plants to NP as an end product, which enters the environment via the sludges (Giger et al. 1984; Di Corcia et al. 1998). Nonylphenol exists of a mixture of isomers with a hydrophilic phenol group and a linear (4-n-NP) or a branched (tNP) carbon chain with nine carbon atoms (Fig. 1). Due to its chemical properties, it adsorbs to and accumulates in sediment (Sturm 1973; Kravetz 1983) as observed in river sediments in, e.g. Spain, Germany and Taiwan (Ding et al. 1999; Fries and Püttmann 2003; Navarro-Ortega et al. 2010). Since NP is an endocrine disruptor, degradation of NP in the sediment to non toxic compounds is of great interest (Nimrod and Benson 1996). Biodegradation under aerobic conditions is well described, as reviewed by Corvini et al. (2006). However, anaerobic degradation of NP is of greater importance since sediments are mainly anaerobic (Middeldorp et al. 2003). So far, tNP seems to be persistent under anaerobic conditions for biodegradation (Ekelund et al. 1993; Hesselsoe et al. 2001). Anaerobic biodegradation of 4-n-NP by bacteria has only been reported in sediment from the Erren River in Taiwan (Chang et al. 2004) under sulphate reducing, methanogenic and nitrate reducing conditions, respectively. Anaerobic bacterial degradation of 4-n-NP in soil and sludge from a waste water treatment plant has also been mentioned and 4-n-NP degrading strains were closely related to Bacillus niacini, Bacillus cereus and Acinetobacter baumanni (Chang et al. 2005; Chang et al. 2007).
Fig. 1.
Molecular structure of a linear nonylphenol and b a branched nonylphenol isomer
To our knowledge, biodegradation of tNP under anaerobic conditions has not been described so far. Stimulation or initiation of the tNP degradation process under anaerobic conditions with a structure analogue of NP is a possibility, as reported in other studies for the degradation of polychlorinated biphenyls initiated by terpenes (Focht 1995; Tandlich et al. 2001). Based on the structure of 4-n-NP and tNP, phenol can be a possible structure analogue for initiation of NP degradation via the aromatic ring, because of the common structure and the biodegradability of phenol under various anaerobic conditions such as denitrifying conditions and sulphate reducing conditions (Bak and Widdel 1986; Tschech and Fuchs 1987; Li et al. 1996; Kuever et al. 2001).
This paper describes the anaerobic degradation of 4-n-NP under denitrifying conditions, as well as the involved microbial populations during the enrichment process. Furthermore, the anaerobic degradation of tNP was tested in the presence and absence of the structure analogue phenol under various anaerobic conditions.
Materials and methods
Chemicals and materials
Nonylphenol technical mixture (tNP) (branched, >94% purity) and 4-n-NP (linear, pestanal, >99% purity) were purchased from Riedel de Haën (Seelze, Germany). Phenol (purity > 99%) was ordered from Merck (Darmstadt, Germany). Other used chemicals were of the highest purity (>99%).
Biodegradation of NP
Nonylphenol polluted sediment was collected in June 2005 from the Spanish Huerva River in Zaragoza (41° 37′ 23″ N, 0° 54′ 28″ W), which is a tributary of the Ebro River. At the moment of sampling, the river water had a temperature of 25.1°C, a redox potential of 525 mV and a pH of 7.82. The water contained 3.8 mg l−1 dissolved oxygen. The dissolved oxygen concentration in the sediment was measured in the lab with an OX-500 micro electrode (Unisense, Aarhus, Denmark) and was below detection limit (<0.01 mg l−1). The redox potential, temperature and pH were not determined in the sediment for practical reasons. Sediment was taken anaerobically with stainless steel cores, and transported on ice to the laboratory. Cores were opened in an anaerobic glove box with ±1% H2-gas and ±99% N2-gas to maintain anaerobic conditions, and the sediment was put in a glass jar. The glass jar was stored at 4°C in an anaerobic box that was flushed with N2-gas.
The sediment contained a mixture of tNP isomers (20 mg kg−1 dry weight), but 4-n-NP was not present in the sediment. The chromatogram of the gas chromatography–mass spectrometry (GC–MS) of the mixture of tNP isomers present in the sediment was comparable to the chromatogram of the tNP technical mixture ordered from Merck. The individual branched isomers were not identified. The total organic carbon fraction of the sediment was 3.5% and contained mainly clay particles with a diameter size < 32 μM.
Biodegradation of 4-n-NP and the mixture of tNP in the sediment was studied with or without the addition of phenol under methanogenic, sulphate reducing and denitrifying conditions. These redox conditions were chosen as they are representative for the conditions in sediments (Berner 1981) and artificial medium was used for the anaerobic incubations instead of the river water because the river water contained oxygen. Briefly, 60 ml bottles were filled with 2 g dry weight sediment and 50 ml methanogenic medium (Holliger et al. 1993), sulphate reducing medium (Stams et al. 1993) or nitrate reducing medium (Evans et al. 1991) was added. The methanogenic medium contained 0.65 g l−1 K2HPO4; 0.20 g l−1 NaH2PO4·2H20; 0.44 g l−1 NH4HCO3; 0.11 g l−1 CaCl2·2H20; 0.10 g l−1 MgCl2·6H20; 3.73 g l−1 NaHCO3; 0.24 g l−1 Na2S·9H20; 0.0005 g l−1 resazurin; 1 ml of trace element solution (containing 2 mg l−1 FeCl2·4H20; 100 mg l−1MnCl2·4H20; 190 mg l−1 CoCl2·6H20; 70 mg l−1 ZnCl2; 2 mg l−1 CuCl2; 10 mg l−1 AIC13·6H20; 6 mg l−1 H3BO3; 36 mg l−1 Na2MoO4; 24 mg l−1 NiCl2·6H20; 500 mg l−1 EDTA, 500 and 1 ml of concentrated HCl); 1 ml of vitamin solution (containing 0.05 mg l−1 biotin; 0.02 mg l−1 folic acid; 0.1 mg l−1 pyridoxine; 0.05 mg l−1 riboflavin; 0.1 mg l−1 thiamine; 0.1 mg l−1 cyanocobalamin; 0.55 mg l−1 nicotinamide; 0.25 mg l−1 p-aminobenzoic acid; 0.05 mg l−1 lipoic acid, 0.05; and 0.05 mg l−1). The sulphate reducing medium contained 0.53 g l−1 Na2HPO4·2H20; 0.41 g l−1 KH2PO4, 0.3 g l−1 NH4Cl; 0.11 g l−1 CaCl2·2H20; 0.1 g l−1 MgCl2·6H20; 0.3 g l−1 NaCl and 4.0 g l−1 NaHCO3. Acid and alkaline trace elements (each, 1 ml l−1) and vitamins (0.2 ml l−1) were added. The acid trace element solution contained 7.5 mM FeCl2; 1 mM H3B04; 0.5 mM ZnCl2; 0.1 mM CuCl2; 0.5 mM MnCl2; 0.5 mM CoCl2; 0.1 mM NiCl2 and 50 mM HCl. The alkaline trace element solution was composed of 0.1 mM Na2SeO3; 0.1 mM Na2WO4; 0.1 mM Na2MoO4 and 10 mM NaOH. The vitamin solution had the following composition: 0.02 g l−1 biotin; 0.2 g l−1 niacin; 0.5 g l−1 pyridoxine; 0.1 g l−1 riboflavin; 0.2 g l−1 thiamine; 0.1 g l−1 cyanocobalamin; 0.1 g l−1 p-aminobenzoic acid and 0.1 g l−1 pantothenic acid. The nitrate reducing medium contained 7.9 g l−1 Na2HPO4·7H20; 1.5 g l−1 KH2PO4; 0.3 g l−1 NH4Cl; 2.02 g l−1 KNO3; 0.1 g l−1 MgSO4·7H20; 5 ml of the trace elements solution and 10 ml of the vitamins solution. The pH of the medium was adjusted to 7.5 with NaOH. The trace elements solution contained: 50 g l−1 EDTA; 22 g l−1 ZnSO4·7H20; 5.54 g l−1 CaCl2; 5.06 g l−1 MnCl2·4H20; 4.99 g l−1 FeSO4·7H20; 1.1 g l−1 (NH4)6Mo7024·4H20; 1.57 g l−1 CuSO4·5H20 and 1.61 g l−1 CoCl2. This solution was adjusted to a pH of 6.0 with KOH. The vitamins solution contained: 0.002 g l−1 biotin; 0.002 g l−1 folic acid; 0.01 g l−1pyridoxine hydrochloride; 0.005 g l−1 riboflavin; 0.005 g l−1 thiamine; 0.005 g l−1 nicotinic acid; 0.005 g l−1 pantothenic acid; 0.0001 g l−1 B12; 0.005 g l−1 p-aminobenzoic acid and 0.005 g l−1 thioctic acid. The pH of the medium was adjusted to 7.5 with NaOH.
Instead of 28 mM Na2SO4 or 20 mM KNO3 in the sulphate reducing and nitrate reducing medium, respectively, 5 mM Na2SO4 or 4 mM KNO3 was added, because both media had an excess in electron acceptor to degrade the present NP. The bottles were filled with sediment and medium in the anaerobic glove box. All bottles were closed with a viton stopper and aluminium cap in the anaerobic glove box, and the headspace was changed to N2/CO2 (80%:20%). Two biotic and one abiotic bottle were prepared per treatment. The abiotic controls were performed by autoclaving one of the three bottles per treatment for 15 min at 121°C and adding 50 mg l−1 HgCl2. Addition and readdition of 4-n-NP and tNP as single carbon source to the degradation experiments (9 μM of final concentration), without unwanted solvent, was done with a high concentrated suspensions of 4-n-NP (1.6 mM) and tNP (3.5 mM) in water. Ultra violet (UV)-sterilized 4-n-NP and tNP were used. During the UV-sterilization, no photo oxidized products or metabolites were formed as confirmed by GC–MS analyses. Before use, the bottle with the suspension was heated at 60°C and shaken to homogenize. Since the used sediment was polluted with tNP, the batches contained a final concentration of 9 μM (2 mg l−1) 4-n-NP and 3.9 μM (2.8 mg l−1) tNP. Phenol was added in a concentration of 53 μM (5 mg l−1). The bottles were incubated in the dark at 30°C, and periodically sampled to measure the NP concentrations. The bottles with the medium and sediment were defined as the first generation enrichments.
Enrichments
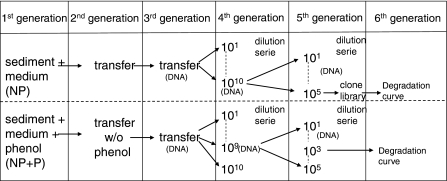
Second to fifth generation enrichments were made of the 4-n-NP degrading batches with nitrate reducing medium. Branched NP was not added since degradation of tNP was not observed. Phenol was not added for further enrichment. The enrichments originating from the first generation enrichment with 4-n-NP were encoded as “NP” and the enrichments originating from the first generation enrichment with 4-n-NP plus phenol were encoded as “NP + P”. Besides transfers also dilutions series were performed to further enrich the 4-n-NP degrading population. An overview of the enrichments and dilution series is shown in Fig. 2.
Fig. 2.
Overview of enrichments, dilution series, DNA samples and clone library sample of batches originating with 4-n-NP (NP) and with 4-n-NP and phenol (NP + P)
Before the transfer to the second generation, 11 μM 4-n-NP was re-added to the first generation enrichment at t = 104 days and this enrichment was transferred at t = 259 days of incubation into fresh nitrate reducing medium with 11 μM (2.5 mg l−1) 4-n-NP and 10% inoculum (v/v). The headspace was formed by pure N2, and 4-n-NP was spiked from a 4-n-NP stock solution.
Dilution series were made to further enrich the 4-n-NP degrading population from the third generation NP and NP + P enrichments with a maximum dilution of 1010 (encoded as fourth generation 1010). To these dilution batches, 11 μM 4-n-NP was added. The 1010 dilution of the NP enrichments and the 109 dilution of NP + P enrichments were the most diluted 4-n-NP degrading enrichments, and were used for a second 10-fold dilution series with a maximum dilution of 105 (fifth generation). Degradation was observed in this fifth generation up to the 105 dilution of the NP enrichment and up to the 103 dilution of the NP + P enrichments.
Degradation studies were performed in duplicate in nitrate reducing medium with 10% inoculum (v/v) of the most diluted active enrichments and 2 μM 4-n-NP (sixth generation). Two sterile controls containing medium, 10% inoculum (v/v) and 4-n-NP were also measured. The medium with inoculum was autoclaved for 15 min. at 121°C and thereafter 4-n-NP from the sterile stock suspension was added. Based on the experiments and the results, first order kinetics were used to calculate the degradation rate constants and half live times of the NP degradation. Student’s t tests were used for comparison of the degradation rates. Probability of significance was set at P < 0.05. The batches were sampled daily for 8 days and the samples were analyzed immediately for the 4-n-NP concentration by GC–MS analyses.
Chemical analyses
Linear nonylphenol (4-n-NP) and tNP concentrations were analyzed by GC–MS. For analysis, a 200 μl slurry sample was taken from the batches with a syringe and a needle with an inner diameter of 500 μm, and no clogging occurred because the sediment existed of particles < 32 μm. The sample was added to a capped 20 ml headspace vial with 1.8 ml MilliQ water containing 7 mg l−1 HgCl2. From the enrichments without sediment, 200 or 500 μl sample was taken depending on the expected NP concentration. The samples were added to a capped 20 ml headspace vial with 1.8 or 1.5 ml MilliQ water containing 7 mg l−1 HgCl2 to a final volume of 2 ml. The headspace vial was capped with a magnetic crimp cap with blue silicon and Teflon coated septum (Grace Davison Discovery Science, Deerfield, IL, USA) and analysed with GC–MS by solid phase micro extraction (SPME), as described previously (De Weert et al. 2008). Briefly, NP was extracted from the headspace for 25 min from the samples with an 85 μM polyacrylate fiber, which is suitable for the extraction of phenolic compounds like NP and possible intermediates. The samples were heated at 100°C and continuously shaken during the extraction. After extraction, the NP was desorbed from the fiber at the injection port of the GC–MS at 300°C. Calibration samples were made with a similar set up and included every time. The calibration samples were extracted by SPME and measured by GC–MS in the same way as the experimental samples. The detection limit was 18 nM (4 μg l−1).
Phenol concentration was analysed by high pressure liquid chromatography (HPLC) with a C18 5 μm reverse column (Alltech, Breda, the Netherlands) and an ultra violet variable wavelength (UV-VAR) detector (Varian Chrompack International BV, Middelburg, the Netherlands) at a wave length of 268 nm. A sample of 0.5 ml was taken from the batches with phenol and centrifuged for 1 min (16.1 rpm). After centrifugation, the supernatant was transferred to a HPLC vial. The eluent consists of 40% acetonitrile and 60% 0.05 M KH2PO4 (pH 3) with a constant flow rate of 0.8 ml min−1. The injection volume was 25 μl.
DNA extraction and nested polymerase chain reaction (PCR)
DNA was extracted from the third generation, the 1010 dilution NP enrichment of the fourth generation, the 109 dilution NP + P enrichment of the fourth generation, the 101, 103 and 105 dilution NP enrichment of the fifth generation, and the 101 and 103 dilution NP + P enrichment of the fifth generation (Fig. 2). To extract total DNA, 5 ml culture volume from the enrichments was filtrated through a 0.2 μm pore size cellulose acetate filter (Whatman GmbH, Dassel, Germany). DNA was extracted from the filters by bead-beating protocol by using a BIO101 Systems Fast DNA®Kit for Soil (Qbiogene, Inc, CA) according to the manufacturer’s instructions. Bead-beat step was modified from 30 to 45 s to optimize the destruction of the cells. DNA was stored at −20°C until further analysis.
A nested PCR protocol, with two successive PCR steps and two sets of primers, was performed to investigate the development and population dynamics of the microbial populations in the different enrichment and dilution steps by denaturing gradient gel electrophoresis (DGGE).
Clone library construction
A 16S rRNA gene clone library was constructed from the fifth generation 105 dilution NP enrichment genomic DNA. This enrichment was used because it was the most enriched batch that degraded 4-n-NP. The first PCR reaction was performed to amplify the complete 16S rRNA genes with two bacterial primer mixtures, fD1/fD2 and rP1/rP2, based on the method as described by Weisburg et al. (1991). Ten-fold diluted PCR amplicons of the complete 16S rRNA genes were used to carry out a clone library. Amplifications were performed with 0.4 U Taq-polymerase (Fermentas International inc, Burlington, Canada), and were carried out with an ICycler (Bio-Rad laboratories, Hercules, CA).
The PCR amplicons of the 16S rRNA genes were purified with the QIA Quick® PCR purification kit (Qiagen, Hilden, Germany), and cloned by using the TOPO TA cloning Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The plasmids were purified with the Qiagen Qia prep® spin mini prep kit and sequenced by Eurofins MWG Operon (Ebersberg, Germany) with M13 uni (−21) sequencing primer. Sequences were inspected for ambiguous base assignments and were compared with sequences deposited in public accessible databases using the NICB BLAST search tool at htpp://www.ncbi.nlm.nih.gov/blast. Alignment of the sequences was carried out by using Clustalw on-line software at http://www.ebi.ac.uk/tools/clustalw2.
DGGE
The amplicons of the 16S rRNA genes (amplified by the primer mixtures Fd1/Fd 2 and rP1/rP2) were amplified in a second (nested) PCR step by using the primers F341-GC and R534 to perform DGGE. This nested PCR was necessary as direct amplification of the DNA with these primers was not successful. For this second PCR reaction, 1 μl from 10-fold diluted PCR amplicons from 16S RNA gene were used as template DNA. The reaction mix had the same composition as for the amplification of the 16S rRNA gene and the PCR conditions were the same as described above.
The DGGE was performed based on the method described by Yu and Morrison (2004). Approximately 400 ng of nested PCR product (25 μl) was loaded on an 8% (w/v) polyacrylamide gel with a denaturing gradient of 30–70% denaturant (100% denaturant contained 7 mM urea and 40% (v/v) formamide). DGGE was performed in a 1× TAE buffer (40 mM tris, 20 mM sodium acetate, 1 mM EDTA; pH 7.4) using a DcodeTM Universal Mutation Detection System (BioRad) at 100 V at 60°C for 16 h. The gels were stained for 45 min with SybrGold (Molecular Probes, Inc, Eugene, OR) in 20 ml of 1× TAE, and viewed under UV light. DGGE bands were processed using Quantity-one version 4.6.2 image analysis software (Bio-Rad Laboratories, Hercules, CA) and corrected manually when needed. After normalization of the gels, bands with a relative peak area intensity above 2% were included in further analyses, and were digitalized and analyzed.
Bacterial diversity, based on DGGE gel analysis, were assessed by using Shannon–Weaver diversity index (H′ = −∑P i log P i) (Shannon and Weaver 1949). P i is the relative peak intensity of a DGGE band, calculated as P i = n i /N, where n i is the peak area of the band and N the sum of all the peak areas in DGGE lane. Furthermore, a covariance principal component analysis (PCA) was performed on band type and peak height with the Excel application StatistiXL, version 1.8 to identify significant shifts and similarities in microbial populations during the enrichment of batches originating from NP with and without phenol.
Predominating present bands of DNA, which show differences in the pattern between the fifth generation 105 dilution of the NP enrichment and the fifth generation 103 dilution of the NP + P enrichment, were excised from the gel. A sterile razor blade was used to excise the bands. The bands were resuspended in 50 μl sterilized MilliQ water and incubated overnight at 4°C. Desorbed DNA from the excised bands was re-amplified by PCR by using the F341 primer, without a GC-clamp and R534 primer. Amplifications were performed with 2.5 U Takara Ex Taq DNA polymerase (Takara Bio, Otsu, Shiga, Japan). The PCR amplicons were purified with the QIA Quick® PCR purification kit (Qiagen, Hilden, Germany) and stored at −20°C for further use. The reamplified PCR amplicons of part of the excised bands were sequenced directly by Eurofins MWG Operon (primer F341). Bands that contained DNA of more than one specie were cloned first by using the TOPO-TA cloning Kit. The plasmids were purified with the Qiagen Qia prep® spin mini prep kit and send for sequencing to Eurofins MWG Operon (primer M13 uni (−21)). Sequences were inspected for ambiguous base assignments with Bio Edit software, version 4.8.7 and were compared with sequences deposited in publicly accessible databases using the NICB BLAST search tool at htpp://www.ncbi.nlm.nih.gov/blast. Alignment of the sequences was done by using Clustalw on line software at http://www.ebi.ac.uk/tools/clustalw2.
GenBank accession numbers
The 16S rRNA gene nucleotide sequences determined in this study have been deposited into the GenBank database under accession numbers FJ626744–FJ626782.
Results
Anaerobic degradation of 4-n-NP, tNP and phenol
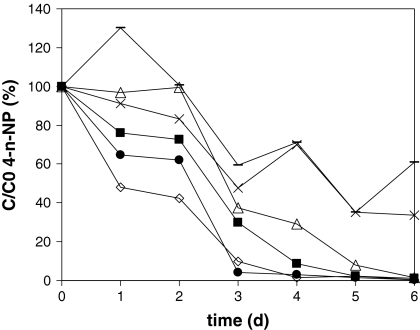
Degradation of 4-n-NP, tNP and phenol was investigated under methanogenic, sulphate reducing and denitrifying conditions during 703 days. The sediment did not contain 4-n-NP, and therefore this isomer was added to the sediment. Degradation of the added 4-n-NP was detected in nitrate reducing medium (Table 1), and the added 4-n-NP was degraded in 91–104 days. The presence of the structure analogue phenol did not significantly enhance the degradation of 4-n-NP. The sixth generation enrichments NP (originating on NP) and NP + P (originating on NP and phenol) degraded 4-n-NP within 4–6 days (Fig. 3). Decrease of the NP concentration in the first 2 days is partly due to adsorption of 4-n-NP to the incubation bottles, which is also observed in the sterile controls (0–20%). However, after a lag phase of 1–3 days the 4-n-NP concentration in the active incubates decreased significantly faster than in the sterile controls. The NP and NP + P enrichments degraded 4-n-NP with half life time of 0.43 ± 0.2 and 0.38 ± 0.06 day, respectively, which were not significant different. No intermediate metabolites were detected by GC–MS during the degradation of 4-n-NP.
Table 1.
Degradation of linear nonylphenol (4-n-NP), branched nonylphenol (tNP) and phenol in sediment under various anaerobic conditions during 703 days
| 4-n-NP | tNP | Phenol | |
|---|---|---|---|
| 4-n-NP + tNP | |||
| Methanogenic | − | − | na |
| Sulphate reducing | − | − | na |
| Nitrate reducing | + (<91–104 days) | − | na |
| Phenol + 4-n-NP + tNP | |||
| Methanogenic | − | − | + (19 days) |
| Sulphate reducing | − | − | + (13 days) |
| Nitrate reducing | + (10–91 days) | − | + (<6 days) |
| Phenol | |||
| Methanogenic | na | na | + (19 days) |
| Sulphate reducing | na | na | + (10 days) |
| Nitrate reducing | na | na | + (<6 days) |
Batches were prepared with 4-n-NP and tNP, phenol, 4-n-NP and tNP, or only phenol
+ Degradation, − no degradation, n.a. not added, () time for complete degradation
Fig. 3.
Degradation of 4-n-NP as C/C0 in nitrate reducing medium in sixth generation enrichments from river sediment from batches originating without phenol (closed symbols), with phenol (open symbols) and sterile controls (minus symbol and multi symbol), all in duplicate
No degradation of 4-n-NP under sulphate reducing and methanogenic conditions was observed. Also, tNP was not degraded under any of the tested conditions. The added phenol was degraded under all tested conditions. The lag phase of phenol degradation in the batches with phenol and nonylphenol or with only phenol was comparable.
Microbial composition of a 4-n-NP-degrading enrichment
The clone library made from 105 diluted enrichment from the fifth generation on 4-n-NP contained 61 clones enclosing 13 different species (Table 2). The major degrading micro organisms (36 out of 61 clones) were closely related to uncultured α-proteobacteria, strains OTU_8 (96–100% similarity) and OTU_10 (98–99% similarity). Strains OTU_8 and OTU_10 are identified in a hexadecane degrading denitrifying consortium (Genbank accession numbers EU083486 and EU083488) (Callaghan et al. 2009). The closest hit with a cultured bacterium was with Parvibaculum lavamentivorans DS-1 (94–98% similarity). From the 61 clones, 9 clones were related to the denitrifying strains γ-proteobacteria Pseudomonas stutzeri (95–99% similarity) and Pseudomonas sp. 42 (99% similarity). Also other denitrifying microorganisms, like the α-proteobacterium Ochrobactrum anthropi and the β-proteobacteria of the Thauera genus (Heylen et al. 2006) were identified in our enrichments.
Table 2.
Phylogenetic affiliations and frequencies of bacterial 16S rRNA gene clones in the clone library of the fifth generation 105 dilution enrichment which degrades 4-n-NP under denitrifying conditions
| No. of clones | Closest related organisms in GenBank (accession no.) | Similarity (%) | Accession no. | Phylogenetic group |
|---|---|---|---|---|
| 20 | Uncultured bacterium clone OTU_8 (EU083486) | 96–100 | FJ626754–FJ626765 | Phyllobacteriaceae; Parvibaculum (α) |
| 16 | Uncultured bacterium clone OTU_10 (EU083488) | 98–99 | FJ626744–FJ626752 | |
| 4 | Pseudomonas stutzeri (U65012) | 95–99 | FJ626774–FJ626777 | Pseudomonadaceae (γ) |
| 5 | Pseudomonas sp. 42 (2008) (EU883660) | 99 | FJ626780–FJ626781 | |
| 1 | Uncultured bacterium clone OTU_9 (EU083487) | 94 | FJ626753 | |
| 3 | Ochrobactrum anthropi ATCC 49188 (CP000759) | 99 | FJ626766–FJ626767 | Brucellaceae (α) |
| 1 | Ochrobactrum tritici strain LMG 2320(t1) (AJ865000) | 99 | FJ626768 | |
| 3 | Thauera sp. R-25071 strain R-25071 (AM084033) | 99 | FJ626772–FJ626773 | Rhodocyclaceae (β) |
| 2 | Stenotrophomonas sp. YC-1 (DQ537219) | 99 | FJ626769–FJ626770 | Xanthomonadaceae (γ) |
| 2 | Uncultured Beijerinckia sp. clone 31 (EF584507) | 97 | FJ626778 | Beijerinckiaceae (α) |
| 1 | Chelatococcus daeguensis strain K106 9AY921846) | 98 | FJ626779 | |
| 2 | Uncultured alpha proteobacterium clone AKYG1898 (AY921846) | 97 | FJ626771 | Proteobacteria (α) |
| 1 | Rhizobium sp. TKW1 (AF345860) | 94 | FJ626782 | Rhizobiaceae (α) |
| Total = 61 | ||||
DGGE profiles of total bacterial community
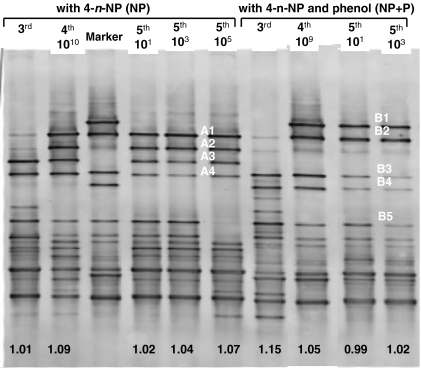
DGGE profiles were made of the third generation, the 1010 dilution fourth generation, and the 101, 103 and 105 dilution fifth generation of the NP enrichment and the third generation, the 109 dilution fourth generation, and the 101 and 103 dilution fifth generation of the NP + P enrichment (Fig. 4). Band quantity and Shannon–Weaver diversity index (H′) of the NP enrichments (originating with 4-n-NP), as analyzed by DGGE (Fig. 4), varied between 12 and 14 bands and 1.01 to 1.09, respectively. This small variety in diversity index indicates that transferring and diluting the cultures did not affect the microbial diversity in these enrichments. The population remained stable. The amount of bands in the DGGE of the NP + P enrichments (originating with 4-n-NP and phenol) varied between 12 and 16, and the H′ index between 0.99 and 1.15. The lower the H′ index the less diverse the pattern is. The largest shift in diversity was observed between the third generation and fourth generation enrichment, as this enrichment step lowered the diversity from 1.15 to 1.05. However, this difference in H′ index is relatively for DGGE. Further enrichment did not affect the population because the H′ index only changed from 0.99 to 1.02 in the fifth generation enrichments.
Fig. 4.
Denaturing gradient gel electrophoresis of PCR-amplified 16S rRNA gene fragments of 4-n-NP degrading enrichments of various generations and dilutions originating from samples with 4-n-NP, and with 4-n-NP and phenol. The marker is equal to fourth generation 109 sample originating with phenol. A1–A4 and B1–B5 are excised bands, and the number below the lanes is the Shannon–Weaver diversity index
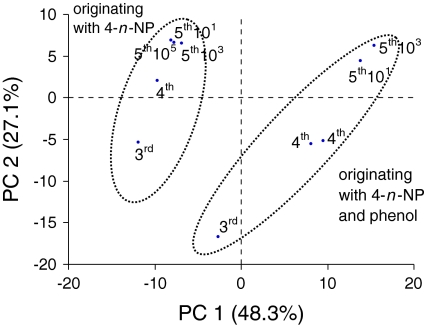
Changes in microbial composition were monitored by principal-component analysis (PCA). The PCA score plot (Fig. 5) shows that the NP enrichments and the NP + P enrichments form two different clusters. The loadings of the different excised dominated bands of the NP and NP + P enrichments are given in Table 3. Within a cluster can be seen that transfer to a new generation had a larger effect on the population than making dilutions series. Dilution of inocula did not change the population significantly in both types of enrichments. However, the population change between the enrichments and dilutions from the batch originating with 4-n-NP and phenol was larger compared to the samples originating with 4-n-NP, mainly on the first component (PC1) which explains 48.3% of the variation in the data.
Fig. 5.
Principal component analysis score plot of denaturing gradient gel electrophoresis profiles (Fig. 4) of a 4-n-NP degrading enrichments of various generations and dilutions originating from samples with 4-n-NP, and with 4-n-NP and phenol. The marker is equal to fourth generation 109 sample originating with 4-n-NP and phenol
Table 3.
DGGE band description: designations, loading PCA values for the DGGE band sequences (Fig. 4), number of clones and levels of similarity to closest related organisms
| DGGE band | PC1 loading | PC2 loading | No. of clones | Length (bp) | Similarity (%) | Closest related organism in GenBank (accession no.)a | Phylogenetic groupb |
|---|---|---|---|---|---|---|---|
| Originating with 4-n-NP | |||||||
| A1 | 0.642 | 0.722 | 4 | 235–239 | 99 | Pseudomonas stutzeri, isolate st104 (AM905852) | Pseudomonadaceae (γ) |
| A2 | −0.602 | 0.602 | 4 | 193–197 | 98 | Pseudomonas stutzeri, isolate st104 (AM905852) | Pseudomonadaceae (γ) |
| 1 | 98 | Uncultured bacterium clone OTU_10 (EU083488) | Phyllobacteriaceae; Parvibaculum (α) | ||||
| A3 | −0.875 | 0.285 | – | 115 | 99 | Uncultured bacterium clone OTU_8 (EU083486) | Phyllobacteriaceae; Parvibaculum (α) |
| A4 | −0.361 | −0.684 | – | 116 | 99 | Uncultured bacterium clone OTU_8 (EU083486) | Phyllobacteriaceae; Parvibaculum (α) |
| Originating with 4-n-NP and phenol | |||||||
| B1 | 0.959 | 0.173 | 1 | 198 | 99 | Pseudomonas aeruginosa strain H13 (AY074894) | Pseudomonadaceae (γ) |
| 2 | 171 | 98–99 | Uncultured bacterium clone OTU_10 (EU083488) | Phyllobacteriaceae; Parvibaculum (α) | |||
| 1 | 196 | 100 | Alcaligenes sp. 22–27 (AY999035) | Alcaligenaceae (ß) | |||
| B2 | 0.642 | 0.722 | 1 | 173 | 98 | Ochrobactrum sp. HPC481 (AY074894) | Rhizobiaceae (α) |
| 1 | 198 | 98 | Pseudomonas aeruginosa strain H13 (AY074894) | Pseudomonadaceae (γ) | |||
| 3 | 173–198 | 98–100 | Uncultured bacterium clone OTU_10 (EU083488) | Phyllobacteriaceae; Parvibaculum (α) | |||
| B3 | −0.361 | −0.684 | – | 117 | 99 | Uncultured bacterium clone OTU_8 (EU083486) | Phyllobacteriaceae; Parvibaculum (α) |
| B4 | 0.572 | −0.757 | – | 114 | 99 | Uncultured bacterium clone OTU_8 (EU083486) | Phyllobacteriaceae; Parvibaculum (α) |
| B5 | −0.300 | −0.217 | – | 114 | 99 | Uncultured bacterium clone OTU_8 (EU083486) | Phyllobacteriaceae; Parvibaculum (α) |
aSequences were matched with the closest relative from the GenBank database
bSequences were matched with the closest relative from the Ribosomal Database Project (Maidak et al. 2000)
α, β and γ represent alpha, beta and gamma Proteobacteria, respectively
In the DGGE pattern from enrichments with NP, and with NP and phenol, different dominant bands were detected. The main differences were the unique presence of bands A2 and A3 in the enrichments NP, and the unique bands B1 and B4 in enrichments NP + P (Fig. 4). Band A1 and B2 were dominantly present in both enrichments. Band B5 was dominantly present in both enrichments but disappeared in the 105 dilution of the fifth generation NP enrichment. Band A1 and A2, which are dominantly present in the NP enrichments, and which became more relatively intense from the 1010 dilution of the fourth generation. Sequencing of the PCR amplicons of bands A1–A4 and B1–B5 showed that the sequence of band A1 belonged to species closely related to Pseudomonas stutzeri isolate st104 (Table 3). Band A2 contained DNA related to the sequence of two different species; Pseudomonas stutzeri isolate st104 (98% similarity) and uncultured bacterium OTU_10 (98% similarity). Alignment of the sequences of the clones of band A1 (235–239 bp) and A2 (193–197 bp) which are closely related to Pseudomonas stutzeri isolate st104 showed 99% similarity. Band A3 and A4 were dominantly present in all NP enrichments. These bands belong to species closely related to uncultured strain OTU_8 (both 99% similarity). Alignment of the sequences of band A3 (115 bp) and A4 (116 bp), which were both closely related to uncultured bacterium OTU_8, showed 100% similarity to each other, although these bands appear at different positions in the DGGE gel. The different band migration could be explained based on PCR bias and by DGGE bias caused by denaturing of duplex DNA into single-chained DNA, generating different DGGE bands.
Band B1 was not present in the DGGE pattern of the third generation NP + P enrichment, and appeared in the fourth enrichment 1010 dilution as a very dominant band. Band B2, had a weak signal in the gel pattern of the third generation NP + P enrichment and became more intense in the enrichment of the fourth generation 109 dilution. These two bands (B1 and B2) contained DNA of three different species. Both bands contained DNA of species closely related to Pseudomonas aeruginosa strain H13, and uncultured bacterium clone OTU_10. Band B1 contained also DNA from species related to Alicaligenes sp.22-27 and band B2 of species closely related to Ochrobactrum sp. HPC481. Alignment showed 99% similarity between the sequences related to Pseudomonas aeruginosa strain H13 of band B1 (198 bp) and B2 (198 bp). Due to further enrichment and dilution of the fourth generation NP + P enrichment, the previous dominant bands B3, B4 and B5 became less dominant. Bands B3, B4 and B5 were identical to each other and related to uncultured bacterium OTU_8 (99% similarity). The different band migration could be explained by PCR bias and DGGE bias as described before.
Discussion
Linear NP (4-n-NP) was degraded under nitrate reducing conditions. The degradation rate increased during the enrichment of the 4-n-NP degrading population. Enhancement of 4-n-NP degradation due to adaptation to 4-n-NP was also reported in sediment from the Erren river (Chang et al. 2004). Degradation in our enrichments is approximately 40 times faster than degradation under denitrifying conditions in batches with sediment from the Erren River. Degradation of 4-n-NP was not observed in batches under sulphate reducing or methanogenic conditions. This implies that bacteria which can degrade 4-n-NP under sulphate reducing or methanogenic conditions were not present in the sediment, and that the biodegradation pathway of 4-n-NP under nitrate reducing conditions is different from the pathway under sulphate reducing and methanogenic conditions.
Degradation of tNP was not observed after 703 days of incubation of the sediment under the different tested conditions, even not under nitrate reducing conditions. Although the sediment was polluted with tNP, the present bacteria were not adapted to degrade tNP. Our results agree with other studies, which did not observe anaerobic degradation of tNP either (Ekelund et al. 1993; Hesselsoe et al. 2001).
Linear NP and the tNP isomers vary in molecular structure of the nonyl chain. As degradation of 4-n-NP was shown under nitrate reducing conditions in our sediment, but not of the tNP, it is likely that the degradation of 4-n-NP starts with the degradation of the linear alkyl chain and not with the phenol ring. This is confirmed by the microbial population in our enrichments, as the major degrading bacteria are most closely related to uncultured hexadecane degrading bacteria under denitrifying conditions. Hexadecane is a linear alkane like the nonyl chain of 4-n-NP. The closest similar sequence of a cultured strain to these dominant present sequences was Parvibaculum lavamentivorans DS-1. This bacterium converts linear alkylbenzene sulfonate (LAS) to sulfophenylcarboxylate as end product. LAS is a compound which has a similar chemical structure as 4-n-NP with a SO3 −-group instead of an OH-group attached to the aromatic ring. This chemical has a linear alkane structure as well.
Because the degradation starts at the chain, we can conclude that phenol is not useful to initiate degradation of NP-isomers, despite the partly similar chemical structure. If the degradation started at the phenol group of NP, phenol could have initiated the degradation of tNP. The use of another structure analogue for the initiation of the degradation of the mixture of NP isomers, like an aliphatic chain, will be complicated. The chemical structure of the nonyl chain in the tNP isomers is variable, and numerous isomers exist.
The microbial population of the enrichments changed most significantly between the third and fourth generation enrichment as seen in the DGGE profile. Further transfers and dilutions led only to minimal differences in population diversity. The population remained stable and approximately 13 bands were present in the DGGE. This variety of DGGE bands in the fifth generation enrichments shows that different micro-organisms are involved in the degradation of 4-n-NP under denitrifying conditions. Some of the dominant different bands in the DGGE patters from enrichments which started either with 4-n-NP (band A3) or with 4-n-NP and phenol (bands B4 and B5) were all closely related to the uncultured hexadecane degrading bacterium OTU_8. The sequence of the DNA in these bands showed 100% similarity to each other, despite their different positions in the gel. The different band migration can be explained by PCR bias and by DGGE bias caused by denaturing of duplex DNA into single-chained DNA, generating different DGGE bands. Because of these biases it is unclear if these different bands are caused by the absence or presence of phenol in the beginning of the experiments, and if these bands belong to different but related microorganisms.
Sequences related to Pseudomonas species are detected both in the clone library and in the dominant DGGE bands of the enrichments of the batches originating with 4-n-NP, and with 4-n-NP and phenol. Sequences related to Pseudomonas stutzeri are identified in the clone library and in the dominating DGGE bands of the enrichment originating with 4-n-NP. A wide variety of diverse denitrifying strains belongs to Pseudomonas stutzeri species that can degrade many different xenobiotic compounds and have been identified in natural sources. The species Pseudomonas stutzeri contain strains which are able to degrade alkanes and phenol (Lalucat et al. 2006). Furthermore, in the enrichment originating with 4-n-NP and phenol, DNA of strains related to Pseudomonas aeruginosa species are present in the dominant bands. Pseudomonas aeruginosa species are commonly present in soils and sediment (Gamble et al. 1977), and are able to degrade phenol and alkanes under denitrifying conditions (Thu et al. 1999; Chayabutra and Ju 2000). If the presence of strains related to Pseudomonas aeruginosa in enrichment originating with 4-n-NP and phenol, is caused by the presence of phenol in the first generation enrichment is unclear. This difference can also be caused by other unknown factors.
In conclusion, 4-n-NP can be degraded under denitrifying conditions. Enrichments were obtained that degraded 2 μM 4-n-NP within 6 days. Most of the sequences obtained in our 4-n-NP degrading nitrate reducing enrichments are closely related to sequences of uncultured alkane denitrifying microorganisms. The linear chain of 4-n-NP is important, and the degradation of 4-n-NP most probably would start with degradation of the linear chain. Branched NP is recalcitrant to biodegradation under all tested anaerobic conditions. For future studies into the effect of structure analogues to enhance or initiate the degradation of all tNP isomers, one should take into account the complicating fact that a large variety of branched alkanes is needed. This study shows that tNP will not degrade under anaerobic conditions, which implies that the tNP will remain in sediment systems, which are generally anaerobic (Martin et al. 1998; Middeldorp et al. 2003; Huttunen et al. 2006). These amounts of tNP will function as a long lasting secondary source for surface water pollution, which may be remobilised under changed hydrologic conditions posing risks for the environment due to its estrogenic properties.
Acknowledgement
This research was financially supported by the EU FP6 Aquaterra (project number 505428 GOCE).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bak F, Widdel F. Anaerobic degradation of phenol and phenol derivates by Desulfobacterium phenolicum sp. nov. Arch Microbiol. 1986;146:177–180. doi: 10.1007/BF00402347. [DOI] [Google Scholar]
- Berner RA. A new geochemical classification of sedimentary environments. J Sediment Petrol. 1981;51:359–365. [Google Scholar]
- Callaghan AV, Tierney M, Phelps CR, Young LY. Anaerobic biodegradation of n-hexadecane by a nitrate-reducing consortium. Appl Environ Microbiol. 2009;75:1339–1344. doi: 10.1128/AEM.02491-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BV, Yu CH, Yuan SY. Degradation of nonylphenol by anaerobic microorganisms from river sediment. Chemosphere. 2004;55:493–500. doi: 10.1016/j.chemosphere.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Chang BV, Chiang F, Yuan SY. Anaerobic degradation of nonylphenol in sludge. Chemosphere. 2005;59:1415–1420. doi: 10.1016/j.chemosphere.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Chang BV, Chiang BW, Yuan SY. Anaerobic degradation of nonylphenol in soil. J Environ Sci Health Part B. 2007;42:387–392. doi: 10.1080/03601230701312753. [DOI] [PubMed] [Google Scholar]
- Chayabutra C, Ju L-K. Degradation of n-hexadecane and its metabolites by Pseudomonas aeruginosa under microaerobic and anaerobic denitrifying conditions. Appl Environ Microbiol. 2000;66:493–498. doi: 10.1128/AEM.66.2.493-498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvini PFX, Schäffer A, Schlosser D. Microbial degradation of nonylphenol and other alkylphenols—our evolving review. Appl Microbiol Biotechnol. 2006;72:223–243. doi: 10.1007/s00253-006-0476-5. [DOI] [PubMed] [Google Scholar]
- De Weert J, De la Cal A, Van den Berg H, Murk AJ, Langenhoff AAM, Rijnaarts HHM, Grotenhuis JTC. Bioavailability and biodegradation of nonylphenol in sediment determined with chemical and bioanalysis. Environ Toxicol Chem. 2008;27:778–785. doi: 10.1897/07-367.1. [DOI] [PubMed] [Google Scholar]
- Di Corcia A, Constantino A, Crescenzi C, Marinoni E, Samperi R. Characterization of recalcitrant intermediates from biotransformation of the branched alkyl side chain of nonylphenol ethoxylate surfactants. Environ Sci Technol. 1998;32:2401–2409. doi: 10.1021/es9801285. [DOI] [Google Scholar]
- Ding W-H, Tzing S-H, Lo J-H. Occurrence and concentrations of aromatic surfactants and their degradation products in river waters of Taiwan. Chemosphere. 1999;38:2597–2606. doi: 10.1016/S0045-6535(98)00467-6. [DOI] [Google Scholar]
- Ekelund R, Granmo A, Magnusson K, Berggren M. Biodegradation of 4-nonylphenol in seawater and sediment. Environ Pollut. 1993;79:59–61. doi: 10.1016/0269-7491(93)90178-Q. [DOI] [PubMed] [Google Scholar]
- Evans PJ, Mang DT, Young LY. Degradation of toluene and m-xylene and transformation of o-xylene by denitrifying enrichment cultures. Appl Environ Microbiol. 1991;57:450–454. doi: 10.1128/aem.57.2.450-454.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht DD. Strategies for improvement of aerobic metabolism of polychlorinated biphenyls. Curr Opinion Biotechnol. 1995;6:341–346. doi: 10.1016/0958-1669(95)80057-3. [DOI] [Google Scholar]
- Fries E, Püttmann W. Occurrence and behaviour of 4-nonylphenol in river water of Germany. J Environ Monit. 2003;5:598–603. doi: 10.1039/b302229n. [DOI] [PubMed] [Google Scholar]
- Gamble TN, Betlach MR, Tiedje JM. Numerically dominant denitrifying bacteria from world soils. Appl Environ Microbiol. 1977;33:926–939. doi: 10.1128/aem.33.4.926-939.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger W, Brunner PH, Schaffner C. 4-Nonylphenol in sewage sludge: accumulation of toxic metabolites from nonionic surfactants. Science. 1984;225:623–625. doi: 10.1126/science.6740328. [DOI] [PubMed] [Google Scholar]
- Hesselsoe M, Jensen D, Skals K, Olesen T, Moldrup P, Roslev P, Mortensen GK, Hendriksen K. Degradation of 4-nonylphenol in homogeneous and nonhomogeneous mixtures of soil and sewage sludge. Environ Sci Technol. 2001;35:3695–3700. doi: 10.1021/es010024l. [DOI] [PubMed] [Google Scholar]
- Heylen B, Vanparys B, Wittebolle L, Verstraete W, Boon N, Vos Pd. Cultivation of denitrifying bacteria: optimization of isolation conditions and diversity studies. Appl Environ Microbiol. 2006;72:2637–2643. doi: 10.1128/AEM.72.4.2637-2643.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliger C, Schraa G, Stams AJM, Zehnder AJB. A highly purified enrichment culture couples by reductive dechlorination of tetrachlorethene to growth. Appl Environ Microbiol. 1993;59:2991–2997. doi: 10.1128/aem.59.9.2991-2997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen JT, Väisänen TS, Helsten SK, Martikainen PJ. Methane fluxes at the sediment–water interface in some boreal lakes and reservoirs. Boreal Environ Res. 2006;11:27–34. [Google Scholar]
- Kravetz L. Biodegradation of nonionic surfactants: alcohol ethoxylates vs nonylphenol ethoxylates. Text Chem Color. 1983;15:57–62. [Google Scholar]
- Kuever J, Könneke M, Galushko A, Drzyzga O. Reclassification of Desulfobacterium phenolicum as Desulfobacula phenolica comb. nov. and description of strain SaxT as Desulfotignum balticum gen. nov., sp. nov. Int J Syst Evol Microbiol. 2001;51:171–177. doi: 10.1099/00207713-51-1-171. [DOI] [PubMed] [Google Scholar]
- Lalucat J, Bennasar A, Bosch R, García-Valdés E, Palleroni NJ. Biology of Pseudomonas stutzeri. Microbiol Mol Biol Rev. 2006;70:510–547. doi: 10.1128/MMBR.00047-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Bisaillon J-G, Villemur R, Létourneau L, Bernard K, Lépine F, Beaudet R. Isolation and characterization of a new bacterium carboxylating phenol to benzoic acid under anaerobic conditions. J Bacteriol. 1996;178:2551–2558. doi: 10.1128/jb.178.9.2551-2558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liber K, Knuth ML, Stay FS. An integrated evaluation of the persistence and effects of 4-nonylphenol in an experimental littoral ecosystem. Environ Toxicol Chem. 1999;18:357–362. doi: 10.1002/etc.5620180301. [DOI] [Google Scholar]
- Maidak BL, Cole JR, Lilburn TG, Parker CT Jr, Saxman PR, Stredwick JM, Garrity GM, Li B, Olsen GJ, Pramanik S, Schmidt TM, Tiedje JM (2000) The RDP (Ribosomal Database Project) continues. Nucleic Acids Res 28:173–174 [DOI] [PMC free article] [PubMed]
- Martin P, Granina L, Materns K, Goddeeris B. Oxygen concentration profiles in sediments of two ancient lakes: Lake Baikal (Siberia, Russia) and Lake Malawi (East Africa) Hydrobiology. 1998;367:163–174. doi: 10.1023/A:1003280101128. [DOI] [Google Scholar]
- Middeldorp P, Staps S, Rijnaarts HHM, Roelofsen F, Valstar J, Smits J. Natural attenuation of organic contaminants at the interface between groundwater and surface water. Belgium: ICC Gent; 2003. [Google Scholar]
- Navarro-Ortega A, Tauler R, Lacorte S, Barcelo D. Occurence and transport of PAHs, pesticides and alkylphenols in sediment samples along the Ebro River Basin. J Hydrol. 2010;383:5–17. doi: 10.1016/j.jhydrol.2009.12.031. [DOI] [Google Scholar]
- Nimrod AC, Benson WH. Environmental estrogenic effects of alkylphenol ethoxylates. Crit Rev Microbiol. 1996;26:335–364. doi: 10.3109/10408449609012527. [DOI] [PubMed] [Google Scholar]
- Shannon C, Weaver W. The mathematical theory information. Urbana, IL: University of Illnios Press; 1949. [Google Scholar]
- Stams AJM, Van Dijk JB, Dijkema C, Plugge CM. Growth of syntrophic propionate-oxidizing bacteria with fumerate in the absence of methanogenic bacteria. Appl Environ Microbiol. 1993;59:1114–1119. doi: 10.1128/aem.59.4.1114-1119.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm RN. Biodegradability of nonionic surfactants: screening test for predicting rate and ultimate biodegradation. J Am Oil Chem Soc. 1973;50:159–167. doi: 10.1007/BF02640470. [DOI] [PubMed] [Google Scholar]
- Tandlich R, Brezná B, Dercová K. The effect of terpenes on the biodegradation of polychlorinated biphenyls by Pseudomonas stutzeri. Chemosphere. 2001;11:1547–1555. doi: 10.1016/S0045-6535(00)00523-3. [DOI] [PubMed] [Google Scholar]
- Thu STT, Blaszczyk M, Przyticka-Jusiak M. Growth and phenol activity of Pseudomonas aeruginosa strain 101/1 in batch cultures. Acta Microbiol Pol. 1999;48:297–306. [PubMed] [Google Scholar]
- Tschech A, Fuchs G. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch Microbiol. 1987;148:213–217. doi: 10.1007/BF00414814. [DOI] [PubMed] [Google Scholar]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Morrison M. Comparisons of different hypervariable regions of rrS genes for use in fingerprinting of microbial communities by PCR-denaturing gradient gel electrophoresis. Appl Environ Microbiol. 2004;70:4800–4806. doi: 10.1128/AEM.70.8.4800-4806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]