Abstract
Infections are the leading cause of hospitalization in transplant recipients. The increased risk of new onset diabetes after transplantation, cardiovascular disease, post-transplant lymphoproliferative disorders adversely affects allograft outcomes. Risk is determined by epidemiologic exposure, immunosuppressive therapy and prophylaxis. The predictable sequence of appearance of infections helps in making management decisions. High likelihood of infections with unusual and multiple organisms necessitates aggressive use of imaging techniques and invasive procedures. Serologic tests depend upon antibody response and are unreliable. Nucleic acid based assays are sensitive, rapid, and allow detection of subclinical infection and assessment of response to therapy. Preventive steps include screening of donors and recipients and vaccination. All indicated vaccines should be administered before transplantation. Inactivated vaccines can be administered after transplantation but produce weak and transient antibody response. Boosters may be required once antibody titers wane. Post-transplant chemoprophylaxis includes cotrimoxazole for preventing urinary tract infections, pneumocystis and Nocardia infections; ganciclovir, valganciclovir, or acyclovir for cytomegalovirus related complications in at-risk recipients; and lamivudine for prevention of progressive liver disease in HBsAg positive recipients. Viral load monitoring and pre-emptive treatment is used for BK virus infection. Infection with new organisms has recently been reported, mostly due to inadvertent transmission via the donor organ.
Keywords: Infections, kidney transplantation, prevention
Introduction
Optimal use of immunosuppressive drugs in a renal transplant recipient (RTR) requires a careful balancing act. Availability of potent and specific immunosuppressive agents has reduced the incidence of acute rejection to about 10–15% in most centers. However, despite refinements in diagnostic techniques and discovery of new anti-microbial drugs, the risk of infection amongst transplant recipients has not come down.[1] About 70% of all RTRs experience at least one infection episode by 3 years. The 2008 USRDS report[2] showed increase in hospitalization rates for infection from 5.9% per 100 patient years in 2001–2003 to 6.5% per 100 patient years in 2004–2006; hospitalization rates for other causes decreased over the same period. Infections are responsible for 18% of all deaths with functioning grafts in the US, and are the leading cause of death in the developing countries.
Infection risk is even greater in the pediatric transplant population. Data from the North American Pediatric Renal Transplant Cooperative study show that 38–42% patients transplanted between 1987 and 2002 required hospitalization for infections. Infection was the primary cause of hospitalization in the first 2 years after transplantation, exceeding that for rejection.[3] The frequency of admissions due to infections during the first 6 months after transplantation remained unchanged over time, but increased in the 6–24 month period in patients of more recent vintage. Infections also increase the risk of new onset diabetes after transplantation (NODAT), cardiovascular events, post-transplant lymphoproliferative disorders (PTLD) and adversely affect allograft outcomes.
Bacterial infections are approximately twice as frequent as viral infections in RTR. About 13% of all patients transplanted between 1996 and 2000 in the US required hospitalization for bacterial infections in the first 3 years compared to 6% for viral infections.[4] Vascular access and urinary tract infections (UTIs) were the most frequent bacterial infections, whereas cytomegalovirus (CMV) was the commonest viral infection. Extremes of recipient age, female gender, deceased donor source, older donor age, CMV+ve donor, time on dialysis and systemic lupus erythematosus (SLE) as the cause of kidney disease increased the infection risk.[4]
The infection risk at any given time after transplant is determined by the overall balance between the nature and intensity of epidemiologic exposure, net status of immunosuppression and the current nature of protection as determined by the vaccination and chemoprophylaxis status. Evaluation of exposure requires obtaining a history of travel to areas where certain infections may be endemic, dietary habits (e.g., cryptosporidium from well water and Salmonella and Lisetria from uncooked meat or dairy products), and details regarding work and hobbies (Aspergillus from construction sites, saprophytic fungi from gardening and leptospirosis in field workers). The overall status of immunosuppression is determined by complex and dynamic interactions between the recipient (age, gender, genetic background, underlying clinical condition), the transplanted organ and drugs. It is also affected by other complications such as a breach in the integrity of muco-cutaneous barriers, leukopenia, NODAT, poor graft function, liver dysfunction and malnutrition.[5]
No consistent relationship has been shown between a specific immunosuppressive agent and overall infection risk. Mycophenolate mofetil (MMF) has been linked to an overall increase in infections, especially viral,[4] and antilymphocyte antibody to CMV reactivation.[6] Higher incidence of BKV nephropathy has been noted amongst those on the “potent” combination of tacrolimus and MMF.
The right level of immunosuppression that affords protection against rejection while minimizing infection risk is achieved in clinical practice by trial and error, based on monitoring of drug levels, leukocyte counts and surveillance for metabolic complications. Studies on evaluation of biomarkers for immune monitoring have focused toward identification of rejection.[7] No reliable method exists currently for objective evaluation of net status of immune system to predict infection risk.
Attempts to develop such a measure have relied on determination of the functional status of T lymphocytes. The Cylex ImmuKnow assay measures the ability of T lymphocytes to respond to non-specific immunostimulation with phytohemagglutinin by producing ATP. Response is quantified in terms of the amount of ATP released in the supernatant. In one study,[8] recipients with ImmuKnow values of 25 ng/ml were 12 times more likely to develop an infection compared to those with a stronger response. Values ≥700 ng/ml conferred a 30-fold increase in rejection risk. RTR with BK viremia showed lower ImmuKnow values in comparison to BKV negative recipients.[9] Serial studies in patients with viral infection have shown increase in values along with viral clearance following reduction of immunosuppression.[10] This test has been cleared by US Food and Drug Administration (FDA) for immune cell function monitoring in immunosuppressed patients. Its value, however, needs to be determined in prospective studies. Recently, an association was shown in a cohort of heart transplant recipients between low circulating levels of soluble CD30, a cell-surface marker expressed by a subset of memory T cells, and infection.[11]
General Considerations in Diagnosis and Management of Infections in RTR
The broadly predictable pattern of the nature of infections encountered following transplantation gave rise to the concept of a “timetable of infections” that divides the risk period into three overlapping zones [Figure 1]. The table helps in making informed decisions about the likely nature of infections and tailoring of diagnostic and therapeutic resources.[5]
Figure 1.
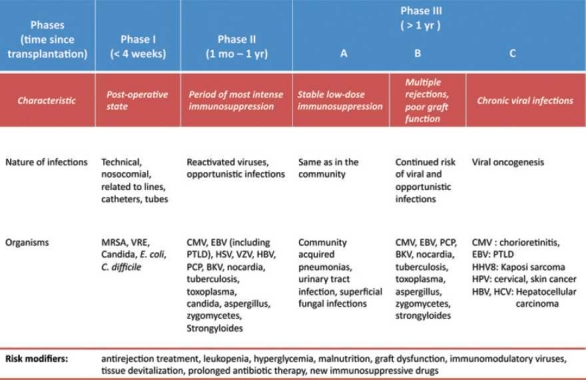
The phases in the “timetable of infections” according to time elapsed since transplantation and the risk status of the patient. The risk status changes in any stage if any of the modifiers are present
The possibility of infection needs to be considered in all febrile presentations of RTR. Fever may occasionally be absent, and symptoms may solely be related to one or more organ systems. The presentation may be different in RTR compared to the general population. For example, parvovirus B19 infection presents as pure red cell aplasia in this group, in contrast to erythema infectiosum in immunocompetent individuals. BK polyoma virus infection, asymptomatic in general population, causes renal allograft dysfunction.
The possibility of infections with unusual, often exotic, organisms and the high likelihood of polymicrobial infections necessitate a multidisciplinary approach with involvement of other specialists including the ID team. Early and aggressive use of imaging techniques such as ultrasound, computed tomography (CT) scans or magnetic resonance imaging (MRI), and invasive procedures like bronchoalveolar lavage, imaging guided aspiration and/or biopsies for obtaining specimens for histological and/or microbiological examination are essential for accurate diagnosis.
Serologic tests are of limited value since antibody response is attenuated in the immunocompromised host. Quantitative nucleic acid based assays are sensitive, quick, and useful for detection of subclinical infection, assessing response to therapy and identifying drug resistance. Studies have documented the adverse impact of subclinical CMV and Epstein–Barr virus (EBV) viremia on graft function.[12] Viral load monitoring is used to guide therapy; failure to clear the virus is associated with strong risk of recurrence of CMV disease.[13] Multiplex assays allow simultaneous quantitative determination of several microorganisms including CMV, EBV, human herpesvirus (HHV)-6 and BK virus and a number of fungi. The main problem with these assays is lack of reproducibility across different laboratories.
The non-specific nature of presentation often necessitates the initiation of broad-spectrum therapy before a specific etiologic diagnosis can be made. Development of CMV or EBV disease indicates over immunosuppression and should prompt reduction in immunosuppressive drug dosage.
Prevention of Post-transplant Infections
Adoption of preventive strategies has considerably reduced the burden of infection in RTR. This process starts before transplantation with pre-transplant screening of donors and recipients, avoidance of use of blood products, use of leukocyte filters during transfusions, treatment of pre-existing infections, immunoprophylaxis (vaccination), and continues after transplantation with tailored chemoprophylaxis and surveillance.
Donor screening is aimed at preventing transmission of latent infections including locally prevalent ones, e.g., tuberculosis and schistosomiasis, via the infected organ.[14] Organs from with hepatitis (B or C) (HBV or HBC) or HIV infected donors are not used for transplant. Recently, some centers have started using organs from HCV or HIV positive donors for recipients who already harbor these infections after informed consent is obtained.[15] A recent analysis showed that the adjusted hazard ratio for death among HCV positive recipients of kidneys from HCV antibody positive donors was lower compared to those who remained on dialysis.[16] HBV core antibody-positivity indicates a low risk of transmission, and kidneys from these donors can be used in HBV antibody-positive recipients.[17,18]
Issues related to timing (if performed during the window period, i.e., between the infection and seroconversion) host (poor antibody response in the immunocompromised patient with end stage renal disease) or organism (genetic change, e.g., HBV precore mutant) can result in a false negative serologic test. Nucleic acid based assays are not subject to these errors. Uncommon pathogens for which screening is not performed routinely (rabies, SARS, West Nile virus) can be transmitted through a contaminated allograft.
Vaccination
Recommendations for vaccination in transplant recipients are based largely on data from general population. Vaccination status should be reviewed at initial evaluation of chronic kidney disease (CKD), and all vaccinations recommended for the general population should be administered. Pediatric CKD patients should be vaccinated against varicella, influenza, hepatitis B and Pneumococcus. Vaccines should be administered early to CKD patients, since poor immune memory in advanced stages of CKD and after transplantation results in weak antibody response.[19] Pre-transplant vaccination may not be feasible in children and in areas with limited dialysis facilities, necessitating post-transplant vaccination. Experts agree that inactivated vaccines are safe when administered after transplantation. Use of live vaccines, however, is controversial. A couple of studies[20,21] demonstrated the safety of varicella and measles vaccines in small number of patients after transplantation, but the balance of opinion suggests that the risks of live vaccines outweigh potential benefits and hence should not be used.[22]
Data on the clinical efficacy of individual vaccines is limited. Observational studies have documented the salutary effect of pre-transplant vaccination on the course of varicella infection after transplantation.[23,24] Post-transplant influenza and pneumococcal vaccinations lead to protective antibody titers in a majority of RTRs.[19] The antibody response is weak for post-transplant hepatitis B vaccine. Antibody titers should be monitored with booster vaccination once the titers fall below 10 IU/ml.
The American Society of Transplantation[22] suggests delaying resumption of vaccinations after transplantation until the immunosuppressive drug dosage has been reduced to the lowest maintenance levels and documentation of vaccine efficacy by serologic assays. There is no consensus on the frequency of monitoring; annual verification is sufficient in most instances. Vaccination is desirable for pathogens that may be encountered while traveling to endemic areas as long as the recommended vaccinations are inactivated. Recommended vaccines in transplant candidates and recipients are shown in Table 1.
Table 1.
Recommended vaccines for renal transplant recipients
| Vaccine | Monitoring required? |
|---|---|
| Can be given before/after transplantation | |
| Influenza | No |
| Hepatitis B | Yes |
| Hepatitis A | Yes |
| Inactivated polio | No |
| Pneumococcal polysaccharide vaccine | Yes |
| Meningococcus | No |
| Tetanus | No |
| Conjugated pneumococcal vaccine* | Yes |
| Pertussisa | No |
| Diphtheriaa | No |
| Haemophilus influenzaa | Yes |
| Japanese encephalitisb | Yes |
| Salmonella typhi Vib | Yes |
| Rabiesc | No |
| Should be given only before transplantation | |
| BCG | No |
| Varicella | No |
| Measlesa | Yes |
| Mumpsa | Yes |
| Rubellaa | Yes |
For children <2 years of age
Recommended only for pediatric recipients
Recommended if traveling to an endemic area
Recommended in case of exposure
Chemoprophylaxis
Drugs provide effective protection against a variety of potential pathogens in RTR. One single strength tablet of cotrimoxazole protects against bacterial UTI, Pneumocystis carinii pneumonia (PCP), Toxoplasma, Listeria and Nocardia. It is be used for 6–12 months after transplantation, the period of maximum risk for PCP and Nocardia, and graft pyelonephritis, bacteremia and poor graft function following UTI.[25] The risk of UTIs increases after stoppage of prophylaxis, but late infections are usually benign.[26] Ciprofloxacin also provides effective prophylaxis for UTI and cotrimoxazole protects against PCP even when taken three times a week, but once a day cotrimoxazole is preferred due to its convenience.
Cytomegalovirus
CMV impacts the course of RTR in several ways. CMV disease presents with a “flu-like” illness, with or without tissue invasion, manifested as bone marrow suppression, hepatitis, colitis, interstitial pneumonia or CNS involvement. Through its immunomodulatory properties, CMV infection also increases the risk of invasion by opportunistic organisms and allograft rejection.[5,6]
Without prophylaxis, 10–60% of RTR develop CMV disease, but risk is not equal in all. Risk stratification is on the basis of recipient and donor CMV serostatus at the time of transplantation.[6] Seronegative recipients who receive organs from seropositive donors (D+R–) have a 40–50% chance of developing the disease. Endogenous reactivation leading to CMV disease occurs in 10–15% of seropositive recipients (D+/–R+). The figure may be higher in those who receive antilymphocyte therapy. The risk is negligible in with D–R– transplants.
Systematic reviews have shown that prophylaxis with oral or intravenous ganciclovir, valganciclovir, acyclovir or valacyclovir reduces the incidence of CMV disease, CMV-associated mortality, all cause mortality and clinically important opportunistic infections.[27,28] One analysis found that prophylaxis significantly reduced the rate of graft rejection,[28] but the other did not.[27] Oral valganciclovir and intravenous ganciclovir are equally efficacious in preventing CMV infection and disease.[29] Similarly, oral and intravenous ganciclovir yielded similar results. Ease of administration makes oral valganciclovir the preferred agent. The recommended dose is 900 mg/d, but recent studies have shown that 450 mg/d is also effective for prevention.[30,31] Prophylaxis also reduces the risk of herpes simplex and zoster disease.[27] Acyclovir is less effective and should be restricted to situations where ganciclovir/valganciclovir cannot be used due to economic reasons.
The exact duration of prophylaxis is not clear. The current recommendations suggest 3 months,[6,32] extended to 6 months in those receiving antilymphocyte induction. A recent meta-analysis did not find a difference in outcomes whether the treatment was for less or more than 6 weeks. A recently recognized effect of prophylaxis has been to delay the onset of CMV disease. Over 90% of disease in patients who receive prophylaxis is now seen after 90 days. Late onset disease is an independent predictor of mortality and graft loss.[33,34] Widespread prophylaxis also carries the risk of development of resistance. In a recent study,[35] 15% of late onset disease was due to drug resistant strains.
An alternative approach of CMV disease prevention is pre-emptive therapy that relies upon CMV viral load monitoring and institution of treatment with ganciclovir at a predetermined threshold.[36] The potential advantages of this strategy are its cost-effectiveness and avoidance of potential toxicities of antiviral agents. An randomized clinical trial[37] showed this approach to be as effective as routine prophylaxis in preventing disease, but without any cost advantage, probably because of the added cost of monitoring. A recent study, however, found pre-emptive therapy to be inferior to universal prophylaxis in terms of rejection risk and preservation of renal function.[38] On balance, therefore, the pre-emptive approach has been suggested to be restricted to low risk (D–/R) recipients. Use of antilymphocyte antibody therapy for acute rejection increases the risk of CMV disease, which can be effectively prevented by use of ganciclovir.[39,40]
Epstein–Barr Virus
The most important clinical consequence of EBV infection in RTR is development of PTLD. The risk is highest in recipients who are EBV-antibody negative at the time of transplantation. A case-control study showed that ganciclovir and acyclovir were effective in preventing PTLD,[41] possibly through suppressing subclinical EBV viremia.[12] Data from the Collaborative Transplant Registry, however, did not confirm this finding, but showed a protective effect of anti-CMV immunoglobulin.[42]
Hepatitis B
HBsAg positive RTRs have a high likelihood of developing chronic liver disease.[43] Lamivudine, a cytosine analog started at the time of transplantation, stabilizes liver function. A meta-analysis[44] showed alanine transaminase (ALT) normalization, and HBV-DNA and HBeAg clearance with lamivudine prophylaxis in 81, 91 and 27%, respectively, of HBsAg positive RTRs. Recipients not on lamivudine experienced deterioration in liver enzymes and increasing HBV DNA levels within a few months, necessitating initiation of therapy.[45] The benefits are directly proportional to the duration of prophylaxis. Withdrawal can lead to increased viral replication and relapse of liver disease, even resulting in liver failure. Development of resistance, reflected by a secondary increase in the HBV DNA titers, is the major risk of long-term use. The optimal duration that ensures long-term remission of viremia, maintenance of normal liver function and minimizes the development of resistance remains unclear. Newer antiviral agents like adefovir and entecavir are effective in those with lamivudine resistance.[46,47] Whether substitution of lamivudine with entecavir for primary prophylaxis will prevent development of resistance is currently unknown.
BK Virus
BK virus, a member of polyoma group of viruses, is ubiquitous in humans. After primary infection, it establishes latency in the urothelium. Reactivation occurs in the setting of immunosuppression, leading to clinically insignificant urinary shedding in 30–60% of RTR.[48] The infection disseminates in about 5–10% and produces the syndrome of BK virus nephropathy (BKN). It is seen as cytopathic changes in renal tubules and interstitial infiltration along with a positive staining for SV40 antigen on allograft biopsy. Over 50% grafts are lost in the first year after diagnosis despite reduction in immunosuppression and treatment with cidofovir and/or leflunomide.[49] Histological changes may not be always obvious because of the focal nature of the disease.[50] Viral load monitoring can identify patients at risk of allograft damage. Demonstration of >104 copies of virus in plasma or >107 copies in urine identifies those likely to develop BKN.[48,51,52] Three-monthly screening is recommended; any high value should be reconfirmed within 3 weeks.[53] Serial monitoring is also useful in following patient after reduction of immunosuppression and in those being considered for re-transplantation to document resolution.
Antifungal Prophylaxis
The risk of Candida infection as a result of increased oral colonization is heightened in the early post-transplant period, during periods of intensified immunosuppression such as after treatment for rejections or after prolonged courses of broad-spectrum antibiotics. Prophylactic topical antifungals such as nystatin or clotrimazole help eradicate the colonization without producing systemic adverse effects.
Emerging Infections in Transplantation
Recent years have seen the identification of disease due to a number of organisms hitherto not seen in RTR [Table 2][54,55] Clinical syndrome may be a result of primary infection due to transmission via the donor organ or following environmental exposure, or secondary to reactivation of latent infection following immunosuppression. Infection may be asymptomatic, or present with either mild self-limiting febrile illness or severe multisystem disease. Diagnosis is usually made by molecular techniques. Treatment includes lowering of immunosuppression and use of intravenous immunoglobulin or antiviral agents.
Table 2.
Emerging infections in transplant recipients
| Pathogen | Mode of transmission | Usual time of presentation | Presenting features | Diagnosis | Treatment |
|---|---|---|---|---|---|
| HHV-6 | Reactivation of latent infection | Commonest in first 2–4 weeks, may occur up to 2 years | Fever, rash, myelosuppression, hepatitis, pneumonitis, encephalitis ↑ risk of CMV and opportunistic infections | PCR | Ganciclovir |
| HHV-7 | Transmission from donor | Histopathology | Cidofovir Foscarnate | ||
| Adenovirus | Reactivation, nosocomial transmission | Commonest in first 3 months, may occur until several years | Interstitial nephritis, hemorrhagic cystitis, pneumonitis | Immunohistochemistry PCR in plasma | IVIG Cidofovir |
| West Nile virus | Transmission from donor,blood transfusion, environmental exposure | Fever, meningoencephalitis,hyporeflexic paralysis | PCR (short viremic phase)Serology (may be delayed) IgM antibody in CSF | IVIG | |
| LCM | Transmission from donor, | First 4 weeks | Fever, diarrhea, asepticmeningitis, interstitial pneumonia, hepatitis, multisystem failure | Cerebrospinal fluid PCR, serology | |
| Parainfluenza and metapneumovirus | Environmental and nosocomial transmission | After 1 year illness, pneumonia | Fever, upper respiratory | PCR Antigen detection on respiratory secretions | Ribavirin |
| Parvovirus B19 | Transmission from donor | First year | Fever, joint pain, pure redcell aplasia, hepatitis, pneumonitis | PCR Bone marrow examination | IVIG |
| Respiratory syncytial virus | Nosocomial transmission | Any time | Upper respiratory tract infection, interstitial pneumonia | PCR Antigen testing on respiratory secretions | Ribacirin IVIG |
| Rotavirus | Environmental transmission | Any time | Self-limiting diarrhea, lowergastrointestinal bleeding | None |
HHV: human herpesvirus; LCM: lymphocyte choriomeningitis virus, CSF: cerebrospinal fl uid, IVIG: intravenous immunoglobulin; PCR: polymerase chain reaction
In conclusion, infections remain a major problem in the transplant population. They are a main cause of death with functioning graft, and cause a number of other complications that increase morbidity. Molecular diagnostic techniques have allowed earlier identification and better monitoring of infections. Prophylactic strategies include vaccination and targeted post-transplant chemoprophylaxis. Use of drugs carries the risk of late and resistant infections. A high index of suspicion and early and aggressive use of diagnostic techniques are essential for accurate diagnosis and improved outcomes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Snyder JJ, Israni AK, Peng Y, Zhang L, Simon TA, Kasiske BL, et al. Rates of first infection following kidney transplant in the United States. Kidney Int. 2009;75:317–26. doi: 10.1038/ki.2008.580. [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System: Annual Data Report. 2008. Available from: http://www.usrds.org/adr [Last accessed on 2008 Dec 15]
- 3.Dharnidharka VR, Stablein DM, Harmon WE. Post-transplant infections now exceed acute rejection as cause for hospitalization: A report of the NAPRTCS. Am J Transplant. 2004;4:384–9. doi: 10.1111/j.1600-6143.2004.00350.x. [DOI] [PubMed] [Google Scholar]
- 4.Dharnidharka VR, Agodoa LY, Abbott KC. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients--an analysis of USRDS data. Am J Transplant. 2007;7:653–61. doi: 10.1111/j.1600-6143.2006.01674.x. [DOI] [PubMed] [Google Scholar]
- 5.Fishman JA. Infection in renal transplant recipients. Semin Nephrol. 2007;27:445–61. doi: 10.1016/j.semnephrol.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Kotton CN, Fishman JA. Viral infection in the renal transplant recipient. J Am Soc Nephrol. 2005;16:1758–74. doi: 10.1681/ASN.2004121113. [DOI] [PubMed] [Google Scholar]
- 7.Schaub S, Wilkins JA, Rush D, Nickerson P. Developing a tool for noninvasive monitoring of renal allografts. Expert Rev Proteomics. 2006;3:497–509. doi: 10.1586/14789450.3.5.497. [DOI] [PubMed] [Google Scholar]
- 8.Kowalski RJ, Post DR, Mannon RB, Sebastian A, Wright HI, Sigle G, et al. Assessing relative risks of infection and rejection: A meta-analysis using an immune function assay. Transplantation. 2006;82:663–8. doi: 10.1097/01.tp.0000234837.02126.70. [DOI] [PubMed] [Google Scholar]
- 9.Batal I, Zeevi A, Heider A, Girnita A, Basu A, Tan H, et al. Measurements of global cell-mediated immunity in renal transplant recipients with BK virus reactivation. Am J Clin Pathol. 2008;129:587–91. doi: 10.1309/23YGPB1E758ECCFP. [DOI] [PubMed] [Google Scholar]
- 10.Gautam A, Morrissey PE, Brem AS, Fischer SA, Gohh RY, Yango AF, et al. Use of an immune function assay to monitor immunosuppression for treatment of post-transplant lymphoproliferative disorder. Pediatr Transplant. 2006;10:613–6. doi: 10.1111/j.1399-3046.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- 11.Nikaein A, Spiridon C, Hunt J, Rosenthal J, Anderson A, Eichhorn E, et al. Pre-transplant level of soluble CD30 is associated with infection after heart transplantation. Clin Transplant. 2007;21:744–7. doi: 10.1111/j.1399-0012.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Chaudhuri A, Weintraub LA, Hsieh F, Shah S, Alexander S, et al. Subclinical cytomegalovirus and Epstein-Barr virus viremia are associated with adverse outcomes in pediatric renal transplantation. Pediatr Transplant. 2007;11:187–95. doi: 10.1111/j.1399-3046.2006.00641.x. [DOI] [PubMed] [Google Scholar]
- 13.Franco A, Serrano R, Gimeno A, de Juan J, Merino E, Jiménez del Cerro L, et al. Evaluation of viral load and antigenemia as markers for relapse cytomegalovirus infection in renal transplant recipients. Nefrologia. 2007;27:202–8. [PubMed] [Google Scholar]
- 14.Schaffner A. Pretransplant evaluation for infections in donors and recipients of solid organs. Clin Infect Dis. 2001;33:S9–14. doi: 10.1086/320898. [DOI] [PubMed] [Google Scholar]
- 15.Veroux P, Veroux M, Puliatti C, Cappello D, Macarone M, Gagliano M, et al. Kidney transplantation from hepatitis C virus-positive donors into hepatitis C virus-positive recipients: A safe way to expand the donor pool? Transplant Proc. 2005;37:2571–3. doi: 10.1016/j.transproceed.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 16.Abbott KC, Lentine KL, Bucci JR, Agodoa LY, Peters TG, Schnitzler MA. The impact of transplantation with deceased donor hepatitis c-positive kidneys on survival in wait-listed long-term dialysis patients. Am J Transplant. 2004;4:2032–7. doi: 10.1046/j.1600-6143.2004.00606.x. [DOI] [PubMed] [Google Scholar]
- 17.Veroux M, Puliatti C, Gagliano M, Cappello D, Macarone M, Vizcarra D, et al. Use of hepatitis B core antibody-positive donor kidneys in hepatitis B surface antibody-positive and -negative recipients. Transplant Proc. 2005;37:2574–5. doi: 10.1016/j.transproceed.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 18.Madayag RM, Johnson LB, Bartlett ST, Schweitzer EJ, Constantine NT, McCarter RJ, Jr, et al. Use of renal allografts from donors positive for hepatitis B core antibody confers minimal risk for subsequent development of clinical hepatitis B virus disease. Transplantation. 1997;64:1781–6. doi: 10.1097/00007890-199712270-00027. [DOI] [PubMed] [Google Scholar]
- 19.Neuhaus TJ. Immunization in children with chronic renal failure: A practical approach. Pediatr Nephrol. 2004;19:1334–9. doi: 10.1007/s00467-004-1597-7. [DOI] [PubMed] [Google Scholar]
- 20.Rand EB, McCarthy CA, Whitington PF. Measles vaccination after orthotopic liver transplantation. J Pediatr. 1993;123:87–9. doi: 10.1016/s0022-3476(05)81545-8. [DOI] [PubMed] [Google Scholar]
- 21.Zamora I, Simon JM, Da Silva ME, Piqueras AI. Attenuated varicella virus vaccine in children with renal transplants. Pediatr Nephrol. 1994;8:190–2. doi: 10.1007/BF00865476. [DOI] [PubMed] [Google Scholar]
- 22.Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. 2004;4:160–3. doi: 10.1111/j.1600-6135.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- 23.Furth SL, Fivush BA. Varicella vaccination in pediatric kidney transplant candidates. Pediatr Transplant. 2002;6:97–100. doi: 10.1034/j.1399-3046.2002.01059.x. [DOI] [PubMed] [Google Scholar]
- 24.Olson AD, Shope TC, Flynn JT. Pretransplant varicella vaccination is cost-effective in pediatric renal transplantation. Pediatr Transplant. 2001;5:44–50. doi: 10.1034/j.1399-3046.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 25.de Souza RM, Olsburgh J. Urinary tract infection in the renal transplant patient. Nat Clin Pract Nephrol. 2008;4:252–64. doi: 10.1038/ncpneph0781. [DOI] [PubMed] [Google Scholar]
- 26.Senger SS, Arslan H, Azap OK, Timurkaynak F, Cağir U, Haberal M. Urinary tract infections in renal transplant recipients. Transplant Proc. 2007;39:1016–7. doi: 10.1016/j.transproceed.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 27.Hodson EM, Barclay PG, Craig JC, Jones C, Kable K, Strippoli GF, et al. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2005;19:CD003774. doi: 10.1002/14651858.CD003774.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-analysis: The efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005;143:870–80. doi: 10.7326/0003-4819-143-12-200512200-00005. [DOI] [PubMed] [Google Scholar]
- 29.Asberg A, Humar A, Rollag H, Jardine AG, Mouas H, Pescovitz MD, et al. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2007;7:2106–13. doi: 10.1111/j.1600-6143.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 30.Avidan YP, Paul M, Rahamimov R, Bishara J, Samra Z, Edna S, et al. Selective low-dose valganciclovir for prevention of cytomegalovirus disease following kidney transplantation. J Infect. 2008;57:236–40. doi: 10.1016/j.jinf.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Weng FL, Patel AM, Wanchoo R, Brahmbhatt Y, Ribeiro K, Uknis ME, et al. Oral ganciclovir versus low-dose valganciclovir for prevention of cytomegalovirus disease in recipients of kidney and pancreas transplants. Transplantation. 2007;83:290–6. doi: 10.1097/01.tp.0000251371.34968.ca. [DOI] [PubMed] [Google Scholar]
- 32.Taber DJ, Ashcraft E, Baillie GM, Berkman S, Rogers J, Baliga PK, et al. Valganciclovir prophylaxis in patients at high risk for the development of cytomegalovirus disease. Transpl Infect Dis. 2004;6:101–9. doi: 10.1111/j.1399-3062.2004.00066.x. [DOI] [PubMed] [Google Scholar]
- 33.Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, et al. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008;46:840–6. doi: 10.1086/528718. [DOI] [PubMed] [Google Scholar]
- 34.Limaye AP, Bakthavatsalam R, Kim HW, Randolph SE, Halldorson JB, Healey PJ, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645–52. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 35.Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: Therapeutic implications and outcomes. Clin Transplant. 2008;22:162–70. doi: 10.1111/j.1399-0012.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 36.Strippoli GF, Hodson EM, Jones CJ, Craig JC. Pre-emptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2006;1:CD005133. doi: 10.1002/14651858.CD005133.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Khoury JA, Storch GA, Bohl DL, Schuessler RM, Torrence SM, Lockwood M, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant. 2006;6:2134–43. doi: 10.1111/j.1600-6143.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- 38.Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F. Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: Results of a randomized clinical trial. Am J Transplant. 2008;8:975–83. doi: 10.1111/j.1600-6143.2007.02133.x. [DOI] [PubMed] [Google Scholar]
- 39.Conti DJ, Freed BM, Singh TP, Gallichio M, Gruber SA, Lempert N. Preemptive ganciclovir therapy in cytomegalovirus-seropositive renal transplants recipients. Arch Surg. 1995;130:1217–21. doi: 10.1001/archsurg.1995.01430110075014. [DOI] [PubMed] [Google Scholar]
- 40.Hibberd PL, Tolkoff-Rubin NE, Conti D, Stuart F, Thistlethwaite JR, Neylan JF, et al. Preemptive ganciclovir therapy to prevent cytomegalovirus disease in cytomegalovirus antibody-positive renal transplant recipients.A randomized controlled trial. Ann Intern Med. 1995;123:18–26. doi: 10.7326/0003-4819-123-1-199507010-00002. [DOI] [PubMed] [Google Scholar]
- 41.Funch DP, Walker AM, Schneider G, Ziyadeh NJ, Pescovitz MD. Ganciclovir and acyclovir reduce the risk of post-transplant lymphoproliferative disorder in renal transplant recipients. Am J Transplant. 2005;5:2894–900. doi: 10.1111/j.1600-6143.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 42.Opelz G, Daniel V, Naujokat C, Fickenscher H, Döhler B. Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post-transplant non-Hodgkin lymphoma: A multicentre retrospective analysis. Lancet Oncol. 2007;8:212–8. doi: 10.1016/S1470-2045(07)70040-2. [DOI] [PubMed] [Google Scholar]
- 43.Fabrizi F, Martin P, Dixit V, Kanwal F, Dulai G. HBsAg seropositive status and survival after renal transplantation: Meta-analysis of observational studies. Am J Transplant. 2005;5:2913–21. doi: 10.1111/j.1600-6143.2005.01113.x. [DOI] [PubMed] [Google Scholar]
- 44.Fabrizi F, Dulai G, Dixit V, Bunnapradist S, Martin P. Lamivudine for the treatment of hepatitis B virus-related liver disease after renal transplantation: Meta-analysis of clinical trials. Transplantation. 2004;77:859–64. doi: 10.1097/01.tp.0000116448.97841.6d. [DOI] [PubMed] [Google Scholar]
- 45.Filik L, Karakayali H, Moray G, Dalgiç A, Emiroğlu R, Ozdemir N, et al. Lamivudine therapy in kidney allograft recipients who are seropositive for hepatitis B surface antigen. Transplant Proc. 2006;38:496–8. doi: 10.1016/j.transproceed.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 46.Fontaine H, Vallet-Pichard A, Chaix ML, Currie G, Serpaggi J, Verkarre V, et al. Efficacy and safety of adefovir dipivoxil in kidney recipients, hemodialysis patients, and patients with renal insufficiency. Transplantation. 2005;80:1086–92. doi: 10.1097/01.tp.0000178305.39231.a2. [DOI] [PubMed] [Google Scholar]
- 47.Kamar N, Milioto O, Alric L, El Kahwaji L, Cointault O, Lavayssièr;re L, et al. Entecavir therapy for adefovir-resistant hepatitis B virus infection in kidney and liver allograft recipients. Transplantation. 2008;86:611–4. doi: 10.1097/TP.0b013e3181806c8c. [DOI] [PubMed] [Google Scholar]
- 48.Pang XL, Doucette K, LeBlanc B, Cockfield SM, Preiksaitis JK. Monitoring of polyomavirus BK virus viruria and viremia in renal allograft recipients by use of a quantitative real-time PCR assay: One-year prospective study. J Clin Microbiol. 2007;45:3568–73. doi: 10.1128/JCM.00655-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mischitelli M, Bellizzi A, Anzivino E, Fioriti D, Boldorini R, Miglio U, et al. Complications post renal transplantation: Literature focus on BK virus nephropathy and diagnostic tools actually available. Virol J. 2008;5:38. doi: 10.1186/1743-422X-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drachenberg CB, Papadimitriou JC, Hirsch HH, Wali R, Crowder C, Nogueira J, et al. Histological patterns of polyomavirus nephropathy: Correlation with graft outcome and viral load. Am J Transplant. 2004;4:2082–92. doi: 10.1046/j.1600-6143.2004.00603.x. [DOI] [PubMed] [Google Scholar]
- 51.Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, et al. Polyomavirus-associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277–86. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 52.Randhawa P, Ho A, Shapiro R, Vats A, Swalsky P, Finkelstein S, et al. Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J Clin Microbiol. 2004;42:1176–80. doi: 10.1128/JCM.42.3.1176-1180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drachenberg CB, Papadimitriou JC, Ramos E. Histologic versus molecular diagnosis of BK polyomavirus-associated nephropathy: A shifting paradigm? Clin J Am Soc Nephrol. 2006;1:374–9. doi: 10.2215/CJN.02021205. [DOI] [PubMed] [Google Scholar]
- 54.Smith JM, McDonald RA. Emerging viral infections in transplantation. Pediatr Transplant. 2006;10:838–43. doi: 10.1111/j.1399-3046.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 55.Fischer SA. Emerging viruses in transplantation: There is more to infection after transplant than CMV and EBV. Transplantation. 2008;86:1327–39. doi: 10.1097/TP.0b013e31818b6548. [DOI] [PubMed] [Google Scholar]


