Abstract
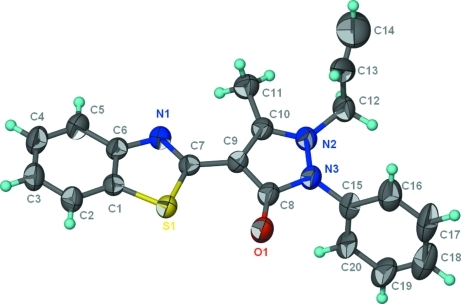
The title compound, C20H17N3OS, is a 1H-pyrazol-3(2H)-one having aromatic 4-(1,3-benzothiazol-2-yl)- and 2-phenyl substituents. The five-membered ring and fused ring system are planar, the r.m.s. deviations being 0.021 and 0.005 Å, respectively. The five-membered ring is aligned at 7.9 (2)° with respect to the fused-ring system. The allyl and phenyl parts of the molecule are both disordered over two positions in a 1:1 ratio. Weak intermolecular C—H⋯O hydrogen bonding is present in the crystal structure.
Related literature
For the structure of a related compound (E)-4-(2,3-dihydro-1,3-benzothiazol-2-ylidene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one, see: Chakibe et al. (2010 ▶).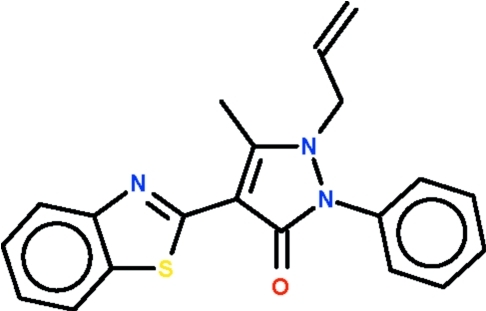
Experimental
Crystal data
C20H17N3OS
M r = 347.43
Orthorhombic,
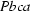
a = 17.8734 (5) Å
b = 10.4297 (2) Å
c = 18.9578 (4) Å
V = 3534.00 (14) Å3
Z = 8
Mo Kα radiation
μ = 0.20 mm−1
T = 293 K
0.30 × 0.30 × 0.25 mm
Data collection
Bruker X8 APEXII diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.944, T max = 0.953
18349 measured reflections
3678 independent reflections
2341 reflections with I > 2σ(I)
R int = 0.032
Refinement
R[F 2 > 2σ(F 2)] = 0.050
wR(F 2) = 0.172
S = 1.00
3678 reflections
227 parameters
17 restraints
H-atom parameters constrained
Δρmax = 0.32 e Å−3
Δρmin = −0.25 e Å−3
Data collection: APEX2 (Bruker, 2008 ▶); cell refinement: SAINT (Bruker, 2008 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810040316/xu5047sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810040316/xu5047Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2⋯O1i | 0.93 | 2.59 | 3.318 (3) | 135 |
| C12—H12A⋯O1ii | 0.97 | 2.51 | 3.404 (4) | 152 |
| C12—H12C⋯O1ii | 0.97 | 2.48 | 3.404 (4) | 159 |
Symmetry codes: (i) 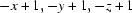 ; (ii)
; (ii) 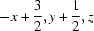 .
.
Acknowledgments
We thank Université Mohammed V-Agdal and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
(E)-4-(2,3-Dihydro-1,3-benzothiazol-2-ylidene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one is an amine that can under a nucleophilic substitution with organo bromides to form 2-substituted derivatives if tetra-n-butyl ammonium bromide is used as catalyst. In this study, the compound is reacted with allyl bromide to yield the title compound (Scheme I, Fig. 1).
Experimental
To a solution of (E)-4-(2,3-dihydro-1,3-benzothiazol-2-ylidene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (1 g, 3.25 mmol) in DMF (50 ml), was added sodium carbonate (2.5 g, 23 mmol), tetra-n-butyl ammonium bromide (0.15 g, 1 mmol) and allyl bromide (5.6 g, 46 mmol). The mixture was stirred for 24 h at room temperature. The solid material was removed by filtration and the solution was evaporated under reduced. The residue was washed with dichloromethane and hexane, and the recrystallized from ethanol to afford the title compound as colorless crystals.
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.93–0.97 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2–1.5Ueq(C).
The allyl and phenyl units are disordered over two positions; the disorder could be refined, and was assumed to be a 1:1 type of disorder. For the allyl unit, the single-bond distances were restrained to 1.50±0.01 Å and the double-bond distances to 1.35±0.01 Å; the anisotropic temperature factors were restrained to be nearly isotropic. The phenyl rings were refined as rigid hexagons of 1.39 Å sides; the N–Cphenyl pair of distances were restrained to within 0.01 Å of each other. Additionally, the temperature factors of the primed atoms were restrained to those of the unprimed ones.
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of C20H17N3OS at the 50% probability level; hydrogen atoms are drawn as arbitrary radius. The disorder is not shown
Crystal data
| C20H17N3OS | F(000) = 1456 |
| Mr = 347.43 | Dx = 1.306 Mg m−3 |
| Orthorhombic, Pbca | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 3745 reflections |
| a = 17.8734 (5) Å | θ = 2.3–22.5° |
| b = 10.4297 (2) Å | µ = 0.20 mm−1 |
| c = 18.9578 (4) Å | T = 293 K |
| V = 3534.00 (14) Å3 | Prism, colorless |
| Z = 8 | 0.30 × 0.30 × 0.25 mm |
Data collection
| Bruker X8 APEXII diffractometer | 3678 independent reflections |
| Radiation source: fine-focus sealed tube | 2341 reflections with I > 2σ(I) |
| graphite | Rint = 0.032 |
| φ and ω scans | θmax = 26.6°, θmin = 2.4° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −22→21 |
| Tmin = 0.944, Tmax = 0.953 | k = −13→12 |
| 18349 measured reflections | l = −23→23 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.050 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.172 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.096P)2 + 0.8646P] where P = (Fo2 + 2Fc2)/3 |
| 3678 reflections | (Δ/σ)max = 0.001 |
| 227 parameters | Δρmax = 0.32 e Å−3 |
| 17 restraints | Δρmin = −0.25 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| S1 | 0.54022 (4) | 0.59623 (7) | 0.57529 (3) | 0.0590 (2) | |
| O1 | 0.63189 (12) | 0.5096 (2) | 0.45761 (10) | 0.0808 (6) | |
| N1 | 0.61613 (12) | 0.7853 (2) | 0.62953 (11) | 0.0590 (6) | |
| N2 | 0.79529 (13) | 0.6762 (2) | 0.48741 (11) | 0.0593 (6) | |
| N3 | 0.75432 (13) | 0.5809 (2) | 0.45335 (11) | 0.0627 (6) | |
| C1 | 0.50073 (15) | 0.6812 (2) | 0.64448 (12) | 0.0531 (6) | |
| C2 | 0.43115 (17) | 0.6640 (3) | 0.67650 (14) | 0.0669 (7) | |
| H2 | 0.3989 | 0.5995 | 0.6617 | 0.080* | |
| C3 | 0.41164 (19) | 0.7448 (3) | 0.73035 (15) | 0.0762 (9) | |
| H3 | 0.3654 | 0.7349 | 0.7522 | 0.091* | |
| C4 | 0.4595 (2) | 0.8412 (3) | 0.75287 (16) | 0.0798 (9) | |
| H4 | 0.4454 | 0.8943 | 0.7900 | 0.096* | |
| C5 | 0.52717 (19) | 0.8589 (3) | 0.72110 (15) | 0.0743 (8) | |
| H5 | 0.5587 | 0.9242 | 0.7362 | 0.089* | |
| C6 | 0.54904 (16) | 0.7785 (2) | 0.66569 (12) | 0.0566 (6) | |
| C7 | 0.61898 (14) | 0.6956 (2) | 0.58142 (11) | 0.0495 (6) | |
| C8 | 0.68208 (17) | 0.5800 (3) | 0.48056 (13) | 0.0603 (7) | |
| C9 | 0.68293 (15) | 0.6721 (2) | 0.53608 (12) | 0.0518 (6) | |
| C10 | 0.75276 (15) | 0.7255 (2) | 0.53898 (12) | 0.0548 (6) | |
| C11 | 0.78327 (19) | 0.8222 (3) | 0.58934 (16) | 0.0760 (8) | |
| H11A | 0.8269 | 0.8617 | 0.5693 | 0.114* | |
| H11B | 0.7965 | 0.7806 | 0.6327 | 0.114* | |
| H11C | 0.7461 | 0.8865 | 0.5984 | 0.114* | |
| C12 | 0.87637 (17) | 0.6862 (3) | 0.48056 (16) | 0.0746 (8) | |
| H12A | 0.8914 | 0.7755 | 0.4806 | 0.090* | 0.50 |
| H12B | 0.8926 | 0.6475 | 0.4366 | 0.090* | 0.50 |
| H12C | 0.8825 | 0.7782 | 0.4852 | 0.090* | 0.50 |
| H12D | 0.8812 | 0.6708 | 0.4303 | 0.090* | 0.50 |
| C13 | 0.9121 (11) | 0.6151 (15) | 0.5440 (7) | 0.101 (3) | 0.50 |
| H13 | 0.8915 | 0.5398 | 0.5616 | 0.121* | 0.50 |
| C14 | 0.9722 (11) | 0.6652 (18) | 0.5718 (9) | 0.139 (4) | 0.50 |
| H14A | 0.9920 | 0.7407 | 0.5535 | 0.166* | 0.50 |
| H14B | 0.9953 | 0.6254 | 0.6099 | 0.166* | 0.50 |
| C13' | 0.9175 (11) | 0.5938 (15) | 0.5294 (7) | 0.101 (3) | 0.50 |
| H13' | 0.9148 | 0.5076 | 0.5171 | 0.121* | 0.50 |
| C14' | 0.9555 (11) | 0.6196 (18) | 0.5854 (8) | 0.139 (4) | 0.50 |
| H14C | 0.9603 | 0.7041 | 0.6004 | 0.166* | 0.50 |
| H14D | 0.9781 | 0.5538 | 0.6107 | 0.166* | 0.50 |
| C15 | 0.7691 (10) | 0.5140 (10) | 0.3885 (4) | 0.0510 (18) | 0.50 |
| C16 | 0.8277 (10) | 0.4304 (14) | 0.3741 (5) | 0.0863 (18) | 0.50 |
| H16 | 0.8561 | 0.3969 | 0.4108 | 0.104* | 0.50 |
| C17 | 0.8437 (10) | 0.3967 (13) | 0.3048 (5) | 0.105 (3) | 0.50 |
| H17 | 0.8829 | 0.3407 | 0.2951 | 0.126* | 0.50 |
| C18 | 0.8012 (11) | 0.4466 (10) | 0.2499 (4) | 0.110 (4) | 0.50 |
| H18 | 0.8119 | 0.4241 | 0.2035 | 0.132* | 0.50 |
| C19 | 0.7426 (10) | 0.5303 (12) | 0.2643 (6) | 0.102 (3) | 0.50 |
| H19 | 0.7142 | 0.5637 | 0.2275 | 0.123* | 0.50 |
| C20 | 0.7265 (9) | 0.5640 (12) | 0.3336 (7) | 0.0714 (19) | 0.50 |
| H20 | 0.6874 | 0.6199 | 0.3432 | 0.086* | 0.50 |
| C15' | 0.7791 (10) | 0.5424 (10) | 0.3848 (5) | 0.0510 (18) | 0.50 |
| C16' | 0.8326 (10) | 0.4458 (14) | 0.3884 (4) | 0.0863 (18) | 0.50 |
| H16' | 0.8571 | 0.4294 | 0.4308 | 0.104* | 0.50 |
| C17' | 0.8494 (11) | 0.3738 (12) | 0.3288 (5) | 0.105 (3) | 0.50 |
| H17' | 0.8852 | 0.3092 | 0.3312 | 0.126* | 0.50 |
| C18' | 0.8127 (11) | 0.3984 (9) | 0.2656 (4) | 0.110 (4) | 0.50 |
| H18' | 0.8240 | 0.3502 | 0.2257 | 0.132* | 0.50 |
| C19' | 0.7593 (10) | 0.4949 (13) | 0.2620 (5) | 0.102 (3) | 0.50 |
| H19' | 0.7347 | 0.5113 | 0.2197 | 0.123* | 0.50 |
| C20' | 0.7425 (9) | 0.5669 (11) | 0.3216 (7) | 0.0714 (19) | 0.50 |
| H20' | 0.7067 | 0.6315 | 0.3192 | 0.086* | 0.50 |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0542 (4) | 0.0698 (4) | 0.0528 (4) | −0.0033 (3) | 0.0062 (3) | −0.0106 (3) |
| O1 | 0.0647 (13) | 0.1020 (15) | 0.0756 (13) | −0.0228 (12) | 0.0166 (10) | −0.0337 (12) |
| N1 | 0.0559 (14) | 0.0630 (12) | 0.0580 (12) | 0.0042 (10) | 0.0020 (10) | −0.0066 (10) |
| N2 | 0.0529 (14) | 0.0655 (12) | 0.0594 (12) | −0.0072 (11) | 0.0080 (10) | −0.0040 (10) |
| N3 | 0.0568 (14) | 0.0740 (13) | 0.0573 (12) | −0.0097 (11) | 0.0118 (11) | −0.0143 (10) |
| C1 | 0.0521 (16) | 0.0634 (14) | 0.0438 (12) | 0.0102 (12) | −0.0007 (11) | 0.0048 (10) |
| C2 | 0.0615 (18) | 0.0812 (18) | 0.0581 (15) | 0.0026 (15) | 0.0106 (13) | 0.0007 (13) |
| C3 | 0.067 (2) | 0.100 (2) | 0.0611 (16) | 0.0151 (18) | 0.0163 (15) | 0.0006 (15) |
| C4 | 0.084 (2) | 0.090 (2) | 0.0657 (17) | 0.0216 (19) | 0.0131 (17) | −0.0169 (16) |
| C5 | 0.075 (2) | 0.0803 (18) | 0.0677 (17) | 0.0077 (16) | 0.0047 (16) | −0.0213 (15) |
| C6 | 0.0589 (17) | 0.0610 (14) | 0.0500 (13) | 0.0111 (13) | −0.0020 (12) | −0.0043 (11) |
| C7 | 0.0514 (15) | 0.0534 (12) | 0.0438 (11) | 0.0041 (11) | −0.0018 (10) | 0.0018 (10) |
| C8 | 0.0599 (17) | 0.0689 (15) | 0.0521 (13) | −0.0055 (14) | 0.0088 (13) | −0.0060 (12) |
| C9 | 0.0538 (16) | 0.0566 (13) | 0.0450 (12) | 0.0004 (12) | 0.0022 (11) | −0.0006 (10) |
| C10 | 0.0558 (16) | 0.0580 (13) | 0.0507 (13) | −0.0014 (12) | 0.0024 (12) | −0.0003 (11) |
| C11 | 0.070 (2) | 0.0852 (19) | 0.0725 (17) | −0.0151 (16) | 0.0033 (15) | −0.0177 (15) |
| C12 | 0.0580 (19) | 0.090 (2) | 0.0763 (19) | −0.0105 (16) | 0.0148 (15) | −0.0134 (16) |
| C13 | 0.072 (3) | 0.124 (5) | 0.108 (6) | 0.039 (3) | −0.021 (4) | −0.065 (4) |
| C14 | 0.136 (8) | 0.165 (9) | 0.115 (6) | 0.033 (7) | 0.001 (5) | 0.023 (6) |
| C13' | 0.072 (3) | 0.124 (5) | 0.108 (6) | 0.039 (3) | −0.021 (4) | −0.065 (4) |
| C14' | 0.136 (8) | 0.165 (9) | 0.115 (6) | 0.033 (7) | 0.001 (5) | 0.023 (6) |
| C15 | 0.059 (4) | 0.031 (4) | 0.0631 (17) | −0.024 (4) | 0.0240 (16) | −0.001 (2) |
| C16 | 0.087 (3) | 0.067 (3) | 0.105 (4) | 0.001 (3) | 0.026 (4) | −0.022 (3) |
| C17 | 0.122 (5) | 0.085 (5) | 0.108 (8) | 0.000 (4) | 0.058 (7) | −0.028 (5) |
| C18 | 0.133 (8) | 0.098 (8) | 0.098 (5) | −0.045 (8) | 0.057 (6) | −0.038 (5) |
| C19 | 0.100 (7) | 0.149 (8) | 0.0579 (19) | −0.034 (6) | 0.029 (3) | −0.017 (3) |
| C20 | 0.069 (6) | 0.102 (2) | 0.043 (4) | −0.020 (3) | 0.028 (3) | 0.007 (2) |
| C15' | 0.059 (4) | 0.031 (4) | 0.0631 (17) | −0.024 (4) | 0.0240 (16) | −0.001 (2) |
| C16' | 0.087 (3) | 0.067 (3) | 0.105 (4) | 0.001 (3) | 0.026 (4) | −0.022 (3) |
| C17' | 0.122 (5) | 0.085 (5) | 0.108 (8) | 0.000 (4) | 0.058 (7) | −0.028 (5) |
| C18' | 0.133 (8) | 0.098 (8) | 0.098 (5) | −0.045 (8) | 0.057 (6) | −0.038 (5) |
| C19' | 0.100 (7) | 0.149 (8) | 0.0579 (19) | −0.034 (6) | 0.029 (3) | −0.017 (3) |
| C20' | 0.069 (6) | 0.102 (2) | 0.043 (4) | −0.020 (3) | 0.028 (3) | 0.007 (2) |
Geometric parameters (Å, °)
| S1—C1 | 1.733 (2) | C12—H12C | 0.9701 |
| S1—C7 | 1.752 (3) | C12—H12D | 0.9701 |
| O1—C8 | 1.238 (3) | C13—C14 | 1.305 (10) |
| N1—C7 | 1.307 (3) | C13—H13 | 0.9300 |
| N1—C6 | 1.383 (3) | C14—H14A | 0.9300 |
| N2—C10 | 1.341 (3) | C14—H14B | 0.9300 |
| N2—N3 | 1.393 (3) | C13'—C14' | 1.289 (9) |
| N2—C12 | 1.459 (3) | C13'—H13' | 0.9300 |
| N3—C8 | 1.391 (4) | C14'—H14C | 0.9300 |
| N3—C15' | 1.430 (6) | C14'—H14D | 0.9300 |
| N3—C15 | 1.438 (6) | C15—C16 | 1.3900 |
| C1—C6 | 1.392 (4) | C15—C20 | 1.3900 |
| C1—C2 | 1.395 (4) | C16—C17 | 1.3900 |
| C2—C3 | 1.369 (4) | C16—H16 | 0.9300 |
| C2—H2 | 0.9300 | C17—C18 | 1.3900 |
| C3—C4 | 1.388 (5) | C17—H17 | 0.9300 |
| C3—H3 | 0.9300 | C18—C19 | 1.3900 |
| C4—C5 | 1.363 (4) | C18—H18 | 0.9300 |
| C4—H4 | 0.9300 | C19—C20 | 1.3900 |
| C5—C6 | 1.399 (4) | C19—H19 | 0.9300 |
| C5—H5 | 0.9300 | C20—H20 | 0.9300 |
| C7—C9 | 1.451 (3) | C15'—C16' | 1.3900 |
| C8—C9 | 1.425 (3) | C15'—C20' | 1.3900 |
| C9—C10 | 1.368 (4) | C16'—C17' | 1.3900 |
| C10—C11 | 1.492 (4) | C16'—H16' | 0.9300 |
| C11—H11A | 0.9600 | C17'—C18' | 1.3900 |
| C11—H11B | 0.9600 | C17'—H17' | 0.9300 |
| C11—H11C | 0.9600 | C18'—C19' | 1.3900 |
| C12—C13' | 1.525 (9) | C18'—H18' | 0.9300 |
| C12—C13 | 1.551 (8) | C19'—C20' | 1.3900 |
| C12—H12A | 0.9700 | C19'—H19' | 0.9300 |
| C12—H12B | 0.9700 | C20'—H20' | 0.9300 |
| C1—S1—C7 | 88.53 (12) | H12A—C12—H12B | 108.5 |
| C7—N1—C6 | 110.1 (2) | C13'—C12—H12C | 121.0 |
| C10—N2—N3 | 108.3 (2) | C13—C12—H12C | 110.8 |
| C10—N2—C12 | 126.9 (2) | C13'—C12—H12D | 116.7 |
| N3—N2—C12 | 122.1 (2) | H12C—C12—H12D | 104.1 |
| C8—N3—N2 | 108.7 (2) | C14—C13—C12 | 117.5 (15) |
| C8—N3—C15' | 128.5 (7) | C14—C13—H13 | 121.3 |
| N2—N3—C15' | 117.3 (6) | C12—C13—H13 | 121.3 |
| C8—N3—C15 | 118.9 (7) | C13—C14—H14A | 120.0 |
| N2—N3—C15 | 130.3 (6) | C13—C14—H14B | 120.0 |
| C6—C1—C2 | 121.4 (2) | H14A—C14—H14B | 120.0 |
| C6—C1—S1 | 109.81 (19) | C14'—C13'—C12 | 128.4 (15) |
| C2—C1—S1 | 128.8 (2) | C14'—C13'—H13' | 115.8 |
| C3—C2—C1 | 118.2 (3) | C12—C13'—H13' | 115.8 |
| C3—C2—H2 | 120.9 | C13'—C14'—H14C | 120.0 |
| C1—C2—H2 | 120.9 | C13'—C14'—H14D | 120.0 |
| C2—C3—C4 | 121.2 (3) | H14C—C14'—H14D | 120.0 |
| C2—C3—H3 | 119.4 | C16—C15—C20 | 120.0 |
| C4—C3—H3 | 119.4 | C16—C15—N3 | 127.6 (11) |
| C5—C4—C3 | 120.6 (3) | C20—C15—N3 | 111.0 (10) |
| C5—C4—H4 | 119.7 | C15—C16—C17 | 120.0 |
| C3—C4—H4 | 119.7 | C15—C16—H16 | 120.0 |
| C4—C5—C6 | 119.9 (3) | C17—C16—H16 | 120.0 |
| C4—C5—H5 | 120.0 | C18—C17—C16 | 120.0 |
| C6—C5—H5 | 120.0 | C18—C17—H17 | 120.0 |
| N1—C6—C1 | 115.6 (2) | C16—C17—H17 | 120.0 |
| N1—C6—C5 | 125.7 (3) | C17—C18—C19 | 120.0 |
| C1—C6—C5 | 118.7 (3) | C17—C18—H18 | 120.0 |
| N1—C7—C9 | 124.4 (2) | C19—C18—H18 | 120.0 |
| N1—C7—S1 | 115.99 (19) | C20—C19—C18 | 120.0 |
| C9—C7—S1 | 119.57 (17) | C20—C19—H19 | 120.0 |
| O1—C8—N3 | 123.1 (2) | C18—C19—H19 | 120.0 |
| O1—C8—C9 | 131.8 (3) | C19—C20—C15 | 120.0 |
| N3—C8—C9 | 105.0 (2) | C19—C20—H20 | 120.0 |
| C10—C9—C8 | 108.3 (2) | C15—C20—H20 | 120.0 |
| C10—C9—C7 | 128.8 (2) | C16'—C15'—C20' | 120.0 |
| C8—C9—C7 | 122.9 (2) | C16'—C15'—N3 | 111.8 (10) |
| N2—C10—C9 | 109.4 (2) | C20'—C15'—N3 | 125.8 (11) |
| N2—C10—C11 | 121.2 (3) | C15'—C16'—C17' | 120.0 |
| C9—C10—C11 | 129.4 (2) | C15'—C16'—H16' | 120.0 |
| C10—C11—H11A | 109.5 | C17'—C16'—H16' | 120.0 |
| C10—C11—H11B | 109.5 | C18'—C17'—C16' | 120.0 |
| H11A—C11—H11B | 109.5 | C18'—C17'—H17' | 120.0 |
| C10—C11—H11C | 109.5 | C16'—C17'—H17' | 120.0 |
| H11A—C11—H11C | 109.5 | C19'—C18'—C17' | 120.0 |
| H11B—C11—H11C | 109.5 | C19'—C18'—H18' | 120.0 |
| N2—C12—C13' | 112.3 (9) | C17'—C18'—H18' | 120.0 |
| N2—C12—C13 | 107.8 (9) | C18'—C19'—C20' | 120.0 |
| N2—C12—H12A | 110.1 | C18'—C19'—H19' | 120.0 |
| C13'—C12—H12A | 118.2 | C20'—C19'—H19' | 120.0 |
| C13—C12—H12A | 110.1 | C19'—C20'—C15' | 120.0 |
| N2—C12—H12B | 110.1 | C19'—C20'—H20' | 120.0 |
| C13—C12—H12B | 110.1 | C15'—C20'—H20' | 120.0 |
| C10—N2—N3—C8 | 5.6 (3) | C12—N2—C10—C11 | 13.0 (4) |
| C12—N2—N3—C8 | 168.3 (2) | C8—C9—C10—N2 | 2.0 (3) |
| C10—N2—N3—C15' | 161.9 (8) | C7—C9—C10—N2 | 179.5 (2) |
| C12—N2—N3—C15' | −35.5 (8) | C8—C9—C10—C11 | −177.2 (3) |
| C10—N2—N3—C15 | 168.7 (9) | C7—C9—C10—C11 | 0.3 (5) |
| C12—N2—N3—C15 | −28.7 (9) | C10—N2—C12—C13' | 77.9 (7) |
| C7—S1—C1—C6 | 1.10 (18) | N3—N2—C12—C13' | −81.3 (7) |
| C7—S1—C1—C2 | 179.7 (2) | C10—N2—C12—C13 | 64.1 (7) |
| C6—C1—C2—C3 | −0.8 (4) | N3—N2—C12—C13 | −95.2 (7) |
| S1—C1—C2—C3 | −179.3 (2) | N2—C12—C13—C14 | −142.4 (14) |
| C1—C2—C3—C4 | −0.1 (4) | C13'—C12—C13—C14 | 106 (7) |
| C2—C3—C4—C5 | 0.8 (5) | N2—C12—C13'—C14' | −108 (2) |
| C3—C4—C5—C6 | −0.6 (5) | C13—C12—C13'—C14' | −35 (6) |
| C7—N1—C6—C1 | 0.3 (3) | C8—N3—C15—C16 | −133.0 (8) |
| C7—N1—C6—C5 | 179.4 (3) | N2—N3—C15—C16 | 65.4 (10) |
| C2—C1—C6—N1 | −179.8 (2) | C15'—N3—C15—C16 | 91 (6) |
| S1—C1—C6—N1 | −1.1 (3) | C8—N3—C15—C20 | 60.6 (8) |
| C2—C1—C6—C5 | 1.0 (4) | N2—N3—C15—C20 | −101.0 (10) |
| S1—C1—C6—C5 | 179.8 (2) | C15'—N3—C15—C20 | −75 (6) |
| C4—C5—C6—N1 | −179.4 (3) | C20—C15—C16—C17 | 0.0 |
| C4—C5—C6—C1 | −0.3 (4) | N3—C15—C16—C17 | −165.3 (9) |
| C6—N1—C7—C9 | −177.2 (2) | C15—C16—C17—C18 | 0.0 |
| C6—N1—C7—S1 | 0.6 (3) | C16—C17—C18—C19 | 0.0 |
| C1—S1—C7—N1 | −1.01 (19) | C17—C18—C19—C20 | 0.0 |
| C1—S1—C7—C9 | 176.89 (19) | C18—C19—C20—C15 | 0.0 |
| N2—N3—C8—O1 | 176.1 (3) | C16—C15—C20—C19 | 0.0 |
| C15'—N3—C8—O1 | 23.3 (8) | N3—C15—C20—C19 | 167.6 (9) |
| C15—N3—C8—O1 | 10.8 (7) | C8—N3—C15'—C16' | −121.9 (9) |
| N2—N3—C8—C9 | −4.3 (3) | N2—N3—C15'—C16' | 87.4 (7) |
| C15'—N3—C8—C9 | −157.0 (7) | C15—N3—C15'—C16' | −71 (6) |
| C15—N3—C8—C9 | −169.5 (7) | C8—N3—C15'—C20' | 40.7 (11) |
| O1—C8—C9—C10 | −178.9 (3) | N2—N3—C15'—C20' | −110.1 (9) |
| N3—C8—C9—C10 | 1.4 (3) | C15—N3—C15'—C20' | 92 (6) |
| O1—C8—C9—C7 | 3.4 (5) | C20'—C15'—C16'—C17' | 0.0 |
| N3—C8—C9—C7 | −176.2 (2) | N3—C15'—C16'—C17' | 163.7 (10) |
| N1—C7—C9—C10 | 7.8 (4) | C15'—C16'—C17'—C18' | 0.0 |
| S1—C7—C9—C10 | −169.9 (2) | C16'—C17'—C18'—C19' | 0.0 |
| N1—C7—C9—C8 | −175.0 (2) | C17'—C18'—C19'—C20' | 0.0 |
| S1—C7—C9—C8 | 7.2 (3) | C18'—C19'—C20'—C15' | 0.0 |
| N3—N2—C10—C9 | −4.7 (3) | C16'—C15'—C20'—C19' | 0.0 |
| C12—N2—C10—C9 | −166.3 (3) | N3—C15'—C20'—C19' | −161.3 (9) |
| N3—N2—C10—C11 | 174.6 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···O1i | 0.93 | 2.59 | 3.318 (3) | 135 |
| C12—H12A···O1ii | 0.97 | 2.51 | 3.404 (4) | 152 |
| C12—H12C···O1ii | 0.97 | 2.48 | 3.404 (4) | 159 |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) −x+3/2, y+1/2, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: XU5047).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bruker (2008). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Chakibe, I., Zerzouf, A., Essassi, E. M., Reichelt, M. & Reuter, H. (2010). Acta Cryst. E66, o1096. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst.43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810040316/xu5047sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810040316/xu5047Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report