Abstract
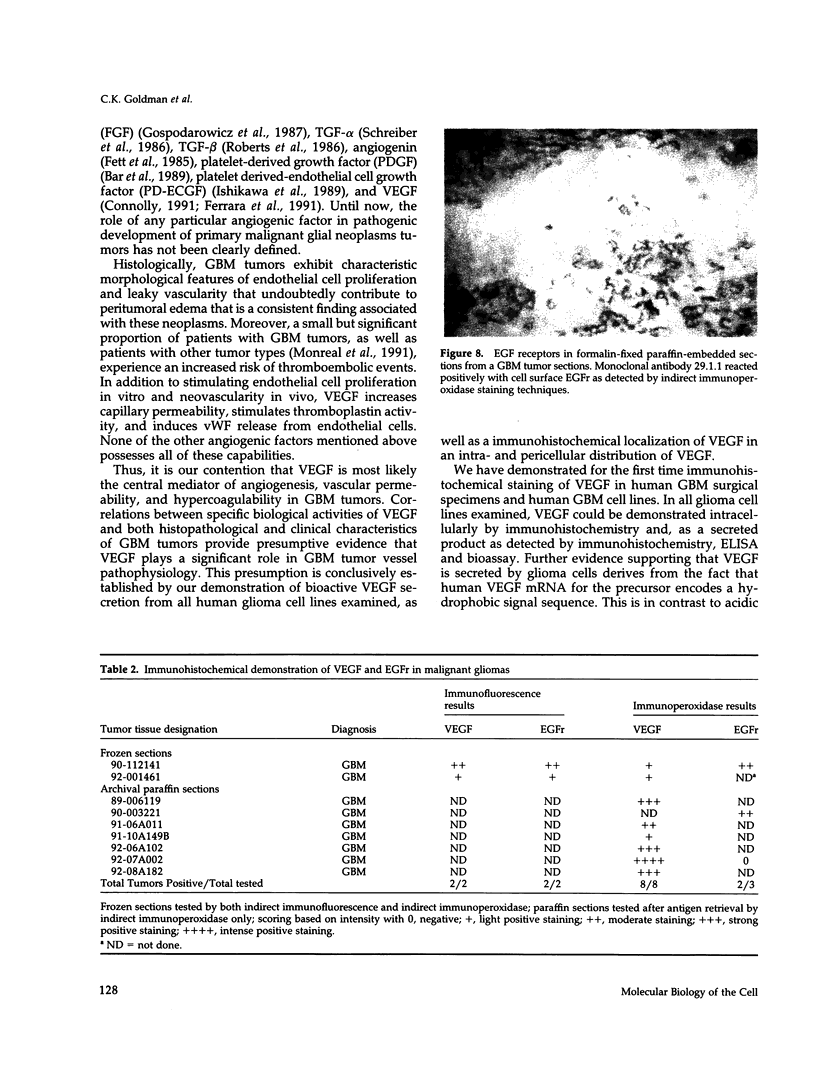
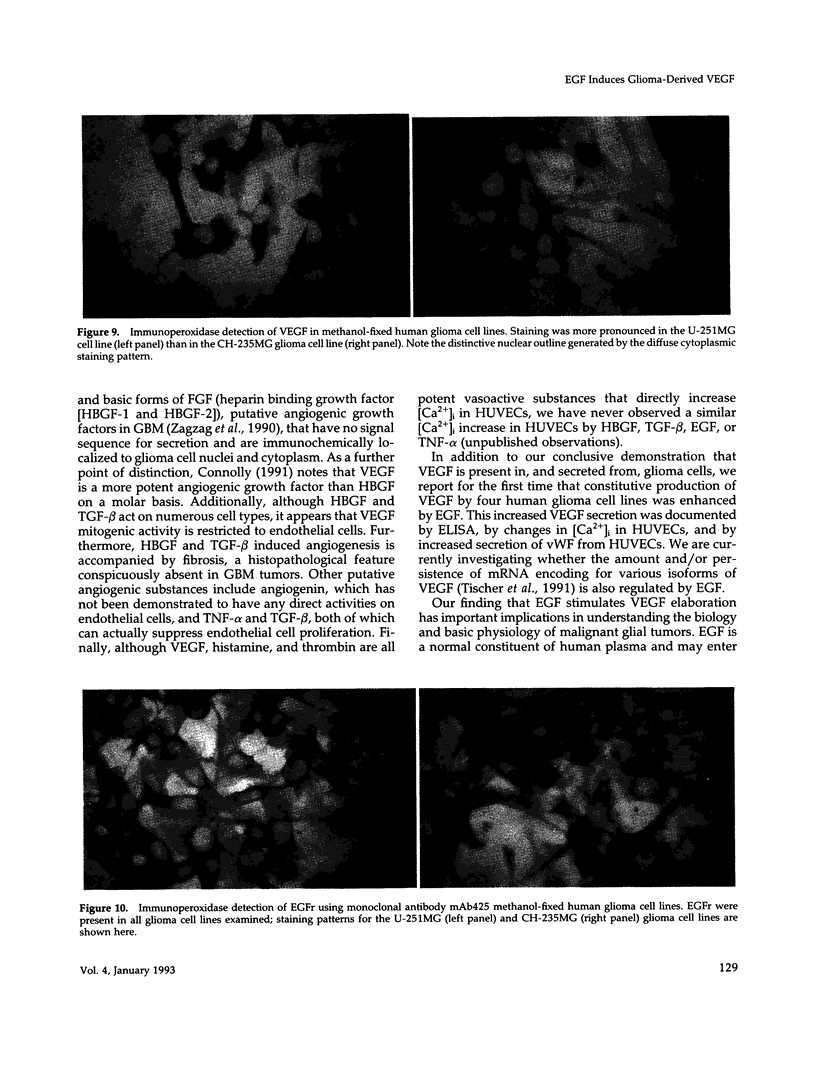
Hypervascularity, focal necrosis, persistent cerebral edema, and rapid cellular proliferation are key histopathologic features of glioblastoma multiforme (GBM), the most common and malignant of human brain tumors. By immunoperoxidase and immunofluorescence, we definitively have demonstrated the presence of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFr) in five out of five human glioma cell lines (U-251MG, U-105MG, D-65MG, D-54MG, and CH-235MG) and in eight human GBM tumor surgical specimens. In vitro experiments with glioma cell lines revealed a consistent and reliable relation between EGFr activation and VEGF production; namely, EGF (1-20 ng/ml) stimulation of glioma cells resulted in a 25-125% increase in secretion of bioactive VEGF. Conditioned media (CM) prepared from EGF-stimulated glioma cell lines produced significant increases in cytosolic free intracellular concentrations of Ca2+ ([Ca2+]i) in human umbilical vein endothelial cells (HUVECs). Neither EGF alone or CM from glioma cultures prepared in the absence of EGF induced [Ca2+]i increases in HUVECs. Preincubation of glioma CM with A4.6.1, a monoclonal antibody to VEGF, completely abolished VEGF-mediated [Ca2+]i transients in HUVECs. Likewise, induction by glioma-derived CM of von Willebrand factor release from HUVECs was completely blocked by A4.6.1 pretreatment. These observations provide a key link in understanding the basic cellular pathophysiology of GBM tumor angiogenesis, increased vascular permeability, and cellular proliferation. Specifically, EGF activation of EGFr expressed on glioma cells leads to enhanced secretion of VEGF by glioma cells. VEGF released by glioma cells in situ most likely accounts for pathognomonic histopathologic and clinical features of GBM tumors in patients, including striking tumor angiogenesis, increased cerebral edema and hypercoagulability manifesting as focal tumor necrosis, deep vein thrombosis, or pulmonary embolism.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi K., Belser P., Bender H., Li D., Rodeck U., Benveniste E. N., Woo D., Schmiegel W. H., Herlyn D. Enhancement of epidermal growth factor receptor expression on glioma cells by recombinant tumor necrosis factor alpha. Cancer Immunol Immunother. 1992;34(6):370–376. doi: 10.1007/BF01741746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar R. S., Boes M., Booth B. A., Dake B. L., Henley S., Hart M. N. The effects of platelet-derived growth factor in cultured microvessel endothelial cells. Endocrinology. 1989 Apr;124(4):1841–1848. doi: 10.1210/endo-124-4-1841. [DOI] [PubMed] [Google Scholar]
- Bethea J. R., Chung I. Y., Sparacio S. M., Gillespie G. Y., Benveniste E. N. Interleukin-1 beta induction of tumor necrosis factor-alpha gene expression in human astroglioma cells. J Neuroimmunol. 1992 Feb;36(2-3):179–191. doi: 10.1016/0165-5728(92)90049-q. [DOI] [PubMed] [Google Scholar]
- Bethea J. R., Gillespie G. Y., Benveniste E. N. Interleukin-1 beta induction of TNF-alpha gene expression: involvement of protein kinase C. J Cell Physiol. 1992 Aug;152(2):264–273. doi: 10.1002/jcp.1041520207. [DOI] [PubMed] [Google Scholar]
- Bethea J. R., Gillespie G. Y., Chung I. Y., Benveniste E. N. Tumor necrosis factor production and receptor expression by a human malignant glioma cell line, D54-MG. J Neuroimmunol. 1990 Nov;30(1):1–13. doi: 10.1016/0165-5728(90)90047-q. [DOI] [PubMed] [Google Scholar]
- Bigner D. D., Bigner S. H., Pontén J., Westermark B., Mahaley M. S., Ruoslahti E., Herschman H., Eng L. F., Wikstrand C. J. Heterogeneity of Genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981 May;40(3):201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J. Chromosome and chromosomal progression of human gliomas in vivo, in vitro and in athymic nude mice. Prog Exp Tumor Res. 1984;27:67–82. doi: 10.1159/000408223. [DOI] [PubMed] [Google Scholar]
- Blumenstock M., Prosenc N., Patt S., Pfanne K., Drum F., Cervos-Navarro J. In contrast to EGFr gene overexpression, H-ras gene expression decreases in human gliomas. Anticancer Res. 1991 May-Jun;11(3):1353–1357. [PubMed] [Google Scholar]
- Boström S., Holmgren E., Jonsson O., Lindberg S., Lindström B., Winsö I., Zachrisson B. Post-operative thromboembolism in neurosurgery. A study on the prophylactic effect of calf muscle stimulation plus dextran compared to low-dose heparin. Acta Neurochir (Wien) 1986;80(3-4):83–89. doi: 10.1007/BF01812279. [DOI] [PubMed] [Google Scholar]
- Boström S., Holmgren E., Jonsson O., Lindström B., Stigendal L. Fibrinopeptide A and fibrinogen fragment B beta 15-42 and their relation to the operative trauma and post-operative thromboembolism in neurosurgical patients. Acta Neurochir (Wien) 1987;88(1-2):49–55. doi: 10.1007/BF01400515. [DOI] [PubMed] [Google Scholar]
- Brady L. W., Miyamoto C., Woo D. V., Rackover M., Emrich J., Bender H., Dadparvar S., Steplewski Z., Koprowski H., Black P. Malignant astrocytomas treated with iodine-125 labeled monoclonal antibody 425 against epidermal growth factor receptor: a phase II trial. Int J Radiat Oncol Biol Phys. 1992;22(1):225–230. doi: 10.1016/0360-3016(92)91009-c. [DOI] [PubMed] [Google Scholar]
- Brock T. A., Capasso E. A. Thrombin and histamine activate phospholipase C in human endothelial cells via a phorbol ester-sensitive pathway. J Cell Physiol. 1988 Jul;136(1):54–62. doi: 10.1002/jcp.1041360107. [DOI] [PubMed] [Google Scholar]
- Brock T. A., Dvorak H. F., Senger D. R. Tumor-secreted vascular permeability factor increases cytosolic Ca2+ and von Willebrand factor release in human endothelial cells. Am J Pathol. 1991 Jan;138(1):213–221. [PMC free article] [PubMed] [Google Scholar]
- Burger P. C., Green S. B. Patient age, histologic features, and length of survival in patients with glioblastoma multiforme. Cancer. 1987 May 1;59(9):1617–1625. doi: 10.1002/1097-0142(19870501)59:9<1617::aid-cncr2820590916>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burger P. C., Kleihues P. Cytologic composition of the untreated glioblastoma with implications for evaluation of needle biopsies. Cancer. 1989 May 15;63(10):2014–2023. doi: 10.1002/1097-0142(19890515)63:10<2014::aid-cncr2820631025>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Clauss M., Gerlach M., Gerlach H., Brett J., Wang F., Familletti P. C., Pan Y. C., Olander J. V., Connolly D. T., Stern D. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990 Dec 1;172(6):1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn G., Soderman D. D., Schaeffer M. T., Wile M., Hatcher V. B., Thomas K. A. Purification of a glycoprotein vascular endothelial cell mitogen from a rat glioma-derived cell line. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1323–1327. doi: 10.1073/pnas.87.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D. T. Vascular permeability factor: a unique regulator of blood vessel function. J Cell Biochem. 1991 Nov;47(3):219–223. doi: 10.1002/jcb.240470306. [DOI] [PubMed] [Google Scholar]
- Constantini S., Kornowski R., Pomeranz S., Rappaport Z. H. Thromboembolic phenomena in neurosurgical patients operated upon for primary and metastatic brain tumors. Acta Neurochir (Wien) 1991;109(3-4):93–97. doi: 10.1007/BF01403001. [DOI] [PubMed] [Google Scholar]
- Criscuolo G. R., Lelkes P. I., Rotrosen D., Oldfield E. H. Cytosolic calcium changes in endothelial cells induced by a protein product of human gliomas containing vascular permeability factor activity. J Neurosurg. 1989 Dec;71(6):884–891. doi: 10.3171/jns.1989.71.6.0884. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Houck K. A., Jakeman L. B., Winer J., Leung D. W. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991 Nov;47(3):211–218. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972 Mar;175(3):409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulling K. H., Nelson J. S. Cerebral astrocytic neoplasms in the adult: contribution of histologic examination to the assessment of prognosis. Semin Diagn Pathol. 1984 May;1(2):152–163. [PubMed] [Google Scholar]
- Gospodarowicz D. Expression and control of vascular endothelial cells: proliferation and differentiation by fibroblast growth factors. J Invest Dermatol. 1989 Aug;93(2 Suppl):39S–47S. doi: 10.1111/1523-1747.ep12580907. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Schweigerer L., Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987 May;8(2):95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hicks C., Breit S. N., Penny R. Response of microvascular endothelial cells to biological response modifiers. Immunol Cell Biol. 1989 Oct;67(Pt 5):271–277. doi: 10.1038/icb.1989.41. [DOI] [PubMed] [Google Scholar]
- Ishikawa F., Miyazono K., Hellman U., Drexler H., Wernstedt C., Hagiwara K., Usuki K., Takaku F., Risau W., Heldin C. H. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989 Apr 13;338(6216):557–562. doi: 10.1038/338557a0. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Li B., Houck K., Winer J., Ferrara N. The vascular endothelial growth factor proteins: identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors. 1992;7(1):53–64. doi: 10.3109/08977199209023937. [DOI] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Maruno M., Kovach J. S., Kelly P. J., Yanagihara T. Transforming growth factor-alpha, epidermal growth factor receptor, and proliferating potential in benign and malignant gliomas. J Neurosurg. 1991 Jul;75(1):97–102. doi: 10.3171/jns.1991.75.1.0097. [DOI] [PubMed] [Google Scholar]
- Maxwell M., Naber S. P., Wolfe H. J., Hedley-Whyte E. T., Galanopoulos T., Neville-Golden J., Antoniades H. N. Expression of angiogenic growth factor genes in primary human astrocytomas may contribute to their growth and progression. Cancer Res. 1991 Feb 15;51(4):1345–1351. [PubMed] [Google Scholar]
- Monreal M., Lafoz E., Casals A., Inaraja L., Montserrat E., Callejas J. M., Martorell A. Occult cancer in patients with deep venous thrombosis. A systematic approach. Cancer. 1991 Jan 15;67(2):541–545. doi: 10.1002/1097-0142(19910115)67:2<541::aid-cncr2820670237>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Murthy U., Basu A., Rodeck U., Herlyn M., Ross A. H., Das M. Binding of an antagonistic monoclonal antibody to an intact and fragmented EGF-receptor polypeptide. Arch Biochem Biophys. 1987 Feb 1;252(2):549–560. doi: 10.1016/0003-9861(87)90062-2. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Sher P. B., Posner J. B., Shapiro W. R. Increased capillary permeability in rat brain induced by factors secreted by cultured C6 glioma cells: role in peritumoral brain edema. J Neurooncol. 1991 Feb;10(1):13–25. doi: 10.1007/BF00151243. [DOI] [PubMed] [Google Scholar]
- Pontén J., Macintyre E. H. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74(4):465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Pontén J., Westermark B. Properties of human malignant glioma cells in vitro. Med Biol. 1978 Aug;56(4):184–193. [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Tischer E., Mitchell R., Hartman T., Silva M., Gospodarowicz D., Fiddes J. C., Abraham J. A. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991 Jun 25;266(18):11947–11954. [PubMed] [Google Scholar]
- Tuomela T. Epidermal growth factor concentrations in submandibular salivary gland, plasma, liver, bile, kidneys and urine of male mice: dynamics after phenylephrine injection. Life Sci. 1990;46(17):1197–1206. doi: 10.1016/0024-3205(90)90494-c. [DOI] [PubMed] [Google Scholar]
- U H. S., Kelley P. Y., Hatton J. D., Shew J. Y. Proto-oncogene abnormalities and their relationship to tumorigenicity in some human glioblastomas. J Neurosurg. 1989 Jul;71(1):83–90. doi: 10.3171/jns.1989.71.1.0083. [DOI] [PubMed] [Google Scholar]
- Wikstrand C. J., Grahmann F. C., McComb R. D., Bigner D. D. Antigenic heterogeneity of human anaplastic gliomas and glioma-derived cell lines defined by monoclonal antibodies. J Neuropathol Exp Neurol. 1985 May;44(3):229–241. doi: 10.1097/00005072-198505000-00002. [DOI] [PubMed] [Google Scholar]
- Zagzag D., Miller D. C., Sato Y., Rifkin D. B., Burstein D. E. Immunohistochemical localization of basic fibroblast growth factor in astrocytomas. Cancer Res. 1990 Nov 15;50(22):7393–7398. [PubMed] [Google Scholar]