Abstract
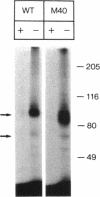
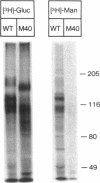
An endothelial cell line (M40) resistant to growth inhibition by transforming growth factor-beta type 1 (TGF beta 1) was isolated by chemical mutagenesis and growth in the presence of TGF beta 1. Like normal endothelial cells, this mutant is characterized by high expression of type II TGF beta receptor and low expression of type I TGF beta receptor. However, the mutant cells display a type II TGF beta receptor of reduced molecular weight as a result of a general defect in N-glycosylation of proteins. The alteration does not impair TGF beta 1 binding to cell surface receptors or the ability of TGF beta 1 to induce fibronectin or plasminogen activator inhibitor-type I production. M40 cells were also resistant to growth inhibition by tumor necrosis factor alpha (TNF alpha) and interleukin-1 alpha (IL-1 alpha) but were inhibited by interferon-gamma (IFN gamma) and heparin. These results imply that TGF beta 1, TNF alpha, and IL-1 alpha act through signal transducing pathways that are separate from pathways for IFN gamma and heparin. Basic fibroblast growth factor was still mitogenic for M40, further suggesting that TGF beta 1, TNF alpha, and IL-1 alpha act by direct inhibition of cell growth rather than by interfering with growth stimulatory pathways.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Dembić Z., Merlin G. Molecular cloning and expression of the human interferon-gamma receptor. Cell. 1988 Oct 21;55(2):273–280. doi: 10.1016/0092-8674(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Baird A., Durkin T. Inhibition of endothelial cell proliferation by type beta-transforming growth factor: interactions with acidic and basic fibroblast growth factors. Biochem Biophys Res Commun. 1986 Jul 16;138(1):476–482. doi: 10.1016/0006-291x(86)90305-0. [DOI] [PubMed] [Google Scholar]
- Boyd F. T., Massagué J. Transforming growth factor-beta inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J Biol Chem. 1989 Feb 5;264(4):2272–2278. [PubMed] [Google Scholar]
- Chinkers M. Isolation and characterization of mink lung epithelial cell mutants resistant to transforming growth factor beta. J Cell Physiol. 1987 Jan;130(1):1–5. doi: 10.1002/jcp.1041300102. [DOI] [PubMed] [Google Scholar]
- Cozzolino F., Torcia M., Aldinucci D., Ziche M., Almerigogna F., Bani D., Stern D. M. Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6487–6491. doi: 10.1073/pnas.87.17.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafeur V., Jiang Z. P., Böhlen P. Signal transduction by bFGF, but not TGF beta 1, involves arachidonic acid metabolism in endothelial cells. J Cell Physiol. 1991 Nov;149(2):277–283. doi: 10.1002/jcp.1041490214. [DOI] [PubMed] [Google Scholar]
- Fafeur V., Terman B. I., Blum J., Böhlen P. Basic FGF treatment of endothelial cells down-regulates the 85-KDa TGF beta receptor subtype and decreases the growth inhibitory response to TGF-beta 1. Growth Factors. 1990;3(3):237–245. doi: 10.3109/08977199009043908. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Friesel R., Komoriya A., Maciag T. Inhibition of endothelial cell proliferation by gamma-interferon. J Cell Biol. 1987 Mar;104(3):689–696. doi: 10.1083/jcb.104.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fràter-Schröder M., Müller G., Birchmeier W., Böhlen P. Transforming growth factor-beta inhibits endothelial cell proliferation. Biochem Biophys Res Commun. 1986 May 29;137(1):295–302. doi: 10.1016/0006-291x(86)91209-x. [DOI] [PubMed] [Google Scholar]
- Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Ploegh H. Inhibitors of oligosaccharide processing. Biochim Biophys Acta. 1985 Jun 24;825(2):95–110. doi: 10.1016/0167-4781(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Geiser A. G., Burmester J. K., Webbink R., Roberts A. B., Sporn M. B. Inhibition of growth by transforming growth factor-beta following fusion of two nonresponsive human carcinoma cell lines. Implication of the type II receptor in growth inhibitory responses. J Biol Chem. 1992 Feb 5;267(4):2588–2593. [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimark R. L., Twardzik D. R., Schwartz S. M. Inhibition of endothelial regeneration by type-beta transforming growth factor from platelets. Science. 1986 Sep 5;233(4768):1078–1080. doi: 10.1126/science.3461562. [DOI] [PubMed] [Google Scholar]
- Hosang M. Recombinant interferon-gamma inhibits the mitogenic effect of platelet-derived growth factor at a level distal to the growth factor receptor. J Cell Physiol. 1988 Mar;134(3):396–404. doi: 10.1002/jcp.1041340310. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Laiho M., Rönnstrand L., Heino J., Decaprio J. A., Ludlow J. W., Livingston D. M., Massagué J. Control of junB and extracellular matrix protein expression by transforming growth factor-beta 1 is independent of simian virus 40 T antigen-sensitive growth-sensitive growth-inhibitory events. Mol Cell Biol. 1991 Feb;11(2):972–978. doi: 10.1128/mcb.11.2.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M., Weis F. M., Boyd F. T., Ignotz R. A., Massagué J. Responsiveness to transforming growth factor-beta (TGF-beta) restored by genetic complementation between cells defective in TGF-beta receptors I and II. J Biol Chem. 1991 May 15;266(14):9108–9112. [PubMed] [Google Scholar]
- Laiho M., Weis M. B., Massagué J. Concomitant loss of transforming growth factor (TGF)-beta receptor types I and II in TGF-beta-resistant cell mutants implicates both receptor types in signal transduction. J Biol Chem. 1990 Oct 25;265(30):18518–18524. [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Tucker A. M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988 Apr;106(4):1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Mundschau L. J., Faller D. V. BALB/c-3T3 fibroblasts resistant to growth inhibition by beta interferon exhibit aberrant platelet-derived growth factor, epidermal growth factor, and fibroblast growth factor signal transduction. Mol Cell Biol. 1991 Jun;11(6):3148–3154. doi: 10.1128/mcb.11.6.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G., Behrens J., Nussbaumer U., Böhlen P., Birchmeier W. Inhibitory action of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5600–5604. doi: 10.1073/pnas.84.16.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukac L. A., Ottlinger M. E., Karnovsky M. J. Heparin suppresses specific second messenger pathways for protooncogene expression in rat vascular smooth muscle cells. J Biol Chem. 1992 Feb 25;267(6):3707–3711. [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Rifkin D. B. The opposing effects of basic fibroblast growth factor and transforming growth factor beta on the regulation of plasminogen activator activity in capillary endothelial cells. J Cell Biol. 1987 Aug;105(2):957–963. doi: 10.1083/jcb.105.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon A., Decker M., Müller T., Armellino D., Kovesdi I., Gluzman Y., Böhlen P. Structure/activity relationships in basic FGF. Ann N Y Acad Sci. 1991;638:98–108. doi: 10.1111/j.1749-6632.1991.tb49021.x. [DOI] [PubMed] [Google Scholar]
- Segarini P. R., Rosen D. M., Seyedin S. M. Binding of transforming growth factor-beta to cell surface proteins varies with cell type. Mol Endocrinol. 1989 Feb;3(2):261–272. doi: 10.1210/mend-3-2-261. [DOI] [PubMed] [Google Scholar]
- Sims J. E., March C. J., Cosman D., Widmer M. B., MacDonald H. R., McMahan C. J., Grubin C. E., Wignall J. M., Jackson J. L., Call S. M. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science. 1988 Jul 29;241(4865):585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. TGF-beta: problems and prospects. Cell Regul. 1990 Nov;1(12):875–882. doi: 10.1091/mbc.1.12.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengers E. D., Kluft C. Plasminogen activator inhibitors. Blood. 1987 Feb;69(2):381–387. [PubMed] [Google Scholar]