Abstract
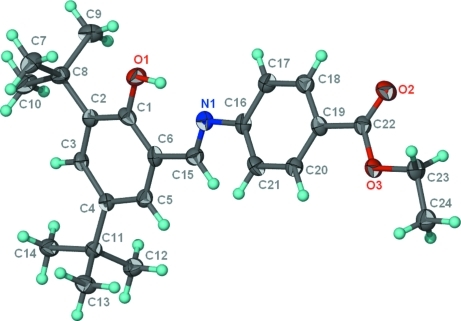
The title compound, a Schiff base, C24H31NO3, has a substituted aromatic ring at both ends of the azomethine linkage and these make a dihedral angle of 24.9 (1)°. There is an intramolecular hydrogen bond between the hydroxy group (donor) and the N atom of themazomethine linkage.
Related literature
For the use of the methyl ester analog of the title compound as a second-harmonic generation material, see: Sliwa et al. (2008 ▶).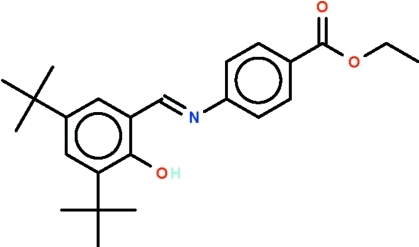
Experimental
Crystal data
C24H31NO3
M r = 381.50
Monoclinic,
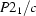
a = 18.4789 (18) Å
b = 10.7194 (11) Å
c = 10.7768 (10) Å
β = 97.437 (2)°
V = 2116.7 (4) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 100 K
0.30 × 0.05 × 0.05 mm
Data collection
Bruker SMART APEX diffractometer
19941 measured reflections
4855 independent reflections
3123 reflections with I > 2σ(I)
R int = 0.065
Refinement
R[F 2 > 2σ(F 2)] = 0.053
wR(F 2) = 0.142
S = 1.01
4855 reflections
257 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.64 e Å−3
Δρmin = −0.26 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810041383/fl2320sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810041383/fl2320Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯N1 | 0.87 (1) | 1.80 (2) | 2.609 (2) | 154 (3) |
Acknowledgments
We thank the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
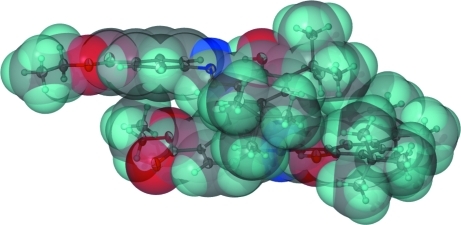
The Schiff base, methyl 4-(di-3,5-tert-butyl-2-hydroxybenzylideneamino)benzoate, is a material suitable for second-harmonic generation as it has electron-donating and electron-withdrawing components that are critical for the manifestation of a permanent dipole (Sliwa et al., 2008). Replacing the methyl group with an ethyl moiety leads to (I), an intensely orange-colored compound (Scheme I, Fig. 1) that crystallizes in a centric space group and is, therefore, not suitable as an SHG material. The azomethine bond has an E-configuration; the two aromatic rings are aligned at 24.9 (1) °. The compound is neutral as the hydroxy group bears a hydrogen atom which is a donor in an intra-molecular H bond to the azomethine nitrogen atom (Table 1). There are no important intermolecular contacts; on the other hand, the compound appears to pack in such a way as to accomodate the bulky t-butyl groups as far as possible (Fig. 2).
Experimental
Ethyl 4-aminobenzoate (0.35 g) dissolved in ethanol (5 ml) was added to 3,5-di-tert-butyl-2-hydroxybenzaldehyde (0.5 g) dissolved in ethanol (20 ml). Several drops of acetic acid were added. The solution was heated for 3 h. The solvent was evaporated and the product recrystallized from ethanol to yield orange prisms in 80% yield that were suitable for data collection.
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.95–0.98 Å) and were included in the refinement in the riding model approximation, with U iso(H) set to 1.2–1.5U eq(C).
The hydroxy H-atom was located in a difference Fourier map, and was refined with the O–H distance restrained to 0.84±0.01 Å; its temperature factor was refined.
In the final difference Fourier map, the largest peak was in the vicinity of an aromatic H-atom.
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of the C24H31NO3 at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Fig. 2.
Van der Waals packing of two adjacent molecules.
Crystal data
| C24H31NO3 | F(000) = 824 |
| Mr = 381.50 | Dx = 1.197 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 18.4789 (18) Å | Cell parameters from 2280 reflections |
| b = 10.7194 (11) Å | θ = 2.2–23.8° |
| c = 10.7768 (10) Å | µ = 0.08 mm−1 |
| β = 97.437 (2)° | T = 100 K |
| V = 2116.7 (4) Å3 | Prism, orange |
| Z = 4 | 0.30 × 0.05 × 0.05 mm |
Data collection
| Bruker SMART APEX diffractometer | 3123 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.065 |
| graphite | θmax = 27.5°, θmin = 2.2° |
| ω scans | h = −24→23 |
| 19941 measured reflections | k = −13→13 |
| 4855 independent reflections | l = −13→14 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.053 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.142 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0558P)2 + 1.1473P] where P = (Fo2 + 2Fc2)/3 |
| 4855 reflections | (Δ/σ)max = 0.001 |
| 257 parameters | Δρmax = 0.64 e Å−3 |
| 1 restraint | Δρmin = −0.25 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.78175 (8) | 0.89233 (13) | 0.62825 (14) | 0.0252 (3) | |
| H1 | 0.7466 (13) | 0.885 (3) | 0.567 (2) | 0.086 (12)* | |
| O2 | 0.42198 (8) | 0.93692 (14) | 0.03223 (14) | 0.0291 (4) | |
| O3 | 0.43875 (7) | 0.73467 (13) | −0.01081 (13) | 0.0237 (3) | |
| N1 | 0.67954 (9) | 0.79668 (16) | 0.46645 (16) | 0.0222 (4) | |
| C1 | 0.78970 (11) | 0.77878 (18) | 0.68363 (19) | 0.0203 (4) | |
| C2 | 0.84179 (10) | 0.76051 (18) | 0.78972 (18) | 0.0186 (4) | |
| C3 | 0.85050 (10) | 0.63866 (18) | 0.83515 (18) | 0.0180 (4) | |
| H3 | 0.8862 | 0.6243 | 0.9054 | 0.022* | |
| C4 | 0.80999 (10) | 0.53616 (18) | 0.78384 (18) | 0.0183 (4) | |
| C5 | 0.75592 (10) | 0.56031 (19) | 0.68469 (18) | 0.0199 (4) | |
| H5 | 0.7253 | 0.4941 | 0.6509 | 0.024* | |
| C6 | 0.74560 (10) | 0.67938 (19) | 0.63368 (18) | 0.0201 (4) | |
| C7 | 0.94204 (12) | 0.9088 (2) | 0.7566 (2) | 0.0290 (5) | |
| H7A | 0.9137 | 0.9359 | 0.6780 | 0.044* | |
| H7B | 0.9735 | 0.8388 | 0.7402 | 0.044* | |
| H7C | 0.9722 | 0.9782 | 0.7930 | 0.044* | |
| C8 | 0.88965 (11) | 0.86740 (19) | 0.84883 (19) | 0.0219 (4) | |
| C9 | 0.84263 (13) | 0.9784 (2) | 0.8811 (2) | 0.0295 (5) | |
| H9A | 0.8124 | 1.0072 | 0.8051 | 0.044* | |
| H9B | 0.8744 | 1.0464 | 0.9158 | 0.044* | |
| H9C | 0.8112 | 0.9521 | 0.9428 | 0.044* | |
| C10 | 0.93590 (13) | 0.8274 (2) | 0.9709 (2) | 0.0304 (5) | |
| H10A | 0.9670 | 0.7569 | 0.9542 | 0.046* | |
| H10B | 0.9037 | 0.8024 | 1.0320 | 0.046* | |
| H10C | 0.9665 | 0.8974 | 1.0044 | 0.046* | |
| C11 | 0.82432 (11) | 0.40232 (18) | 0.82969 (18) | 0.0197 (4) | |
| C12 | 0.75683 (11) | 0.3511 (2) | 0.8825 (2) | 0.0254 (5) | |
| H12A | 0.7465 | 0.4030 | 0.9529 | 0.038* | |
| H12B | 0.7662 | 0.2652 | 0.9111 | 0.038* | |
| H12C | 0.7148 | 0.3526 | 0.8169 | 0.038* | |
| C13 | 0.84080 (12) | 0.3212 (2) | 0.7192 (2) | 0.0259 (5) | |
| H13A | 0.8838 | 0.3540 | 0.6858 | 0.039* | |
| H13B | 0.7988 | 0.3225 | 0.6535 | 0.039* | |
| H13C | 0.8502 | 0.2352 | 0.7479 | 0.039* | |
| C14 | 0.88904 (11) | 0.3930 (2) | 0.9334 (2) | 0.0247 (5) | |
| H14A | 0.9330 | 0.4251 | 0.9025 | 0.037* | |
| H14B | 0.8966 | 0.3056 | 0.9583 | 0.037* | |
| H14C | 0.8789 | 0.4423 | 1.0057 | 0.037* | |
| C15 | 0.68922 (11) | 0.69415 (19) | 0.52586 (19) | 0.0219 (4) | |
| H15 | 0.6589 | 0.6250 | 0.4995 | 0.026* | |
| C16 | 0.62487 (10) | 0.8028 (2) | 0.36008 (19) | 0.0213 (4) | |
| C17 | 0.59190 (11) | 0.9167 (2) | 0.3314 (2) | 0.0235 (5) | |
| H17 | 0.6062 | 0.9876 | 0.3815 | 0.028* | |
| C18 | 0.53831 (11) | 0.9279 (2) | 0.23032 (19) | 0.0228 (5) | |
| H18 | 0.5157 | 1.0064 | 0.2116 | 0.027* | |
| C19 | 0.51707 (10) | 0.82475 (19) | 0.15535 (19) | 0.0202 (4) | |
| C20 | 0.55214 (11) | 0.7112 (2) | 0.18075 (19) | 0.0214 (4) | |
| H20 | 0.5391 | 0.6411 | 0.1286 | 0.026* | |
| C21 | 0.60615 (11) | 0.7002 (2) | 0.28224 (19) | 0.0234 (5) | |
| H21 | 0.6305 | 0.6228 | 0.2990 | 0.028* | |
| C22 | 0.45526 (11) | 0.84073 (19) | 0.05346 (19) | 0.0209 (4) | |
| C23 | 0.37799 (11) | 0.7442 (2) | −0.1100 (2) | 0.0265 (5) | |
| H23A | 0.3908 | 0.8002 | −0.1768 | 0.032* | |
| H23B | 0.3347 | 0.7788 | −0.0767 | 0.032* | |
| C24 | 0.36159 (12) | 0.6163 (2) | −0.1613 (2) | 0.0281 (5) | |
| H24A | 0.3206 | 0.6204 | −0.2286 | 0.042* | |
| H24B | 0.3488 | 0.5616 | −0.0945 | 0.042* | |
| H24C | 0.4046 | 0.5830 | −0.1944 | 0.042* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0265 (8) | 0.0202 (8) | 0.0271 (8) | −0.0029 (6) | −0.0030 (7) | 0.0048 (6) |
| O2 | 0.0308 (8) | 0.0244 (8) | 0.0291 (8) | 0.0063 (7) | −0.0075 (7) | 0.0009 (7) |
| O3 | 0.0216 (7) | 0.0233 (8) | 0.0235 (8) | 0.0036 (6) | −0.0069 (6) | −0.0009 (6) |
| N1 | 0.0198 (8) | 0.0244 (9) | 0.0225 (9) | 0.0007 (7) | 0.0031 (7) | 0.0006 (8) |
| C1 | 0.0218 (10) | 0.0195 (10) | 0.0202 (10) | 0.0052 (8) | 0.0057 (8) | 0.0059 (8) |
| C2 | 0.0173 (9) | 0.0200 (10) | 0.0187 (10) | 0.0001 (8) | 0.0031 (8) | 0.0001 (8) |
| C3 | 0.0162 (9) | 0.0229 (10) | 0.0143 (9) | 0.0016 (8) | −0.0008 (8) | 0.0021 (8) |
| C4 | 0.0165 (9) | 0.0206 (10) | 0.0178 (10) | 0.0008 (8) | 0.0023 (8) | 0.0009 (8) |
| C5 | 0.0173 (9) | 0.0229 (11) | 0.0191 (10) | −0.0008 (8) | 0.0011 (8) | −0.0032 (8) |
| C6 | 0.0154 (9) | 0.0273 (11) | 0.0173 (10) | 0.0025 (8) | 0.0004 (8) | 0.0000 (9) |
| C7 | 0.0294 (12) | 0.0291 (12) | 0.0286 (12) | −0.0085 (9) | 0.0037 (10) | 0.0017 (10) |
| C8 | 0.0252 (10) | 0.0193 (10) | 0.0204 (10) | −0.0026 (8) | 0.0000 (8) | 0.0017 (8) |
| C9 | 0.0393 (13) | 0.0220 (11) | 0.0273 (12) | −0.0012 (10) | 0.0047 (10) | −0.0015 (9) |
| C10 | 0.0351 (12) | 0.0253 (12) | 0.0275 (12) | −0.0087 (10) | −0.0080 (10) | 0.0024 (10) |
| C11 | 0.0201 (10) | 0.0191 (10) | 0.0192 (10) | 0.0004 (8) | 0.0002 (8) | −0.0015 (8) |
| C12 | 0.0262 (11) | 0.0220 (11) | 0.0280 (12) | −0.0025 (9) | 0.0031 (9) | 0.0009 (9) |
| C13 | 0.0309 (11) | 0.0232 (11) | 0.0232 (11) | 0.0051 (9) | 0.0023 (9) | −0.0025 (9) |
| C14 | 0.0269 (11) | 0.0202 (11) | 0.0254 (11) | 0.0022 (9) | −0.0024 (9) | 0.0009 (9) |
| C15 | 0.0212 (10) | 0.0212 (11) | 0.0235 (11) | −0.0011 (8) | 0.0039 (8) | −0.0020 (9) |
| C16 | 0.0170 (9) | 0.0283 (12) | 0.0184 (10) | 0.0001 (8) | 0.0014 (8) | 0.0009 (9) |
| C17 | 0.0216 (10) | 0.0247 (11) | 0.0238 (11) | −0.0014 (9) | 0.0013 (9) | −0.0008 (9) |
| C18 | 0.0226 (10) | 0.0227 (11) | 0.0227 (11) | 0.0021 (8) | 0.0012 (9) | 0.0015 (9) |
| C19 | 0.0187 (10) | 0.0239 (11) | 0.0174 (10) | 0.0028 (8) | 0.0004 (8) | 0.0021 (8) |
| C20 | 0.0205 (10) | 0.0228 (11) | 0.0204 (10) | −0.0005 (8) | 0.0016 (8) | 0.0003 (9) |
| C21 | 0.0216 (10) | 0.0241 (11) | 0.0238 (11) | 0.0060 (9) | 0.0004 (8) | 0.0039 (9) |
| C22 | 0.0194 (10) | 0.0232 (11) | 0.0198 (10) | 0.0001 (8) | 0.0012 (8) | 0.0020 (9) |
| C23 | 0.0240 (11) | 0.0295 (12) | 0.0231 (11) | 0.0018 (9) | −0.0082 (9) | 0.0016 (9) |
| C24 | 0.0261 (11) | 0.0306 (12) | 0.0271 (12) | −0.0001 (10) | 0.0012 (9) | −0.0006 (10) |
Geometric parameters (Å, °)
| O1—C1 | 1.355 (2) | C11—C14 | 1.531 (3) |
| O1—H1 | 0.866 (10) | C11—C13 | 1.537 (3) |
| O2—C22 | 1.207 (2) | C11—C12 | 1.537 (3) |
| O3—C22 | 1.346 (2) | C12—H12A | 0.9800 |
| O3—C23 | 1.450 (2) | C12—H12B | 0.9800 |
| N1—C15 | 1.273 (3) | C12—H12C | 0.9800 |
| N1—C16 | 1.428 (3) | C13—H13A | 0.9800 |
| C1—C6 | 1.406 (3) | C13—H13B | 0.9800 |
| C1—C2 | 1.410 (3) | C13—H13C | 0.9800 |
| C2—C3 | 1.397 (3) | C14—H14A | 0.9800 |
| C2—C8 | 1.535 (3) | C14—H14B | 0.9800 |
| C3—C4 | 1.402 (3) | C14—H14C | 0.9800 |
| C3—H3 | 0.9500 | C15—H15 | 0.9500 |
| C4—C5 | 1.389 (3) | C16—C17 | 1.381 (3) |
| C4—C11 | 1.529 (3) | C16—C21 | 1.399 (3) |
| C5—C6 | 1.393 (3) | C17—C18 | 1.379 (3) |
| C5—H5 | 0.9500 | C17—H17 | 0.9500 |
| C6—C15 | 1.465 (3) | C18—C19 | 1.395 (3) |
| C7—C8 | 1.540 (3) | C18—H18 | 0.9500 |
| C7—H7A | 0.9800 | C19—C20 | 1.389 (3) |
| C7—H7B | 0.9800 | C19—C22 | 1.488 (3) |
| C7—H7C | 0.9800 | C20—C21 | 1.387 (3) |
| C8—C10 | 1.534 (3) | C20—H20 | 0.9500 |
| C8—C9 | 1.539 (3) | C21—H21 | 0.9500 |
| C9—H9A | 0.9800 | C23—C24 | 1.494 (3) |
| C9—H9B | 0.9800 | C23—H23A | 0.9900 |
| C9—H9C | 0.9800 | C23—H23B | 0.9900 |
| C10—H10A | 0.9800 | C24—H24A | 0.9800 |
| C10—H10B | 0.9800 | C24—H24B | 0.9800 |
| C10—H10C | 0.9800 | C24—H24C | 0.9800 |
| C1—O1—H1 | 106 (2) | H12A—C12—H12B | 109.5 |
| C22—O3—C23 | 114.99 (16) | C11—C12—H12C | 109.5 |
| C15—N1—C16 | 118.83 (18) | H12A—C12—H12C | 109.5 |
| O1—C1—C6 | 119.20 (18) | H12B—C12—H12C | 109.5 |
| O1—C1—C2 | 120.37 (18) | C11—C13—H13A | 109.5 |
| C6—C1—C2 | 120.43 (18) | C11—C13—H13B | 109.5 |
| C3—C2—C1 | 116.73 (18) | H13A—C13—H13B | 109.5 |
| C3—C2—C8 | 121.24 (17) | C11—C13—H13C | 109.5 |
| C1—C2—C8 | 121.96 (18) | H13A—C13—H13C | 109.5 |
| C2—C3—C4 | 124.26 (18) | H13B—C13—H13C | 109.5 |
| C2—C3—H3 | 117.9 | C11—C14—H14A | 109.5 |
| C4—C3—H3 | 117.9 | C11—C14—H14B | 109.5 |
| C5—C4—C3 | 116.83 (18) | H14A—C14—H14B | 109.5 |
| C5—C4—C11 | 120.03 (17) | C11—C14—H14C | 109.5 |
| C3—C4—C11 | 123.12 (17) | H14A—C14—H14C | 109.5 |
| C4—C5—C6 | 121.49 (19) | H14B—C14—H14C | 109.5 |
| C4—C5—H5 | 119.3 | N1—C15—C6 | 122.09 (19) |
| C6—C5—H5 | 119.3 | N1—C15—H15 | 119.0 |
| C5—C6—C1 | 120.03 (18) | C6—C15—H15 | 119.0 |
| C5—C6—C15 | 117.32 (18) | C17—C16—C21 | 119.51 (19) |
| C1—C6—C15 | 122.63 (19) | C17—C16—N1 | 117.79 (19) |
| C8—C7—H7A | 109.5 | C21—C16—N1 | 122.64 (19) |
| C8—C7—H7B | 109.5 | C18—C17—C16 | 120.3 (2) |
| H7A—C7—H7B | 109.5 | C18—C17—H17 | 119.8 |
| C8—C7—H7C | 109.5 | C16—C17—H17 | 119.8 |
| H7A—C7—H7C | 109.5 | C17—C18—C19 | 120.5 (2) |
| H7B—C7—H7C | 109.5 | C17—C18—H18 | 119.7 |
| C10—C8—C2 | 112.00 (17) | C19—C18—H18 | 119.7 |
| C10—C8—C7 | 107.87 (18) | C20—C19—C18 | 119.40 (18) |
| C2—C8—C7 | 108.92 (17) | C20—C19—C22 | 122.69 (19) |
| C10—C8—C9 | 106.89 (17) | C18—C19—C22 | 117.87 (18) |
| C2—C8—C9 | 111.03 (17) | C21—C20—C19 | 120.0 (2) |
| C7—C8—C9 | 110.07 (18) | C21—C20—H20 | 120.0 |
| C8—C9—H9A | 109.5 | C19—C20—H20 | 120.0 |
| C8—C9—H9B | 109.5 | C20—C21—C16 | 120.18 (19) |
| H9A—C9—H9B | 109.5 | C20—C21—H21 | 119.9 |
| C8—C9—H9C | 109.5 | C16—C21—H21 | 119.9 |
| H9A—C9—H9C | 109.5 | O2—C22—O3 | 123.23 (18) |
| H9B—C9—H9C | 109.5 | O2—C22—C19 | 124.17 (19) |
| C8—C10—H10A | 109.5 | O3—C22—C19 | 112.57 (17) |
| C8—C10—H10B | 109.5 | O3—C23—C24 | 107.99 (17) |
| H10A—C10—H10B | 109.5 | O3—C23—H23A | 110.1 |
| C8—C10—H10C | 109.5 | C24—C23—H23A | 110.1 |
| H10A—C10—H10C | 109.5 | O3—C23—H23B | 110.1 |
| H10B—C10—H10C | 109.5 | C24—C23—H23B | 110.1 |
| C4—C11—C14 | 112.55 (16) | H23A—C23—H23B | 108.4 |
| C4—C11—C13 | 108.92 (16) | C23—C24—H24A | 109.5 |
| C14—C11—C13 | 108.30 (16) | C23—C24—H24B | 109.5 |
| C4—C11—C12 | 109.85 (16) | H24A—C24—H24B | 109.5 |
| C14—C11—C12 | 107.53 (17) | C23—C24—H24C | 109.5 |
| C13—C11—C12 | 109.64 (17) | H24A—C24—H24C | 109.5 |
| C11—C12—H12A | 109.5 | H24B—C24—H24C | 109.5 |
| C11—C12—H12B | 109.5 | ||
| O1—C1—C2—C3 | −175.11 (17) | C3—C4—C11—C13 | −122.2 (2) |
| C6—C1—C2—C3 | 4.6 (3) | C5—C4—C11—C12 | −64.1 (2) |
| O1—C1—C2—C8 | 1.8 (3) | C3—C4—C11—C12 | 117.6 (2) |
| C6—C1—C2—C8 | −178.52 (18) | C16—N1—C15—C6 | 178.21 (17) |
| C1—C2—C3—C4 | −1.5 (3) | C5—C6—C15—N1 | −174.29 (19) |
| C8—C2—C3—C4 | −178.36 (18) | C1—C6—C15—N1 | 4.0 (3) |
| C2—C3—C4—C5 | −2.7 (3) | C15—N1—C16—C17 | 151.3 (2) |
| C2—C3—C4—C11 | 175.58 (19) | C15—N1—C16—C21 | −31.4 (3) |
| C3—C4—C5—C6 | 3.9 (3) | C21—C16—C17—C18 | 3.2 (3) |
| C11—C4—C5—C6 | −174.44 (18) | N1—C16—C17—C18 | −179.46 (18) |
| C4—C5—C6—C1 | −0.9 (3) | C16—C17—C18—C19 | −0.4 (3) |
| C4—C5—C6—C15 | 177.43 (18) | C17—C18—C19—C20 | −2.2 (3) |
| O1—C1—C6—C5 | 176.18 (18) | C17—C18—C19—C22 | 175.61 (19) |
| C2—C1—C6—C5 | −3.5 (3) | C18—C19—C20—C21 | 2.0 (3) |
| O1—C1—C6—C15 | −2.1 (3) | C22—C19—C20—C21 | −175.68 (18) |
| C2—C1—C6—C15 | 178.18 (18) | C19—C20—C21—C16 | 0.8 (3) |
| C3—C2—C8—C10 | −9.4 (3) | C17—C16—C21—C20 | −3.4 (3) |
| C1—C2—C8—C10 | 173.86 (19) | N1—C16—C21—C20 | 179.43 (18) |
| C3—C2—C8—C7 | 109.8 (2) | C23—O3—C22—O2 | 0.8 (3) |
| C1—C2—C8—C7 | −66.9 (2) | C23—O3—C22—C19 | 178.95 (17) |
| C3—C2—C8—C9 | −128.8 (2) | C20—C19—C22—O2 | 177.1 (2) |
| C1—C2—C8—C9 | 54.5 (2) | C18—C19—C22—O2 | −0.6 (3) |
| C5—C4—C11—C14 | 176.14 (18) | C20—C19—C22—O3 | −1.0 (3) |
| C3—C4—C11—C14 | −2.1 (3) | C18—C19—C22—O3 | −178.74 (18) |
| C5—C4—C11—C13 | 56.0 (2) | C22—O3—C23—C24 | −173.52 (17) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···N1 | 0.87 (1) | 1.80 (2) | 2.609 (2) | 154 (3) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FL2320).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bruker (2009). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sliwa, M., Spangenberg, A., Metivier, R., Letard, S., Nakatani, K. & Yu, P. (2008). Res. Chem. Intermed.34, 181–190.
- Westrip, S. P. (2010). J. Appl. Cryst.43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810041383/fl2320sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810041383/fl2320Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report