SUMMARY
The Akt signalling pathway plays vital roles in controlling cellular responses to insulin as well as in proliferation and survival. Inhibition of Akt signalling leads to insulin resistance and type 2 diabetes, whereas hyperactivation of Akt promotes tumorigenesis. In this study, we investigate how modest changes in the activity of the Akt signalling pathway, to an extent that might be achieved by drug treatment, would impact on insulin resistance and tumorigenesis. Using insulin-resistant PDK1K465E/K465E PH domain knock-in mice, we found that introducing the PTEN+/− mutation to slightly stimulate Akt restored normal insulin sensitivity. Introducing the PDK1K465E/K465E PH domain knock-in mutation into cancer-prone PTEN+/− mice, lowered Akt activity only by about 50%, but led to a delay in tumour onset of ∼4 months in a broad range of tumours. This was also accompanied by slower growth of B cell follicular lymphomas, as monitored by magnetic resonance imaging. Our findings imply that signal transduction inhibitors that lead to a modest reduction in Akt activity would not only delay onset of tumours possessing elevated phosphoinositide 3-kinase pathway activity but would also reduce the growth rate of developed tumours.
INTRODUCTION
The phosphoinositide 3-kinase (PI3K)-Akt signalling pathway plays essential roles in regulating diverse biological responses, including metabolic actions of insulin as well as growth and proliferation (Taniguchi et al., 2006). Insufficient activation of the PI3K-Akt pathway is a hallmark of insulin resistance and can lead to type 2 diabetes. A high fat diet and mutations in signalling components that suppress the PI3K-Akt signalling pathway invariably lead to insulin resistance (Whiteman et al., 2002). By contrast, many cancer-driving mutations stimulate cell proliferation and survival by promoting activation of the PI3K-Akt pathway (Alessi et al., 2009; Manning and Cantley, 2007; Wullschleger et al., 2006). One of the most commonly mutated genes in human cancer is PTEN, which encodes a lipid phosphatase that breaks down phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3], a second messenger produced downstream of PI3K activation (Salmena et al., 2008). Loss of PTEN results in increased levels of PtdIns(3,4,5)P3, thereby promoting the activation of Akt via the upstream 3-phosphoinositide-dependent protein kinase 1 (PDK1) and mTOR complex 2 kinases (Pearce et al., 2010). Furthermore, heterozygous PTEN+/− mice have been shown to spontaneously develop a variety of tumours (Di Cristofano et al., 1998; Haas-Kogan et al., 1998; Maehama and Dixon, 1998; Myers et al., 1998; Podsypanina et al., 1999; Stambolic et al., 1998; Suzuki et al., 1998).
The activation of Akt is mediated by phosphorylation of the T-loop (T308) by PDK1 and requires the interaction of Akt and PDK1 with PtdIns(3,4,5)P3 via their Pleckstrin homology (PH) domains (Pearce et al., 2010). PDK1K465E/K465E PH domain knock-in mice express a form of PDK1 (with lysine 465 exchanged for glutamic acid) that is normally active, but unable to bind PtdIns(3,4,5)P3. These mice display a small size phenotype and are insulin resistant (Bayascas et al., 2008). The insulin resistance in the PDK1K465E/K465E animals is thought to result from partial inhibition of insulin-induced activation of Akt isoforms. Akt belongs to the AGC kinase family, and PDK1 activates other members of this family including S6K and SGK isoforms. The activation of these kinases is not dependent on PDK1-binding to PtdIns(3,4,5)P3 and therefore these enzymes arenormally activated in the PDK1K465E/K465E PH domain knock-in mice (Bayascas et al., 2008). The physiological roles of the Akt signalling pathway have been studied using knockout mouse models (Dummler and Hemmings, 2007); however, the PDK1K465E/K465E PH domain knock-in mice represent a unique mouse model in which Akt isoform activity is not ablated, but only moderately reduced.
There is much effort to develop drugs that inhibit the Akt pathway for the treatment of cancer (Liu et al., 2009). Because the Akt pathway plays vital roles in controlling many diverse essential cellular processes, it is likely that drugs would only be tolerated if they modestly inhibit the Akt-signalling network. In this study, we exploit the PDK1K465E/K465E mouse model to investigate how small changes in the activity of the Akt pathway impact on insulin resistance and tumorigenesis. Our data indicate that drugs that moderately reduce Akt pathway activity would have therapeutic benefit in delaying the onset as well as growth of a broad spectrum of tumours driven by elevated PI3K pathway signalling.
RESULTS
Generation of PDK1K465E/K465EPTEN+/− mice
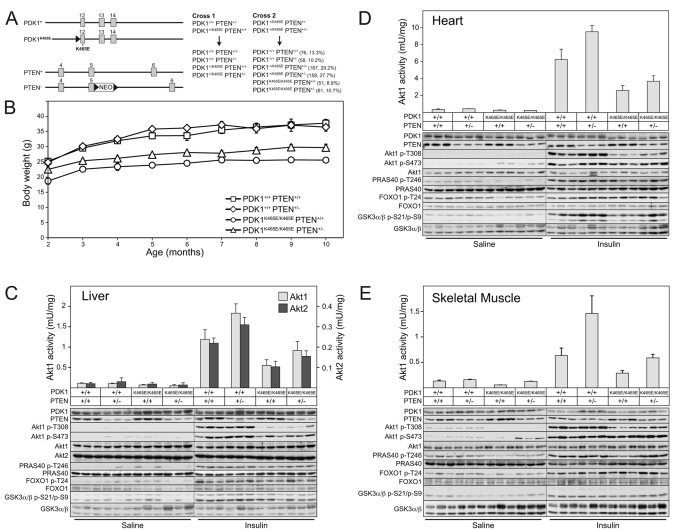
Employing the breeding strategy described in Fig. 1A, we generated littermate strains of mice expressing wild-type PDK1 and wild-type PTEN (PDK1+/+PTEN+/+), PH domain mutant PDK1 and wild-type PTEN (PDK1K465E/K465EPTEN+/+), wild-type PDK1 and deficient PTEN (PDK1+/+PTEN+/−), and PH domain mutant PDK1 and deficient PTEN (PDK1K465E/K465EPTEN+/−). Apart from the PDK1K465E/K465EPTEN+/+ mice that were born at ∼70% of the expected number, the other mouse strains were recovered at around the expected Mendelian frequency (Fig. 1A, Cross 2). Consistent with previous work (Bayascas et al., 2008), PDK1K465E/K465EPTEN+/+ mice were ∼35% smaller than wild-type PDK1+/+PTEN+/+ mice (Fig. 1B), which is probably a result of reduced Akt pathway activity during development. The PDK1K465E/K465EPTEN+/− mice were of an intermediate size and only ∼20% smaller than wild-type PDK1+/+PTEN+/+ mice, suggesting that deficiency of PTEN can partially restore normal Akt pathway activity during development and therefore rescue the small size phenotype.
Fig. 1.
Generation and analysis of Akt activation and phosphorylation. (A) Schematic representation of exons 12 to 14 of the PDK1 gene and 4 to 6 of the PTEN gene, depicting the different alleles used in this study. The grey boxes represent exons, the continuous lines introns, and triangles Cre-loxP excision sites. NEO, neomycin-resistance gene cassette. The asterisk indicates the location of lysine at position 465, which is mutated to glutamic acid in the PDK1K465E knock-in allele. The breeding strategy employed to generate the different genotypes of PDK1 and PTEN mice used in the present study is shown. The number of mice and percentage of each genotype obtained after weaning are indicated. (B) Mean body weight of male mice of the indicated genotype at the indicated age. Values represent the mean ± s.e.m. for each data point. P<0.05 comparing PDK1K465E/K465E PTEN+/− with PDK1K465E/K465E PTEN+/+, as determined by the Student’s t-test. PDK1+/+ PTEN+/+ n=12, PDK1+/+ PTEN+/− n=10, PDK1K465E/K465E PTEN+/+ n=10, PDK1K465E/K465E PTEN+/− n=13. (C-E) Mice of the indicated genotype were fasted overnight and then intravenously injected with either saline or insulin (1 mU/g body weight) for 10 minutes. Liver (C), heart (D) and skeletal muscle (E) were rapidly extracted and frozen in liquid nitrogen. Tissue extracts derived from three different mice of each genotype were subjected to immunoblotting using the indicated antibodies. Akt1 and Akt2 were also immunoprecipitated from tissue lysates and their kinase activities assayed using the Crosstide peptide. The results are presented as the mean specific activity ± s.e.m. derived from three independent mice assayed in duplicate.
Analysis of Akt1 and Akt2 activity and phosphorylation
To establish the effect of PTEN heterozygosity on Akt activation in PDK1K465E/K465E animals, mice were starved overnight and then injected intravenously with a physiological dose of insulin (1 mU/g) for 10 minutes. Insulin-sensitive tissues, including liver (Fig. 1C), heart (Fig. 1D) and skeletal muscle (Fig. 1E) were removed and extracts generated. In the PDK1K465E/K465EPTEN+/− or PDK1+/+PTEN+/− mice, the level of the PTEN protein in liver, heart and skeletal muscle was reduced by half compared with tissues derived from PDK1K465E/K465EPTEN+/+ or PDK1+/+PTEN+/+ mice (Fig. 1C–E). As reported previously, PDK1 expression in all tissues examined was not affected by the K465E knock-in mutation (Bayascas et al., 2008) (Fig. 1C–E). Akt1 (all tissues) and Akt2 (liver) protein kinase activity was measured following immunoprecipitation. We also assessed Akt phosphorylation at the T-loop PDK1 site (T308-Akt1) and phosphorylation of the hydrophobic motif mTOR complex 2 site (S473-Akt1) by immunoblotting with phosphospecific antibodies. Consistent with previous work, insulin induced a 30–50% lower activation of Akt1 and Akt2 in PDK1K465E/K465EPTEN+/+ mice compared with wild-type animals, which was accompanied by reduced phosphorylation of T308, but not S473 (Fig. 1C–E) (Bayascas et al., 2008). The basal activity and phosphorylation of Akt1 and Akt2 were not significantly enhanced in PDK1K465E/K465EPTEN+/− or PDK1+/+PTEN+/− animals (Fig. 1C–E). However, a modest increase in Akt activity and phosphorylation was observed in PDK1K465E/K465EPTEN+/− or PDK1+/+PTEN+/− animals compared with PDK1K465E/K465EPTEN+/+ or PDK1+/+PTEN+/+ mice (Fig. 1C–E). We also monitored phosphorylation of various Akt substrates, including PRAS40, FOXO1 and GSK3 using phosphospecific antibodies against Akt phosphorylation sites of these proteins. We detected a decrease in the phosphorylation of GSK3 in heart, comparing wild-type PDK1+/+PTEN+/+ mice with PDK1K465E/K465EPTEN+/+ animals (Fig. 1D). Reduced GSK3 phosphorylation was partially rescued in heart lysates from PDK1K465E/K465EPTEN+/− mice compared with PDK1K465E/K465EPTEN+/+ mice (Fig. 1D). Similarly, phosphorylation of PRAS40 was reduced in muscle extracts from PDK1K465E/K465EPTEN+/+ mice compared with PDK1+/+PTEN+/+ animals (Fig. 1E). PRAS40 phosphorylation in muscle extracts was restored to wild-type PDK1+/+PTEN+/+ levels in PDK1K465E/K465EPTEN+/− animals (Fig. 1E). We did not detect significant changes in the phosphorylation status of FOXO1 in any of the tissues examined, nor did we observe any significant effects in the liver of phosphorylation of Akt substrates in the PDK1K465E/K465EPTEN+/+ knock-in mice (Fig. 1C). This indicates that, in some tissues, a moderate decrease in Akt activity does not affect phosphorylation of Akt substrates induced by acute insulin stimulation.
Enhanced insulin sensitivity of PDK1K465E/K465EPTEN+/− mice
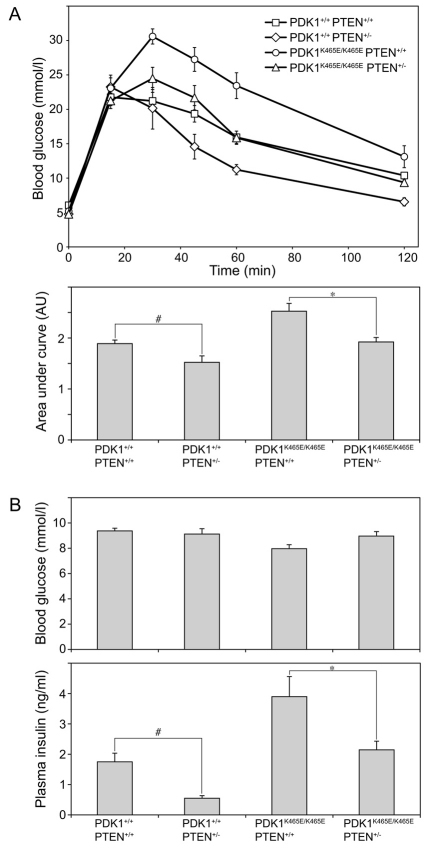
To investigate whether the modestly enhanced Akt activity observed in the PDK1K465E/K465EPTEN+/− compared with the PDK1K465E/K465EPTEN+/+ mice would be sufficient to counteract the insulin resistance, we performed a glucose tolerance test on these animals. Mice were starved overnight and injected with an intraperitoneal bolus of glucose, and blood glucose levels monitored over a 120-minute period. As reported previously (Bayascas et al., 2008), PDK1K465E/K465EPTEN+/+ animals displayed significant glucose intolerance because their blood glucose levels were markedly higher at all time points compared with PDK1+/+PTEN+/+ wild-type mice (Fig. 2A). The insulin resistance in the PDK1K465E/K465EPTEN+/+ animals was also emphasised by the markedly higher levels of plasma insulin compared with PDK1+/+PTEN+/+ mice (Fig. 2B). Strikingly, however, the PDK1K465E/K465EPTEN+/− mice showed markedly improved glucose tolerance compared with PDK1K465E/K465EPTEN+/+ animals. The rises in blood glucose levels at all time points were similar to the wild-type PDK1+/+PTEN+/+ mice in the glucose tolerance test (Fig. 2A). In addition, plasma insulin levels were not elevated in the PDK1K465E/K465EPTEN+/− animals and were similar to those of wild-type mice (Fig. 2B). As reported previously (Wong et al., 2007), we also observed that the PDK1+/+PTEN+/− mice displayed enhanced insulin sensitivity compared with wild-type PDK1+/+PTEN+/+ animals (Fig. 2A). The PDK1+/+PTEN+/− mice also possessed lower plasma insulin levels than wild-type mice (Fig. 2B). These data indicate that lowering the expression of PTEN by only 50% is sufficient to rescue the insulin resistance phenotype observed in PDK1K465E/K465EPTEN+/+ animals.
Fig. 2.
Improved insulin sensitivity in PDK1K465E/K465EPTEN+/− mice. (A) Glucose tolerance test. Male mice aged 4 months of the indicated genotype were deprived of food overnight and then injected intraperitoneally with glucose (2 mg/g body weight). Blood glucose concentration was measured at the indicated times. Data are shown as mean ± s.e.m. Area under the curve was calculated and shown as mean ± s.e.m. of arbitrary units. Data from one experiment are shown; similar results were obtained with a second group of mice. P-values were obtained by the Student’s t-test, #P<0.02 for PDK1+/+ PTEN+/+ compared with PDK1+/+ PTEN+/−. *P<0.005 for PDK1K465E/K465E PTEN+/+ compared with PDK1K465E/K465E PTEN+/−. (B) Blood glucose and plasma insulin levels were measured in male mice aged 4 months fed ad libitum. Data are shown as mean ± s.e.m. Data of one experiment are shown; similar results were obtained with a second group of mice. P-values were obtained by Student’s t-test, #P<0.05 for PDK1+/+ PTEN+/+ compared with PDK1+/+ PTEN+/−. *P<0.05 for PDK1K465E/K465E PTEN+/+ compared with PDK1K465E/K465E PTEN+/−. PDK1+/+ PTEN+/+ n=9, PDK1+/+ PTEN+/− n=4, PDK1K465E/K465E PTEN+/+ n=8, PDK1K465E/K465E PTEN+/− n=8.
Delayed tumour onset in PDK1K465E/K465EPTEN+/− mice
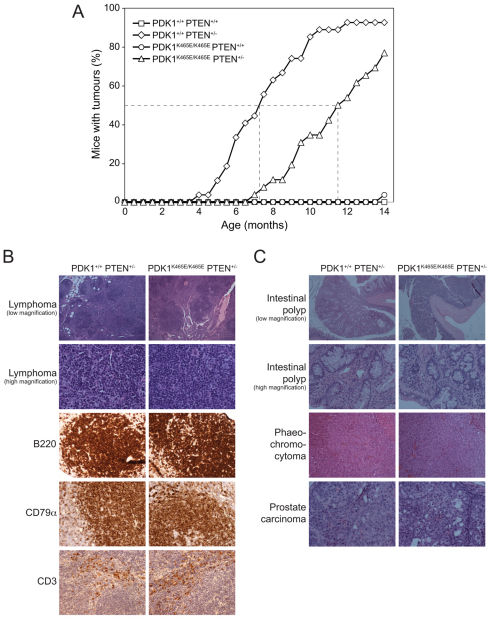
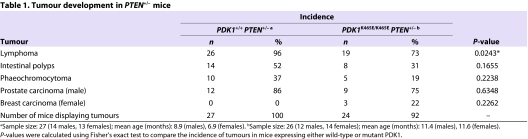
We next sought to define how a modest reduction in Akt signalling, which results from introducing the PDK1-PH domain K465E mutation, impinges on tumour development in PTEN+/− animals. We monitored groups of ∼25 PDK1+/+PTEN+/+, PDK1K465E/K465EPTEN+/+, PDK1+/+PTEN+/− and PDK1K465E/K465EPTEN+/− animals for tumour development. Animals were sacrificed when they exhibited large externally palpable tumours, reduced body weight, became unwell, or reached 14 months of age. A necropsy was performed, the tissues fixed in 10% formalin and subjected to a detailed histopathological examination. By 14 months of age, wild-type PDK1+/+PTEN+/+ and PDK1K465E/K465EPTEN+/+ mice displayed no significant tumours (Fig. 3A). Consistent with previous work (Podsypanina et al., 1999), tumour development in PDK1+/+PTEN+/− mice was observed at ∼5 months of age, and by 10 months of age ∼90% had one or more tumours. Strikingly, the PDK1K465E/K465EPTEN+/− mice developed tumours significantly later, commencing at 7 months of age. At 10 months of age, only ∼35% of the PDK1K465E/K465EPTEN+/− mice had developed tumours (Fig. 3A). These data demonstrate that tumour initiation in PDK1K465E/K465EPTEN+/− was delayed by 3–4 months compared with PDK1+/+PTEN+/− mice. The types and morphology of tumours arising in PTEN+/− mice with either wild-type PDK1 or PDK1-PH domain mutation were similar (Table 1). Comparing the incidence of detected tumours in much younger PDK1+/+PTEN+/−mice with older PDK1K465E/K465EPTEN+/− animals did not suggest a major difference in the frequency of different tumours. We detected a significant difference in tumour formation in B cell follicular lymphomas, which were found at ∼25% lower incidence in the older PDK1K465E/K465EPTEN+/− mice compared with younger PDK1+/+PTEN+/− animals (Table 1). We also found a lower incidence rate for the formation of intestinal polyps, phaeochromocytoma and prostate carcinoma in PDK1K465E/K465EPTEN+/− mice compared with PDK1+/+PTEN+/− mice (Table 1), indicating that inhibition of Akt activity might have a broad spectrum of action in tumour formation.
Fig. 3.
Delayed tumour formation in PDK1K465E/K465EPTEN+/− mice. (A) Incidence curve of tumour formation. Mice were maintained under standard husbandry conditions and the percentage of mice at each age with visible tumours recorded (B cell follicular lymphoma). The broken lines indicate mean age for 50% of animals to develop tumours. P-values were obtained by Fisher’s exact test comparing PDK1+/+ PTEN+/− with PDK1K465E/K465E PTEN+/− mice: P<0.003 for each data point from 7 to 12 months, P<0.05 for each data point from 12.5 to 13.5 months. PDK1+/+ PTEN+/+ n=28, PDK1+/+ PTEN+/− n=27, PDK1K465E/K465E PTEN+/+ n=27, PDK1K465E/K465E PTEN+/− n=26. (B) Representative sections of H&E (hematoxylin and eosin) staining (2× and 20× objective) and immunohistochemisty of lymphomas using the indicated antibodies. The lymphomas were all classified as B cell follicular lymphoma by the Bethesda criteria. (C) Representative sections of H&E staining of intestinal polyps (2× and 20× objective), phaeochromocytoma (20× objective) and prostate carcinoma (20×objective).
Table 1.
Tumour development in PTEN+/− mice
Representative histopathological sections of the tumours found in PDK1+/+PTEN+/− and PDK1K465E/K465EPTEN+/− mice are shown in Fig. 3B,C. Lymphomas in both genotypes had a follicular architecture and showed extension beyond the lymph node capsule (Fig. 3B, low magnification images). The neoplastic follicles were composed of centrocytes and centroblasts (Fig. 3B, high magnification images). Reactivity for B220 and CD79α by immunohistochemistry confirmed that the lymphomas were all B cell follicular lymphomas. Occasional CD3-reactive T cells were seen mostly in interfollicular areas (Fig. 3B). Intestinal polyps could be seen at low magnification, projecting into the bowel lumen (Fig. 3C). High magnification examination showed the composition of these polyps to include mucin secreting epithelium and a smooth muscle cell rich stroma (Fig. 3C). Phaeochromocytoma of the adrenal medulla was seen in both genotypes. There was no evidence of invasion beyond the adrenal medulla (Fig. 3C). Prostate carcinoma in both genotypes consisted of microacinar or cribriform adenocarcinoma, with focal cell death. Microinvasion or frank invasion of muscle was seen in both genotypes (Fig. 3C). These data provide evidence that the onset of tumour formation in PDK1K465E/K465EPTEN+/− mice was significantly reduced compared with PDK1+/+PTEN+/− animals, but the tumour types and morphology arising in the two different genotypes were similar.
Tumour growth is reduced but Akt pathway is unaltered in tumours from PDK1K465E/K465EPTEN+/− mice
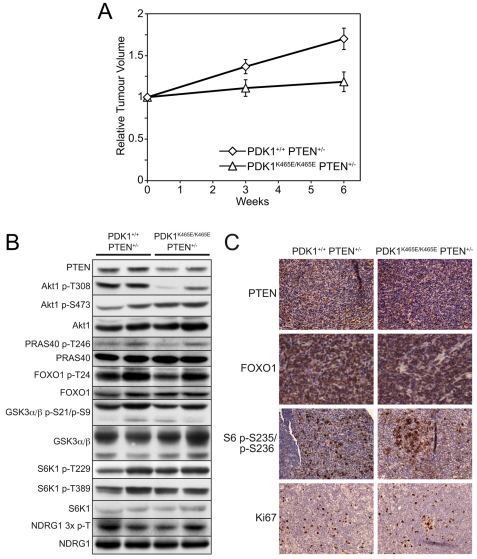
To assess the growth rate of tumours formed in PDK1+/+PTEN+/−and PDK1K465E/K465EPTEN+/− mice, the volumes of B cell follicular lymphomas present in the animals were measured at 3-week intervals over a 6-week period by magnetic resonance imaging (MRI). For these experiments, littermate mice of the same age and possessing tumours were selected. We observed that the volume of B cell follicular lymphomas in PDK1+/+PTEN+/− mice increased 1.7-fold over this period of analysis (Fig. 4A). By contrast, the size of tumours observed in PDK1K465E/K465EPTEN+/− mice did not appreciably increase over 6 weeks. After this period, total cell extracts were generated from the B cell follicular lymphomas studied by MRI. Phospho-immunoblotting revealed that Akt phosphorylation at T308, but not S473, was significantly reduced in the B cell follicular lymphomas derived from PDK1K465E/K465EPTEN+/− mice compared with PDK1+/+PTEN+/−animals (Fig. 4B). We did not detect significant differences in the phosphorylation of Akt substrates, such as PRAS40, FOXO1 and GSK3α/β, comparing lymphomas from PDK1+/+PTEN+/− with those from PDK1K465E/K465EPTEN+/− mice. Similarly, there was no significant difference in the phosphorylation of other PDK1 substrates, S6K and SGK [assessed by phosphorylation of its substrate NDRG1 (Garcia-Martinez and Alessi, 2008)], in tumours derived from PDK1+/+PTEN+/− as compared with PDK1K465E/K465EPTEN+/− mice. To further assess the properties of the tumours formed in PDK1+/+PTEN+/− and PDK1K465E/K465EPTEN+/− mice, sections of B cell follicular lymphomas were analysed by immunohistochemistry. PTEN staining of the lymphoma was detected, which indicates that these lymphomas have not lost all expression of PTEN. We also investigated the subcellular localisation of the FOXO1 transcription factor, which is localised in the cytoplasm after phosphorylation by Akt. In both centroblasts and smaller centrocytes, FOXO1 staining was predominantly cytoplasmic, irrespective of genotype, indicating that Akt was activated. Strong cytoplasmic staining for phosphorylation of S6 in centroblasts was observed, suggesting that S6K was active in tumours of either genotype. There was no significant difference between the genotypes in the proportion of the proliferative marker Ki67. These data indicate that although Akt phosphorylation at T308 was reduced in tumours from PDK1K465E/K465EPTEN+/− mice, once tumours had formed there was no significant difference in the signalling pathway downstream of Akt, which is consistent with previous studies (Bayascas et al., 2005).
Fig. 4.
Reduced tumour growth in PDK1K465E/K465EPTEN+/− mice. (A) The size of B cell follicular lymphomas of 11-month-old littermate PDK1+/+PTEN+/− and PDK1K465E/K465E PTEN+/− mice were determined by MRI imaging. Individual tumours from the same animal were measured at 0-, 3- and 6-week intervals. The data are depicted as relative tumour volume. PDK1+/+ PTEN+/− n=3, PDK1K465E/K465E PTEN+/− n=2. (B) Lysates of B cell follicular lymphomas from PDK1+/+ PTEN+/− and PDK1K465E/K465E PTEN+/− mice were subjected to immunoblot analysis using the indicated antibodies. (C) Immunohistochemical analysis of B cell follicular lymphomas using the indicated antibodies.
DISCUSSION
In this study, we have investigated how modest modulation of the Akt signalling pathway activity impacts on insulin sensitivity and tumorigenesis. We found that lowering the expression of PTEN in insulin-resistant PDK1K465E/K465E mice resulted in a significant improvement in the insulin sensitivity (Figs 1, 2). We also observed that a reduction in Akt activity in PDK1K465E/K465EPTEN+/− animals lead to a considerable delay in the onset and growth rate of tumours (Figs 3, 4).
Earlier studies from our laboratory indicated that lowering the expression of PDK1 has a pronounced effect on tumorigenesis of PTEN+/− mice (Bayascas et al., 2005). Reduction in levels of PDK1 expression is likely to impact on the activity of multiple downstream targets of PDK1. However, the only major signalling defect we observed in the PDK1K465E/K465EPTEN+/− mice was a moderate reduction of Akt T308 phosphorylation, which reduces Akt isoform activity. All other AGC kinases we have studied, including S6K1 and SGK activity (as judged by phosphorylation of NDRG1), are not affected in the tumours that develop in the PDK1K465E/K465EPTEN+/− mice. This indicates that a moderate reduction in Akt isoform activity is sufficient to delay tumour onset and development. The mechanism by which reduction in Akt activity delays tumour onset and development requires further investigation because phosphorylation of the Akt substrates we have investigated is not markedly inhibited in tumours derived from PDK1K465E/K465EPTEN+/− animals. It cannot be ruled out that there are other Akt substrates that we have not investigated whose phosphorylation will be more significantly affected in tumours derived from PDK1K465E/K465EPTEN+/− mice and which could play a key role in regulating tumour initiation and development.
Previous studies have demonstrated that genetic ablation of PI3K (Jia et al., 2008), Akt isoforms (Chen et al., 2006) and mTOR regulatory subunits such as Rictor (Guertin et al., 2009), when combined with loss of PTEN, markedly suppress tumorigenesis. It is unclear whether these mouse models represent the clinical situation of a human cancer patient taking a dose of a drug that might only partially suppress Akt pathway activity. Because the Akt network controls many essential physiological roles, it is likely that inhibitors would only be tolerated at doses that moderately lower Akt activity. The PDK1K465E/K465E mouse model is unique in that it only leads to a slight reduction in the activity of Akt isoforms in all tissues, without affecting the activity of other related kinases such as S6K and SGK (Bayascas et al., 2008). We argue that the PDK1K465E/K465E mice mimic the situation of a patient treated with an Akt inhibitor better than the complete knockout mouse models that have been generated previously. The observation that tumour development and tumour growth is delayed in PDK1K465E/K465EPTEN+/− mice is encouraging and suggests that a drug causing partial inhibition of the Akt pathway would have some efficacy in slowing tumour growth. This could be relevant to the treatment of human tumours because diagnosis is normally made once a tumour has formed. In addition, there is the concern that inhibition of the PI3K signalling pathway would promote insulin resistance and result in overt diabetes. However, as the PDK1K465E/K465E mice display only a mild diabetic phenotype, it is probable that any insulin resistance that develops in human cancer patients treated with an Akt inhibitor could be managed in the clinic. Indeed, measuring increased plasma insulin levels as a marker of insulin resistance in patients administered with drugs that suppress Akt activity is probably an effective biomarker strategy for assessing the efficacy with which such compounds inhibit Akt in patients. Although our data suggest that a moderate inhibition of PTEN would effectively counteract insulin resistance, it is unlikely that drugs that inhibit PTEN will ever be utilised for the treatment of diabetes because recent genetic analysis has elegantly demonstrated that even a 20% reduction in PTEN expression is sufficient to cause significant neoplastic lesions (Alimonti et al., 2010).
Our study demonstrates the importance of the PI3K-Akt signalling pathway in the development of diseases such as diabetes and cancer, and provides insights into how targeting this pathway might be useful for treatment of cancer. Crucially, our study suggests that the inhibition of Akt activity by either an Akt, PDK1, PI3K or mTOR inhibitor would be effective in not only delaying onset of tumours driven by PTEN inactivation or other mutations that stimulate the Akt pathway, but also by suppressing the growth of these tumours once formed. Importantly, although our study focuses on the formation of B cell follicular lymphomas, our results indicate that drugs that suppress Akt might be effective for the treatment of a broader range of common tumour types, such as intestinal polyps, phaeochromocytoma and prostate carcinoma (Table 1). It will be interesting to see how efficacious Akt-pathway inhibitors (now in early stage clinical trials) are at suppressing tumorigenesis of different tumour types (Liu et al., 2009). Finally, this study also suggests that compounds that inhibit the PH domain of PDK1 binding to PtdIns(3,4,5)P3 could be used as an alternative approach to the development of anticancer agents that suppress the Akt pathway. Perhaps these agents could be employed in combination with Akt kinase domain inhibitors or with other drugs that target components of the PI3K signal transduction pathway.
METHODS
Materials and general buffers
Protein G-Sepharose and [γ32P]-ATP were purchased from Amersham Pharmacia Biotech. Human insulin from Novo-Nordisk was obtained from Ninewells Pharmacy, Dundee. Lysis buffer was 50 mM Tris-HCl pH 7.5, 1 mM EDTA, 1 mM EGTA, 1% (w/v) Triton-X 100, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 0.27 M sucrose, 0.1% (v/v) 2-mercaptoethanol and ‘Complete’ protease inhibitor cocktail (Roche).
Antibodies
The following antibodies were raised in sheep and affinity-purified on the appropriate antigen. The total antibody used for immunoprecipitation and immunoblotting of Akt1 (S742B, 3rd bleed) was raised against full-length His-Akt1 expressed in Escherichia coli. Akt2 antibody (S069C, 2nd bleed) was raised against the sequence RYDSLDPLELDQRTH, which corresponds to residues 455–469 of mouse Akt2. The PDK1 antibody (S48A, 3rd bleed) was raised against the sequence RKIQEVWRQQYQSNPDAAVQ, which corresponds to residues 540–559 of mouse PDK1. The S6K1 antibody (S417B, 2nd bleed) was raised against the sequence AGVFDIDLDQPEDAGSEDEL, corresponding to residues 25–44 of human S6K1. The NDRG1 antibody (S276, 2nd bleed) was raised against full length GST-NDRG1. An antibody that recognizes NDRG1 phosphorylated at T346, T356 and T366 (S911B, 2nd bleed, termed NDRG1 3× p-T) was raised against the peptide CRSRSHpTSEG, whose sequence is common to all three sites. The PRAS40 antibody (S115B, 1st bleed) was raised against the sequence DLPRPRLNTSDFQKLKRKY, which corresponds to residues 238–256 of human PRAS40. An antibody that recognizes PRAS40 phosphorylated at T246 (S114B, 2nd bleed) was raised against the peptide CRPRLNpTSDFQK. The FOXO1 antibody (S457, 3rd bleed) was raised against GST-FOXO1, comprising residues 2–655 of human FOXO1. The Akt1 p-T308 (#9275), Akt1 p-S473 (#9271), FOXO1 p-T24 (#9464), GSK3α/β p-S21/p-S9 (#9331), S6K1 pT389 (#9205), PTEN (#9559) and the pan-PDK1 site antibody (#9379) that recognises the phosphorylated T-loop of S6K were purchased from Cell Signaling Technology. The GSK3α/β antibody (#44–610) was purchased from Biosource. Secondary antibodies coupled to horseradish peroxidase were from Pierce.
Mice breeding, genotype analysis and tumour analysis
All animal studies and breeding was approved by the University of Dundee Ethical Committee and performed under a UK Home Office project licence. The generation and genotyping of the PTEN+/− mice (Podsypanina et al., 1999) and the PDK1K465E/K465E knock-in mice (Bayascas et al., 2008) have been described previously. The parental PTEN+/− and PDK1+/K465E mice used for these experiments had been backcrossed with C57BL/6J for over five generations before initiating the crosses for the present study. Littermates with the different genotypes of PTEN and PDK1 were derived as described in Fig. 1A and were maintained under standard husbandry conditions for a period of up to 14 months. The onset of tumour formation was monitored weekly by palpation of the major lymph nodes. According to our UK Home Office licence, animals displaying a tumour of over 1.44 cm2, showing signs of sickness, or having lost over 20% of body weight were culled. The tissues were fixed in 10% formalin and subjected to necropsy and pathological analysis. Tumour slices were generated and analysed as described previously (Bayascas et al., 2005).
Tissue microarray construction
The use of tissue microarrays allows all samples to be simultaneously stained, thus reducing variability between antibody runs. A tissue microarray (TMA) was constructed from the representative lymphoma areas from the original mouse pathology wax blocks. A manual tissue arrayer (Beecher Instruments) was used to bore cores of tissue from the areas of the paraffin blocks marked by the pathologist and transfer them into a recipient paraffin block. Either two or three cores, each 0.6 mm in diameter, were donated from each lymphoma and eight cases of lymphomas from each of the PDK1+/+ PTEN+/− or PDK1K465E/K465E PTEN+/−genotypes were analysed.
IHC staining
Primary antibodies were used to detect B220/CD45R (RA3-6B2, BD Pharmingen), CD79αcy (HM57, Dako), CD3 (F7.2.38, Dako) and Ki67 (VP-K452, Vector Laboratories). Antibodies against PTEN protein (#9188), FOXO1 (#9462) and S6 p-S235/S236 (#4857) were purchased from Cell Signaling Technology. Antibody binding was visualised using Vectastain reagents and protocols performed on a Dako immunostainer. Sections were viewed on a Nikon Eclipse E600 microscope, and digital images captured on a Nikon DXM 1200 digital camera.
Preparation of tissue lysates, immunoblotting and kinase assays
Following an overnight fast, a bolus of insulin (1 mU/g body weight) was intravenously injected through the inferior vena cava to mice that had been terminally anaesthetised by pentobarbital (86 μg/g body weight, intraperitoneally injected). At 10 minutes after injection, tissues were extracted, frozen in liquid nitrogen and stored at −80°C. Tissues were homogenised on ice in a tenfold mass excess of ice-cold lysis buffer using a Kinematica Polytron. Tissue lysates were centrifuged at 18,000 g for 15 minutes at 4°C and the supernatant snap-frozen and stored at −80°C. The activity of Akt1 was assessed either by immunoblotting of tissue lysate (20 μg) or by kinase activity assays. Briefly, Akt1 or Akt2 was immunoprecipitated from 1 mg of tissue lysate and the kinase activity measured using the Crosstide peptide (GRPRTSSFAEG) as described previously (Williams et al., 2000).
Glucose tolerance test
Mice were deprived of food overnight and then basal blood glucose levels determined using the Ascensia Breeze 2 blood glucose monitoring system (Bayer) following tail incision. Mice were injected intraperitoneally with 2 mg glucose/g body weight. Blood glucose levels were measured 15, 30, 45, 60 and 120 minutes after glucose injection. The area under the curve was calculated using GraphPad PRISM software.
Plasma insulin measurement
Blood was collected from mice following tail incision and using Na-heparinised capillary tubes (Hawksley). The blood was centrifuged at 3000 g for 15 minutes and the supernatant collected. Plasma insulin levels were determined using a rat/mouse insulin ELISA kit from Millipore (EZRMI-13K) according to the instructions of the manufacturer. Rat insulin of 0–10 ng/ml was used as a standard.
Micro-magnetic resonance imaging of tumours
Micro-magnetic resonance imaging (micro-MRI) data were acquired on a Bruker Avance FT NMR spectrometer (Bruker Biospin, Rheinstetten, Germany) with a wide-bore 7.1-Tesla vertical magnet resonating at 300.15 MHz for 1H, fitted with Bruker micro-imaging magnetic field gradients. A MicroMouse birdcage radio-frequency resonator with an internal diameter of 30 mm was used. Three dimensional (3D) 128×128×128 rapid acquisition relaxation enhanced (RARE) MRI experiments were performed with RARE factor of 4, taken from the Paravision library. The recycle time (TR) was 250 milliseconds and echo time (TE) was 10 milliseconds. One acquisition sequence was acquired to minimise the experimental time, which was 17 minutes. The field of view was 30 mm, the isotropic image spatial resolution was 234 μm per pixel. Mice were anesthetised with isoflurane (1.5% in 100% oxygen) using a nose cone with scavenging. The mice were monitored during imaging and recovery, and then returned to their cage. The MRI data were Fourier-transformed and visualised using Amira PC-based software (Visage Imaging, San Diego, CA). The tumours were digitally segmented and then 3D finite element meshes generated and surface rendered to produce 3D surface representations. The volumes of the tumours were calculated from these images.
Acknowledgments
We thank Jose Bayascas (Institute of Neurosciences, University Autonoma of Barcelona, Barcelona, Spain) for providing the PDK1K465E/K465E knock-in mice and for helpful advice. We also thank Ramon Parsons (Institute for Cancer Genetics, Department of Pathology and Cell Biology, Columbia University, New York, NY) for the PTEN+/− mice; Gail Fraser (MRC Protein Phosphorylation Unit, University of Dundee) for assistance with genotyping of mice; the Tissue Bank of the University of Dundee for histological preparation; the antibody purification team (Division of Signal Transduction Therapy, University of Dundee) co-ordinated by Hilary McLauchlan and James Hastie; and Laura Pearce for critical reading of the manuscript. We are also grateful to the Medical Research Council (MRC), Wellcome Trust ( SLD-WT081039) and the pharmaceutical companies supporting the Division of Signal Transduction Therapy (AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck-Serono and Pfizer) for financial support.
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
S.W. and D.R.A. conceived of the project. All authors were involved in planning and analysing the experimental data. S.W. undertook most of the experimentation. K.S. helped with insulin studies, S.D. undertook the MRI analysis and S.F. and L.J. performed the immunohistochemistry and tumour classifications. S.W. and D.R.A. wrote manuscript. D.R.A. supervised the project.
REFERENCES
- Alessi D.R., Pearce L.R., Garcia-Martinez J.M. (2009). New insights into mTOR signaling: mTORC2 and beyond. Sci. Signal. 2, pe27. [DOI] [PubMed] [Google Scholar]
- Alimonti A., Carracedo A., Clohessy J.G., Trotman L.C., Nardella C., Egia A., Salmena L., Sampieri K., Haveman W.J., Brogi E., et al. (2010). Subtle variations in Pten dose determine cancer susceptibility. Nat. Genet. 42, 454–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayascas J.R., Leslie N.R., Parsons R., Fleming S., Alessi D.R. (2005). Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN(+/−) mice. Curr. Biol. 15, 1839–1846 [DOI] [PubMed] [Google Scholar]
- Bayascas J.R., Wullschleger S., Sakamoto K., Garcia-Martinez J.M., Clacher C., Komander D., van Aalten D.M., Boini K.M., Lang F., Lipina C., et al. (2008). Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Mol. Cell. Biol. 28, 3258–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.L., Xu P.Z., Peng X.D., Chen W.S., Guzman G., Yang X., Di Cristofano A., Pandolfi P.P., Hay N. (2006). The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes. Dev. 20, 1569–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A., Pesce B., Cordon-Cardo C., Pandolfi P.P. (1998). Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19, 348–355 [DOI] [PubMed] [Google Scholar]
- Dummler B., Hemmings B.A. (2007). Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc. Trans. 35, 231–235 [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez J.M., Alessi D.R. (2008). mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 416, 375–385 [DOI] [PubMed] [Google Scholar]
- Guertin D.A., Stevens D.M., Saitoh M., Kinkel S., Crosby K., Sheen J.H., Mullholland D.J., Magnuson M.A., Wu H., Sabatini D.M. (2009). mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell 15, 148–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas-Kogan D., Shalev N., Wong M., Mills G., Yount G., Stokoe D. (1998). Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr. Biol. 8, 1195–1198 [DOI] [PubMed] [Google Scholar]
- Jia S., Liu Z., Zhang S., Liu P., Zhang L., Lee S.H., Zhang J., Signoretti S., Loda M., Roberts T.M., et al. (2008). Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454, 776–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Cheng H., Roberts T.M., Zhao J.J. (2009). Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 8, 627–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T., Dixon J.E. (1998). The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 [DOI] [PubMed] [Google Scholar]
- Manning B.D., Cantley L.C. (2007). AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M.P., Pass I., Batty I.H., Van der Kaay J., Stolarov J.P., Hemmings B.A., Wigler M.H., Downes C.P., Tonks N.K. (1998). The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc. Natl. Acad. Sci. USA 95, 13513–13518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L.R., Komander D., Alessi D.R. (2010). The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11, 9–22 [DOI] [PubMed] [Google Scholar]
- Podsypanina K., Ellenson L.H., Nemes A., Gu J., Tamura M., Yamada K.M., Cordon-Cardo C., Catoretti G., Fisher P.E., Parsons R. (1999). Mutation of Pten/MmaC1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. USA 96, 1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L., Carracedo A., Pandolfi P.P. (2008). Tenets of PTEN tumor suppression. Cell 133, 403–414 [DOI] [PubMed] [Google Scholar]
- Stambolic V., Suzuki A., de la Pompa J.L., Brothers G.M., Mirtsos C., Sasaki T., Ruland J., Penninger J.M., Siderovski D.P., Mak T.W. (1998). Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 [DOI] [PubMed] [Google Scholar]
- Suzuki A., de la Pompa J.L., Stambolic V., Elia A.J., Sasaki T., del Barco Barrantes I., Ho A., Wakeham A., Itie A., Khoo W., et al. (1998). High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr. Biol. 8, 1169–1178 [DOI] [PubMed] [Google Scholar]
- Taniguchi C.M., Emanuelli B., Kahn C.R. (2006). Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 [DOI] [PubMed] [Google Scholar]
- Whiteman E.L., Cho H., Birnbaum M.J. (2002). Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13, 444–451 [DOI] [PubMed] [Google Scholar]
- Williams M.R., Arthur J.S., Balendran A., van der Kaay J., Poli V., Cohen P., Alessi D.R. (2000). The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr. Biol. 10, 439–448 [DOI] [PubMed] [Google Scholar]
- Wong J.T., Kim P.T., Peacock J.W., Yau T.Y., Mui A.L., Chung S.W., Sossi V., Doudet D., Green D., Ruth T.J., et al. (2007). Pten (phosphatase and tensin homologue gene) haploinsufficiency promotes insulin hypersensitivity. Diabetologia 50, 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S., Loewith R., Hall M.N. (2006). TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]