Abstract
The asymmetric unit of the title compound, [Na2(C8H8O8)]n, contains one Na+ ion and half of a 2,3-bis(carboxymethyl)butanedioate (H2BTC2−) dianion, which lies on a center of symmetry. The dianion exhibits a μ10-bridging mode. Each Na atom lies in a NaO6 octahedron defined by six O atoms from five dianions. Adjacent NaO6 octahedra share a common O—O edge, generating a bioctahedron; adjacent bioctahedra are O—O edge-connected to one another, building up a chain along [001]. The chains are connected by adjacent H2BTC2− anions into a three-dimensional framework. The structure is further stabilized by O—H⋯O hydrogen bonds.
Related literature
For related structures, see: Delgado et al. (2007 ▶); Liu et al. (2008 ▶); Wang et al. (2005 ▶); Zheng et al. (2004 ▶); Zhu & Zheng (2010 ▶).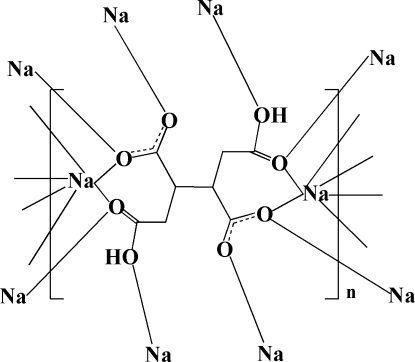
Experimental
Crystal data
[Na2(C8H8O8)]
M r = 278.12
Orthorhombic,

a = 8.9053 (18) Å
b = 8.6395 (17) Å
c = 12.527 (3) Å
V = 963.8 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.24 mm−1
T = 293 K
0.44 × 0.36 × 0.32 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
Absorption correction: multi-scan (ABSCOR; Higashi, 1995 ▶) T min = 0.900, T max = 0.925
8610 measured reflections
1097 independent reflections
1000 reflections with I > 2σ(I)
R int = 0.021
Refinement
R[F 2 > 2σ(F 2)] = 0.038
wR(F 2) = 0.111
S = 1.10
1097 reflections
86 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.43 e Å−3
Δρmin = −0.22 e Å−3
Data collection: RAPID-AUTO (Rigaku, 1998 ▶); cell refinement: RAPID-AUTO; data reduction: CrystalStructure (Rigaku/MSC, 2004 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks ptcLa, I. DOI: 10.1107/S1600536810040857/ng5044sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810040857/ng5044Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2C⋯O4i | 0.85 (2) | 1.67 (3) | 2.5097 (18) | 177 (2) |
Symmetry code: (i)  .
.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (grant No. 20072022), the Expert Project of Key Basic Research of the Ministry of Science and Technology of China (grant No. 2003CCA00800), the Science and Technology Department of Zhejiang Province (grant No. 2006 C21105) and the Education Department of Zhejiang Province. Thanks are also extended to the K. C. Wong Magna Fund of Ningbo University.
supplementary crystallographic information
Comment
Recently, the aliphatic multi-carboxylic acids have attractived considerable attention due to both its conformational flexibility and a variety of coordination fashions (Wang et al., 2005; Zheng et al., 2004).The butane-1,2,3,4-tetracarboxylic acid (H4BTC) ligand possesses four ionizable protons that can be removed gradually to form a series of deprotonated anions such as H3BTC-, H2BTC2-, HBTC3-, BTC4-, which have allowed the preparation of a variety of complexes with differents metals (Delgado et al., 2007; Liu et al., 2008; Zhu et al., 2010). In this contribution, we report the synthesis and crystal structure of the title compound.
The asymmetric unit of the title compound contains one Na+ ion and half a H2BTC2- anion(Figure 1). The H2BTC2-ligand is diprotonated, which is crystallographically imposed by symmetry of center with inversion centers at the midpoints of the central C3—C3i bond with the Wyckoff 4b site. Each H2BTC2- anions coordinate ten sodium ions through eight carboxyl oxygen atoms. The carboxylate group and carboxylic group all coordinates to two metal atoms in a syn/anti µ2η2 bridging fashion, and two seven-membered chelating rings are concomitantly formed. Each Na atom is in a distorted octahedra NaO6 gemetry defined by six O atoms from five H2BTC2- ligands, the Na—O contact distances are all within the normal ranges. The adjacent two NaO6 octahedra are fused via common edge O1—O1 and O3—O3, generating a one-dimensional sodium-oxide chains (Figure 2), and the resulting chains are further interlinked by H2BTC2- anions into three-dimensional frameworks (Figure 3).
Experimental
All chemicals were obtained from commerical sources and were used as obtained. NaOH (0.079 g, 1.98 mmol) was added to a stirred mixture solution of butane-1,2,3,4-tetracarboxylic acid (0.1173 g, 0.50 mmol) in 10 ml H2O and 10 ml me thanol, and the resulting mixture was stirred for 5 min. Colorless crystals were obtained from the solution (pH = 7.13) after standing at room temperature for five weeks.
Refinement
H atoms bonded to C atoms were palced in geometrically calculated position and were refined using a riding model, with Uiso(H) = 1.2 Ueq(C). H atoms attached to O atoms were found in a difference Fourier synthesis and refined with the O—H distance restranied to 0.83 (1) Å.
Figures
Fig. 1.
The content of asymmetric unit showing the atomic numbering and 45% probability dispalcement ellipsoids.[Symmetry codes: (i) -x + 2, -y + 1, -z + 1. (ii) -x + 2, -y, -z + 1. (iii) -x + 2, y, -z + 1.5. (iv) x - 1/2,y - 1/2, -z + 1.5. (v) x - 1/2, -y + 1/2, -z + 1.]
Fig. 2.
The one-dimensional sodium-oxide chains with the common edges O1—O1 and O3—O3.
Fig. 3.
The three-dimensional metal-organic framework in the title compound.
Crystal data
| [Na2(C8H8O8)] | F(000) = 568 |
| Mr = 278.12 | Dx = 1.917 Mg m−3 |
| Orthorhombic, Pbcn | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2n 2ab | Cell parameters from 7116 reflections |
| a = 8.9053 (18) Å | θ = 3.3–27.4° |
| b = 8.6395 (17) Å | µ = 0.24 mm−1 |
| c = 12.527 (3) Å | T = 293 K |
| V = 963.8 (3) Å3 | Block, colorless |
| Z = 4 | 0.44 × 0.36 × 0.32 mm |
Data collection
| Rigaku R-AXIS RAPID diffractometer | 1097 independent reflections |
| Radiation source: fine-focus sealed tube | 1000 reflections with I > 2σ(I) |
| graphite | Rint = 0.021 |
| Detector resolution: 0 pixels mm-1 | θmax = 27.4°, θmin = 3.3° |
| ω scan | h = −11→11 |
| Absorption correction: multi-scan (ABSCOR; Higashi, 1995) | k = −11→11 |
| Tmin = 0.900, Tmax = 0.925 | l = −16→16 |
| 8610 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.038 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.111 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.10 | w = 1/[σ2(Fo2) + (0.0658P)2 + 0.5154P] where P = (Fo2 + 2Fc2)/3 |
| 1097 reflections | (Δ/σ)max < 0.001 |
| 86 parameters | Δρmax = 0.43 e Å−3 |
| 1 restraint | Δρmin = −0.22 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Na | 0.91916 (8) | 0.06655 (8) | 0.62976 (5) | 0.0292 (2) | |
| O1 | 1.12521 (17) | 0.21959 (14) | 0.69249 (10) | 0.0391 (4) | |
| O2 | 1.30500 (16) | 0.37558 (13) | 0.75029 (10) | 0.0325 (3) | |
| C1 | 1.19167 (19) | 0.34236 (18) | 0.68752 (12) | 0.0243 (3) | |
| C2 | 1.15019 (18) | 0.46906 (17) | 0.61037 (11) | 0.0219 (3) | |
| H2A | 1.2386 | 0.4963 | 0.5692 | 0.026* | |
| H2B | 1.1207 | 0.5598 | 0.6508 | 0.026* | |
| C3 | 1.02287 (16) | 0.42768 (14) | 0.53277 (10) | 0.0161 (3) | |
| H3A | 0.9358 | 0.3927 | 0.5741 | 0.019* | |
| C4 | 1.07185 (16) | 0.29671 (16) | 0.45770 (11) | 0.0177 (3) | |
| O3 | 1.01257 (15) | 0.16760 (12) | 0.46431 (9) | 0.0300 (3) | |
| O4 | 1.17325 (15) | 0.33112 (14) | 0.39020 (10) | 0.0300 (3) | |
| H2C | 1.310 (4) | 0.308 (3) | 0.7989 (19) | 0.088 (11)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Na | 0.0336 (4) | 0.0244 (4) | 0.0296 (4) | −0.0038 (3) | 0.0023 (3) | −0.0031 (2) |
| O1 | 0.0481 (8) | 0.0278 (7) | 0.0415 (7) | −0.0062 (6) | −0.0172 (6) | 0.0104 (5) |
| O2 | 0.0432 (7) | 0.0262 (6) | 0.0282 (6) | 0.0011 (5) | −0.0177 (5) | 0.0041 (5) |
| C1 | 0.0305 (8) | 0.0216 (7) | 0.0208 (7) | 0.0046 (6) | −0.0054 (6) | −0.0007 (5) |
| C2 | 0.0278 (7) | 0.0185 (7) | 0.0194 (7) | 0.0020 (6) | −0.0054 (6) | 0.0003 (5) |
| C3 | 0.0208 (7) | 0.0137 (6) | 0.0138 (6) | 0.0032 (5) | 0.0005 (5) | 0.0002 (5) |
| C4 | 0.0212 (7) | 0.0160 (6) | 0.0159 (6) | 0.0039 (5) | −0.0012 (5) | −0.0008 (5) |
| O3 | 0.0448 (7) | 0.0166 (5) | 0.0286 (6) | −0.0050 (5) | 0.0081 (5) | −0.0041 (4) |
| O4 | 0.0340 (6) | 0.0247 (6) | 0.0314 (6) | −0.0026 (5) | 0.0148 (5) | −0.0082 (5) |
Geometric parameters (Å, °)
| Na—O4i | 2.3748 (15) | O2—H2C | 0.843 (10) |
| Na—O1 | 2.3943 (15) | C1—C2 | 1.506 (2) |
| Na—O3 | 2.3978 (13) | C2—C3 | 1.536 (2) |
| Na—O3ii | 2.4188 (13) | C2—H2A | 0.9700 |
| Na—O2iii | 2.4522 (14) | C2—H2B | 0.9700 |
| Na—O1iv | 2.6196 (15) | C3—C4 | 1.5346 (18) |
| Na—Naiv | 3.3388 (14) | C3—C3vi | 1.550 (2) |
| Na—Naii | 3.7369 (14) | C3—H3A | 0.9800 |
| O1—C1 | 1.216 (2) | C4—O3 | 1.2368 (18) |
| O1—Naiv | 2.6196 (15) | C4—O4 | 1.2723 (19) |
| O2—C1 | 1.311 (2) | O3—Naii | 2.4188 (13) |
| O2—Nav | 2.4522 (14) | O4—Navii | 2.3748 (15) |
| O4i—Na—O1 | 122.39 (5) | C1—O1—Na | 146.90 (11) |
| O4i—Na—O3 | 95.36 (5) | C1—O1—Naiv | 123.86 (11) |
| O1—Na—O3 | 79.44 (5) | Na—O1—Naiv | 83.38 (5) |
| O4i—Na—O3ii | 119.46 (5) | C1—O2—Nav | 146.49 (11) |
| O1—Na—O3ii | 115.41 (6) | C1—O2—H2C | 109 (2) |
| O3—Na—O3ii | 78.24 (5) | Nav—O2—H2C | 90 (2) |
| O4i—Na—O2iii | 86.14 (5) | O1—C1—O2 | 122.32 (14) |
| O1—Na—O2iii | 119.22 (5) | O1—C1—C2 | 123.18 (14) |
| O3—Na—O2iii | 156.69 (5) | O2—C1—C2 | 114.49 (14) |
| O3ii—Na—O2iii | 80.80 (5) | C1—C2—C3 | 114.71 (13) |
| O4i—Na—O1iv | 76.26 (5) | C1—C2—H2A | 108.6 |
| O1—Na—O1iv | 63.76 (7) | C3—C2—H2A | 108.6 |
| O3—Na—O1iv | 127.10 (5) | C1—C2—H2B | 108.6 |
| O3ii—Na—O1iv | 150.95 (5) | C3—C2—H2B | 108.6 |
| O2iii—Na—O1iv | 75.89 (5) | H2A—C2—H2B | 107.6 |
| O4i—Na—Naiv | 119.52 (4) | C4—C3—C2 | 110.47 (11) |
| O1—Na—Naiv | 51.20 (4) | C4—C3—C3vi | 110.16 (13) |
| O3—Na—Naiv | 129.07 (5) | C2—C3—C3vi | 109.98 (14) |
| O3ii—Na—Naiv | 109.34 (4) | C4—C3—H3A | 108.7 |
| O2iii—Na—Naiv | 68.03 (4) | C2—C3—H3A | 108.7 |
| O1iv—Na—Naiv | 45.42 (3) | C3vi—C3—H3A | 108.7 |
| O4i—Na—Naii | 112.22 (4) | O3—C4—O4 | 123.93 (13) |
| O1—Na—Naii | 99.22 (5) | O3—C4—C3 | 120.17 (12) |
| O3—Na—Naii | 39.32 (3) | O4—C4—C3 | 115.89 (12) |
| O3ii—Na—Naii | 38.92 (3) | C4—O3—Na | 122.30 (9) |
| O2iii—Na—Naii | 119.06 (4) | C4—O3—Naii | 127.90 (10) |
| O1iv—Na—Naii | 162.35 (5) | Na—O3—Naii | 101.76 (5) |
| Naiv—Na—Naii | 128.23 (4) | C4—O4—Navii | 144.24 (11) |
Symmetry codes: (i) x−1/2, −y+1/2, −z+1; (ii) −x+2, −y, −z+1; (iii) x−1/2, y−1/2, −z+3/2; (iv) −x+2, y, −z+3/2; (v) x+1/2, y+1/2, −z+3/2; (vi) −x+2, −y+1, −z+1; (vii) x+1/2, −y+1/2, −z+1.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2C···O4viii | 0.85 (2) | 1.67 (3) | 2.5097 (18) | 177 (2) |
Symmetry codes: (viii) −x+5/2, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: NG5044).
References
- Delgado, L. C., Fabelo, O., Pasàn, J., Delgado, F. S., Lloret, F., Julve, M. & Ruiz-Pérez, C. (2007). Inorg. Chem.46, 7458–7465. [DOI] [PubMed]
- Higashi, T. (1995). ABSCOR Rigaku Corporation, Tokyo, Japan.
- Liu, Y. Y., Ma, J. F., Yang, J., Ma, J. C. & Su, Z. M. (2008). CrystEngComm, 10, 894–904.
- Rigaku (1998). RAPID-AUTO Rigaku Corporation, Tokyo, Japan.
- Rigaku/MSC (2004). CrystalStructure Rigaku/MSC Inc., The Woodlands, Texas, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wang, M. S., Guo, G. C., Fu, M. L., Xu, L., Cai, L. Z. & Huang, J. S. (2005). J. Chem. Soc. Dalton Trans. pp. 2899–2907. [DOI] [PubMed]
- Zheng, Y. Q., Lin, J. L. & Kong, Z. P. (2004). Inorg. Chem.43, 2590–2596. [DOI] [PubMed]
- Zhu, H. L. & Zheng, Y. Q. (2010). J. Mol. Struct.970, 27–35.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks ptcLa, I. DOI: 10.1107/S1600536810040857/ng5044sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810040857/ng5044Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report





