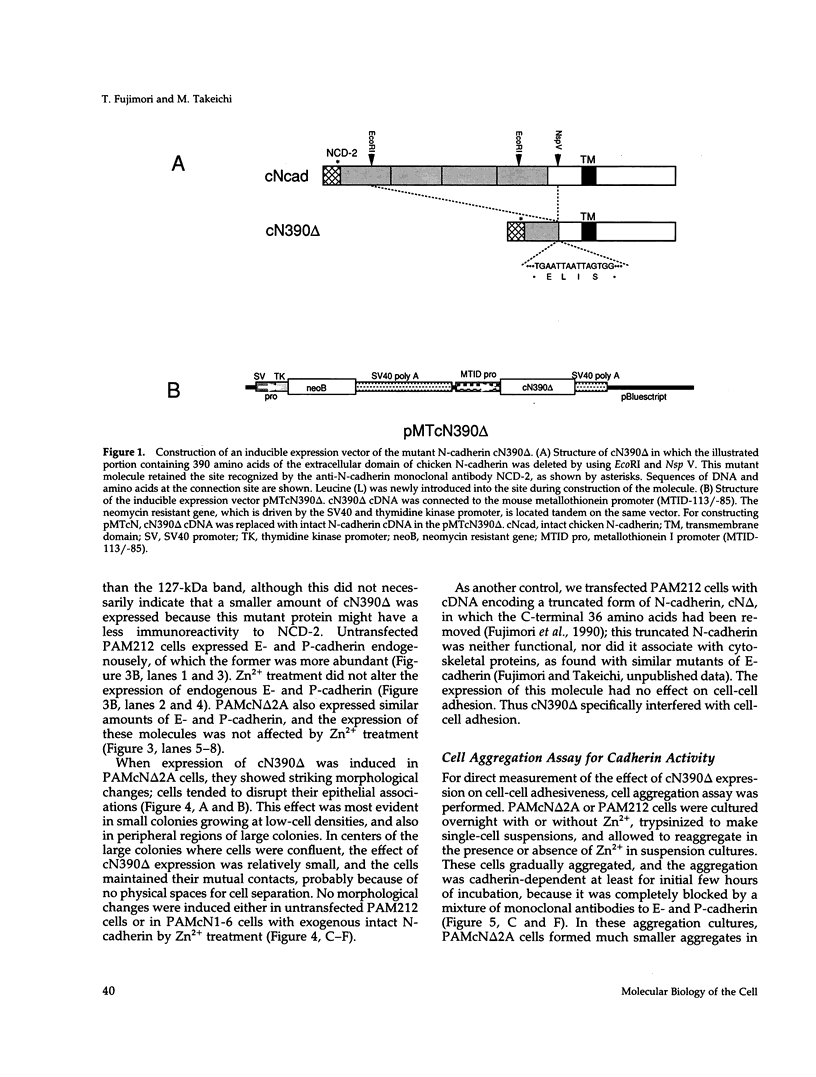
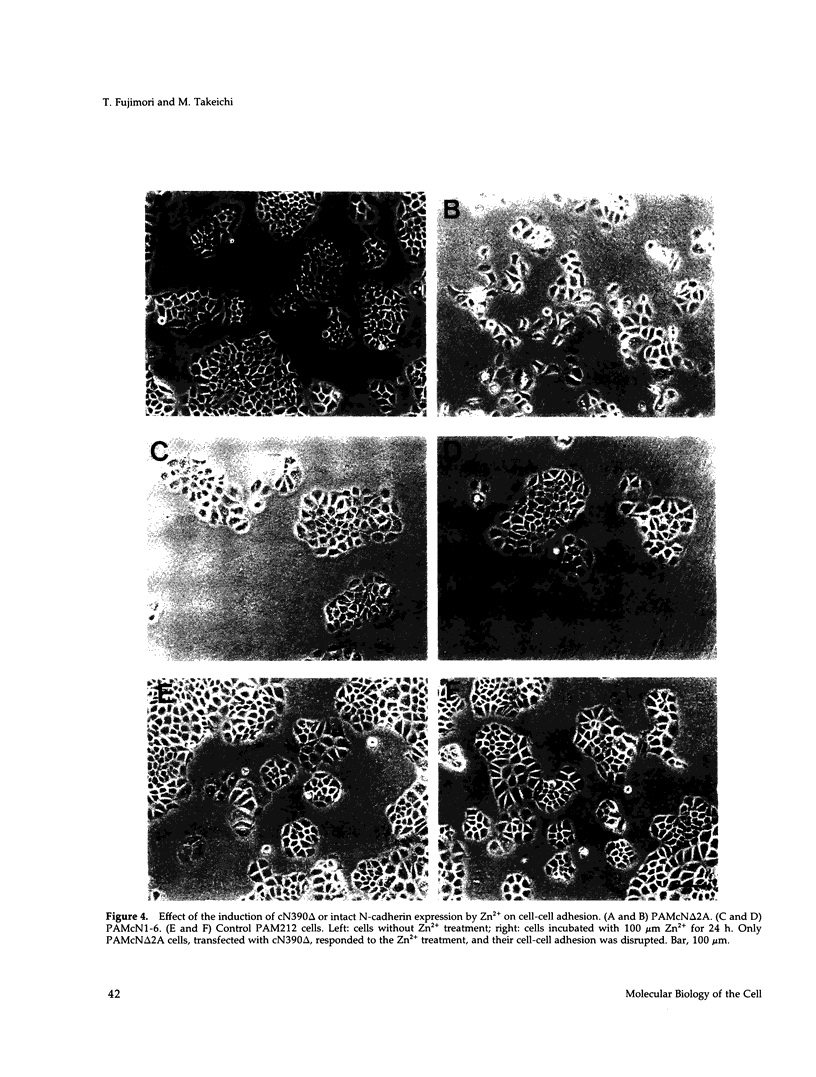
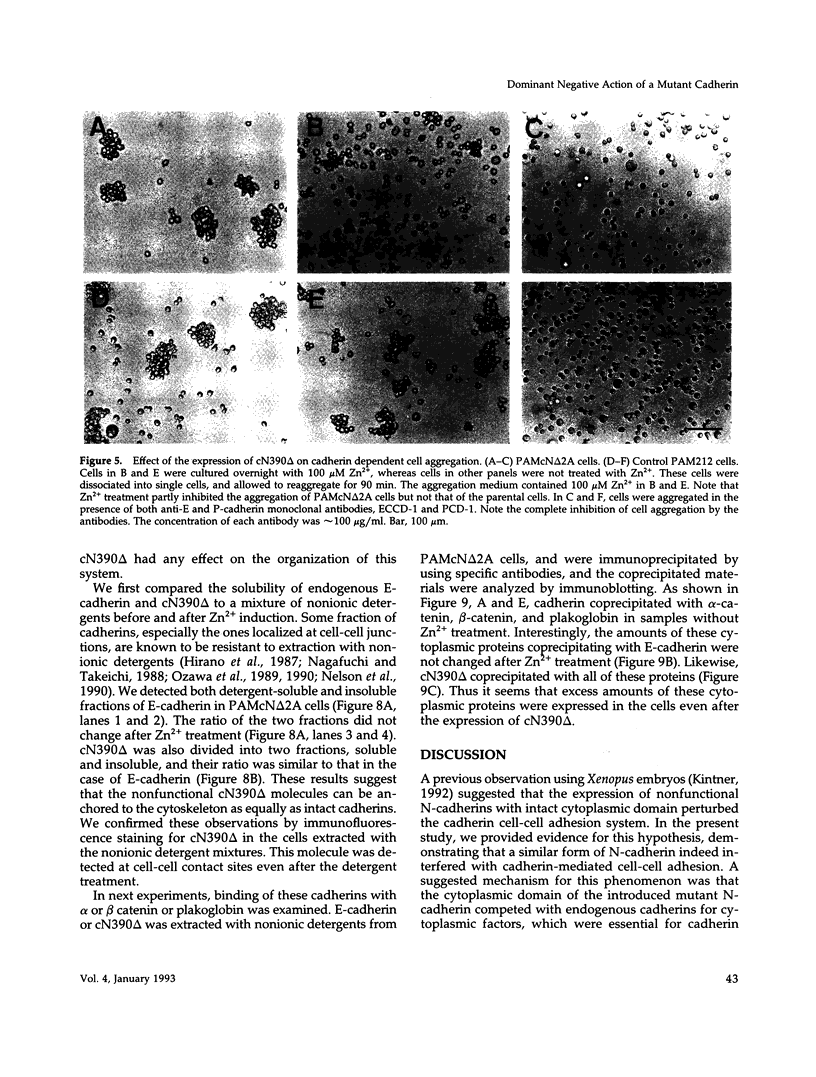
Abstract
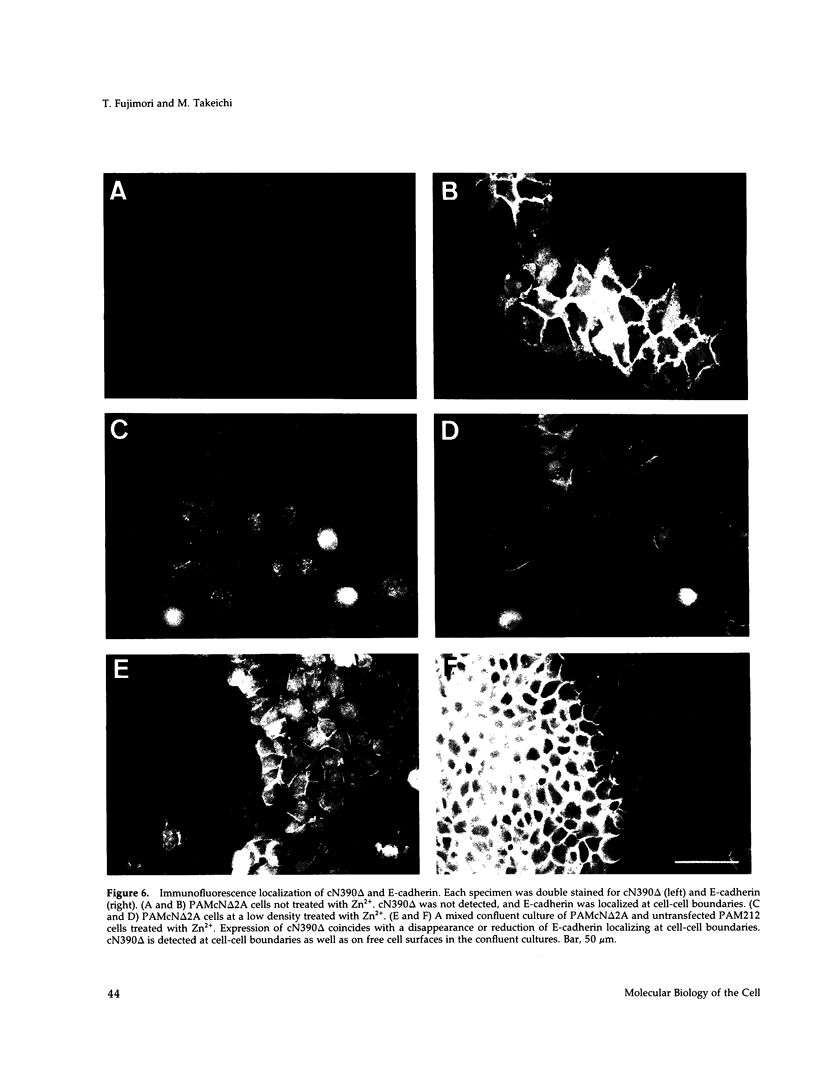
Cadherins, a family of transmembrane cell-cell adhesion receptors, require interactions with the cytoskeleton for normal function. To assess the mechanisms of these interactions, we studied the effect of exogenous expression of a mutant N-cadherin, cN390 delta; on epithelial cell-cell adhesion. The intracellular domain of cN390 delta was intact but its extracellular domain was largely deleted so that this molecule was not functional for cell adhesion. cDNA of cN390 delta was attached to the metallothionein promoter, and introduced into the keratinocyte line PAM212 expressing endogenous E- and P-cadherin. When the expression of cN390 delta was induced by Zn2+, cadherin-dependent adhesion of the transfected cells was inhibited, resulting in the dispersion of cell colonies, although their contacts were maintained under high cell density conditions. In these cultures, cN390 delta was expressed not only on the free surfaces of the cells but also at cell-cell junctions. The endogenous cadherins were concentrated at cell-cell junctions under normal conditions. As a result of cN390 delta expression, however, the endogenous cadherins localizing at the cell-cell junctions were largely diminished, suggesting that these molecules were replaced by the mutant molecules at these sites. As a control, we transfected the same cell line with cDNA of a truncated form of N-cadherin cadherin whose intracellular C terminus had been deleted leaving the extracellular domain intact. This molecule had no effect on cell-cell adhesion, nor did it localize to cell-cell contact sites. We also found that the association of the endogenous cadherins with alpha- and beta-catenins and plakoglobin was not affected by the expression of cN390 delta, which also formed a complex with these molecules, suggesting that no competition occurred between the endogenous and exogenous cadherins for these cytoplasmic proteins. These and other additional results suggest that the nonfunctional cadherins whose intracellular domain is intact occupy the sites where the endogenous cadherins should localize, through interactions with the cytoskeleton, and inhibit the cadherin adhesion system.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Buxton R. S., Magee A. I. Structure and interactions of desmosomal and other cadherins. Semin Cell Biol. 1992 Jun;3(3):157–167. doi: 10.1016/s1043-4682(10)80012-1. [DOI] [PubMed] [Google Scholar]
- Ferns M. J., Hall Z. W. How many agrins does it take to make a synapse? Cell. 1992 Jul 10;70(1):1–3. doi: 10.1016/0092-8674(92)90525-h. [DOI] [PubMed] [Google Scholar]
- Fujimori T., Miyatani S., Takeichi M. Ectopic expression of N-cadherin perturbs histogenesis in Xenopus embryos. Development. 1990 Sep;110(1):97–104. doi: 10.1242/dev.110.1.97. [DOI] [PubMed] [Google Scholar]
- Geiger B., Ginsberg D. The cytoplasmic domain of adherens-type junctions. Cell Motil Cytoskeleton. 1991;20(1):1–6. doi: 10.1002/cm.970200102. [DOI] [PubMed] [Google Scholar]
- Hatta K., Nose A., Nagafuchi A., Takeichi M. Cloning and expression of cDNA encoding a neural calcium-dependent cell adhesion molecule: its identity in the cadherin gene family. J Cell Biol. 1988 Mar;106(3):873–881. doi: 10.1083/jcb.106.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K., Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986 Apr 3;320(6061):447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Herrenknecht K., Ozawa M., Eckerskorn C., Lottspeich F., Lenter M., Kemler R. The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S., Nose A., Hatta K., Kawakami A., Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol. 1987 Dec;105(6 Pt 1):2501–2510. doi: 10.1083/jcb.105.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Kanamori A., Kondoh H. Rapid and transient decrease of N-myc expression in retinoic acid-induced differentiation of OTF9 teratocarcinoma stem cells. Mol Cell Biol. 1990 Feb;10(2):486–491. doi: 10.1128/mcb.10.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Takahashi Y., Hayashi S., Kondoh H. Improved mammalian vectors for high expression of G418 resistance. Cell Struct Funct. 1987 Dec;12(6):575–580. doi: 10.1247/csf.12.575. [DOI] [PubMed] [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992 Apr 17;69(2):225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Knudsen K. A., Wheelock M. J. Plakoglobin, or an 83-kD homologue distinct from beta-catenin, interacts with E-cadherin and N-cadherin. J Cell Biol. 1992 Aug;118(3):671–679. doi: 10.1083/jcb.118.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme S. E., Akiyama S. K., Yamada K. M. Regulation of fibronectin receptor distribution. J Cell Biol. 1992 Apr;117(2):437–447. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyoshi N., Hamaguchi M., Taniguchi S., Nagafuchi A., Tsukita S., Takeichi M. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992 Aug;118(3):703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea P. D., Gumbiner B. M. Purification of a 92-kDa cytoplasmic protein tightly associated with the cell-cell adhesion molecule E-cadherin (uvomorulin). Characterization and extractability of the protein complex from the cell cytostructure. J Biol Chem. 1991 Mar 5;266(7):4514–4520. [PubMed] [Google Scholar]
- McCrea P. D., Turck C. W., Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991 Nov 29;254(5036):1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Shirayoshi Y., Okazaki K., Yasuda K., Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987 Sep 24;329(6137):341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988 Dec 1;7(12):3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Transmembrane control of cadherin-mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989 Nov;1(1):37–44. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M., Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991 May 31;65(5):849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Shore E. M., Wang A. Z., Hammerton R. W. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1990 Feb;110(2):349–357. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986 Dec;103(6 Pt 2):2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Tsuji K., Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990 Apr 6;61(1):147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol. 1992 Feb;116(4):989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Ringwald M., Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santerre R. F., Allen N. E., Hobbs J. N., Jr, Rao R. N., Schmidt R. J. Expression of prokaryotic genes for hygromycin B and G418 resistance as dominant-selection markers in mouse L cells. Gene. 1984 Oct;30(1-3):147–156. doi: 10.1016/0378-1119(84)90115-x. [DOI] [PubMed] [Google Scholar]
- Shirayoshi Y., Nose A., Iwasaki K., Takeichi M. N-linked oligosaccharides are not involved in the function of a cell-cell binding glycoprotein E-cadherin. Cell Struct Funct. 1986 Sep;11(3):245–252. doi: 10.1247/csf.11.245. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Chen H. Y., Brinster R. L., Palmiter R. D. A 12-base-pair DNA motif that is repeated several times in metallothionein gene promoters confers metal regulation to a heterologous gene. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7318–7322. doi: 10.1073/pnas.81.23.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977 Nov;75(2 Pt 1):464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa S. H., Hawley-Nelson P., Koehler B., Stanley J. R. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980 Dec;40(12):4694–4703. [PubMed] [Google Scholar]